Abstract
Understanding dispersal and habitat selection behaviours is central to many problems in ecology, evolution and conservation. One factor often hypothesized to influence habitat selection by dispersers is the natal environment experienced by juveniles. Nonetheless, evidence for the effect of natal environment on dispersing, wild vertebrates remains limited. Using 18 years of nesting and mark–resight data across an entire North American geographical range of an endangered bird, the snail kite (Rostrhamus sociabilis), we tested for natal effects on breeding-site selection by dispersers and its consequences for reproductive success and population structure. Dispersing snail kites were more likely to nest in wetlands of the same habitat type (lacustrine or palustrine) as their natal wetland, independent of dispersal distance, but this preference declined with age and if individuals were born during droughts. Importantly, dispersing kites that bred in natal-like habitats had lower nest success and productivity than kites that did not. These behaviours help explain recently described population connectivity and spatial structure across their geographical range and reveal that assortative breeding is occurring, where birds are more likely to breed with individuals born in the same wetland type as their natal habitat. Natal environments can thus have long-term and large-scale effects on populations in nature, even in highly mobile animals.
Keywords: connectivity, dispersal, evolutionary trap, natal habitat preference induction, snail kite, spatial network
1. Introduction
The influence of early-life events on later adult behaviours has long attracted the interest of biologists, psychologists and the general public. Early-life events can influence a variety of ecological and evolutionary processes later in life, such as habitat selection, dispersal, foraging and the formation of host races [1,2]. In particular, it is often hypothesized that individuals may show preferences for habitats similar to those experienced in their natal environment [3]. Such preferences are often assumed to be important, in part, because settling in habitats similar to natal environments could confer several fitness benefits. Benefits can arise through a variety of mechanisms, such as habitat familiarity or more rapid settlement [4,5]. If natal habitat preferences by dispersing animals occur, this process can be highly relevant for interpreting animal distribution [3], population structure [6] and conservation strategies [7].
Nonetheless, natal habitat preferences of dispersers have been rarely documented in wild populations of vertebrates for at least two reasons [8,9]. First and foremost, determining natal habitat preferences later in life requires the ability to track dispersal and subsequent breeding across entire landscapes, which is challenging in many vertebrates [10,11]. Second, to interpret natal habitat preferences, it is necessary to disentangle effects of dispersal distance and natal habitat preferences [9,12]. Because habitat variation is often spatially autocorrelated [13] and dispersal probability decreases as a function of distance [14], habitats selected may appear to be consistent with natal habitat preference yet could more parsimoniously be driven simply by geographical constraints on dispersal.
Several hypotheses have been proposed regarding factors that may alter natal habitat preferences. For example, Stamps et al. [15] hypothesize that if individuals have unfavourable natal experiences (e.g. little food or shelter), individuals should be less likely to have natal habitat preferences than individuals that have favourable experiences. In addition, it is often hypothesized that natal habitat preferences should diminish with age [8], because as individuals grow older they should be more likely to use recent information in guiding habitat selection decisions [16]. While such age- and experience-related hypotheses are relevant for interpreting the ecological and evolutionary consequences of natal habitat preferences, these hypotheses have been rarely tested.
Here, we use 18 years of mark–recapture and nesting data on a highly mobile, endangered bird, the snail kite (Rostrhamus sociabilis), to determine the influence of natal environments on habitat preferences of dispersing kites later in life. Snail kites exhibit regional fidelity in movements that appears to be linked to natal habitats [17], but these patterns have not been linked to nesting decisions. We first test whether natal dispersal is influenced by natal habitat preferences. We then test hypotheses regarding age and environmental conditions mediating the natal habitat preferences by dispersers. Finally, we link observed preferences to reproductive success and ask whether such preferences can explain recently reported patterns of population structure and connectivity in this critically endangered bird [18].
2. Methods
(a). Focal species and study area
In North America, the snail kite is a critically endangered bird, confined to central and south Florida. The snail kite is dependent upon shallow, freshwater wetland ecosystems dominated by sparsely emergent vegetation. This species is highly mobile and can move across its entire geographical range over short periods of time [19]. Nonetheless, recent analyses suggest a tendency for movements to generate potential spatial population structure, with two functional regions being identified [18,20].
Snail kites breed in different types of wetlands [21], including both lacustrine (littoral habitats along the perimeters of lakes) and palustrine habitats (freshwater marshes; see [22] for all criteria used to delineate wetland types). Key differences in the criteria delineating these wetland types for the region include: (i) lacustrine wetlands occur in either topographic depressions or a dammed river channel; (ii) lacustrine wetlands lack trees, shrubs or persistent emergent herbaceous vegetation with greater than 30% areal coverage in wetlands, whereas palustrine wetlands are dominated by persistent emergent herbaceous vegetation; and (iii) water depths in the deepest part of basins at low water are greater than 2 m for lacustrine and less than 2 m for palustrine wetlands, respectively [22]. In this region, lacustrine habitats are dominated by cattail (Typha spp.) and bulrush (Scirpus spp.), and tend to have more stable hydrology than palustrine habitats. Palustrine habitats are frequently dominated by sawgrass (Cladium jamaicense) and have more dynamic hydrology that is more sensitive to precipitation and drought [23]. Snail kites primarily place nests in Scirpus and Typha in lacustrine wetlands, whereas they tend to place nests in woody substrates (Annona glabra, Salix spp.) in palustrine wetlands [21].
(b). Measuring dispersal and nesting
Snail kites have been monitored for decades, but here we focus on standardized mark–recapture and nest monitoring data that were collected at all known breeding wetlands across the entire geographical range, in 1996–2013 [24,25]. In each wetland, airboat surveys were conducted every 18–21 days during the peak of the breeding season (1 March–30 June; four to six surveys each year). During these surveys, attempts were made to find all nests and monitor them until completion (failure or success). Nest monitoring began before and continued beyond the duration of the standardized surveys (typically January–October each year). Nests were checked every two to three weeks until completion (successful nests are active approx. 56 days; [21]). At each nest visit, observers recorded whether adults were banded and their band combinations, as well as other relevant metrics, such as the nesting substrate. At approximately 24 days of age, nestlings were banded with unique colour combinations. Between 1996 and 2013, we monitored 947 nests where at least one parent was banded (approx. one-third of all nests monitored). Birds were marked as nestlings just prior to fledging, such that natal location was known for all banded birds.
(c). Analysis
(i). Natal dispersal
We first contrasted different potential factors that may influence natal habitat preferences for natal dispersal, defined as the movement of individuals from their natal origin to a site that they attempt to reproduce in their first potential breeding season, or when they were 1-year old [26]. We first focus on natal dispersal, because such movements and decisions are not based on previous breeding decisions or outcomes. We contrasted potential natal habitat preferences of these dispersing kites to potential preference for larger wetlands [23], palustrine wetlands [27] or nearby wetlands (simply distance-driven dispersal) [17,23].
For testing potential natal habitat preferences, we determined the similarity of natal sites to current nest sites using similarity measures [8,28]. We considered three factors of relevance to potential natal habitat preferences: (i) wetland vegetation types; (ii) nesting substrates used in wetlands; and (iii) spatial characteristics of wetlands [29]. For wetland vegetation types, we used the National Wetlands Inventory database [22] to extract information regarding wetland type—palustrine versus lacustrine—at each nest location and the proportion of each wetland type within 2 km of nest sites. Two kilometres was used here, because breeding snail kites have been observed to forage primarily within 2 km of nest sites, and such scales have proved useful for interpreting snail kite reproduction elsewhere [25]. With these two variables, we calculated a Euclidean distance matrix among sites and then performed a cluster analysis (partitioning around Medoids analysis, related to k-means clustering but more robust to outliers [30]) to classify sites. We contrasted one to seven cluster alternatives using silhouette plots [30]; a two-cluster solution was most supported by the data, explaining 94% of the variation in wetland categories measured at the nest and landscape scale (2 km scale). This two-cluster solution resulted in classifying wetlands as either predominantly lacustrine or palustrine wetlands (see the electronic supplementary material, figures S1–S2). Using this classification, we then used a binary covariate of whether the current wetland was the same or different type of wetland as the natal wetland. For nesting substrates, we used a Bray–Curtis dissimilarity matrix among sites based on the dominant plant species used as a nesting substrate for each nest. For spatial characteristics, we focused on wetland area, because this factor has been shown to explain variation in mark–resight data [23]. Based on wetland area, we calculated a Euclidean dissimilarity matrix among sites.
With these measures, we used conditional logistic regression [31] to test if dispersing juveniles selected sites based on: distance to natal wetland, wetland area and wetland type, and contrasted these factors to whether the selected site was similar to their natal site, in terms of wetland type, nest substrates or wetland spatial characteristics. In this context, conditional logistic regression uses a matched, case–control response design to compare each site selected by individual dispersers to sites not chosen but were surveyed for kites (8–17 available sites depending on sampling effort each year). Consequently, conditional logistic regression can be a powerful technique to assess disperser preferences [31,32], and it can account for distance-related effects that can arise from either spatially autocorrelated resource distribution or constraints on dispersal when assessing other covariates [33]. We contrasted univariate models for each factor and also considered models that included both distance and habitat preference covariates to determine if natal habitat preferences persisted when controlling for dispersal distance effects. We compared models using Akaike's information criterion, adjusted for sample size.
(ii). Dispersal and natal habitat preferences over time
Based on the results for natal dispersal, we then addressed hypotheses regarding variation in natal habitat preference—age and natal environmental conditions—using all observed nesting events of marked birds that dispersed from their natal wetland. To do so, we considered models that allowed for natal habitat preferences to change with age (natal × age) and for annual variation in natal environmental conditions, accounting for repeated measures of individuals over time through the use of robust standard errors (generalized estimating equations) in inferences from conditional logit models [32]. Because all individuals were marked as nestlings, calculating age (years) of the individuals observed nesting was straightforward.
Annual variation in drought conditions can impact prey availability for snail kites [24], thus reducing habitat quality, and previously we have shown that drought conditions can have strong impacts on both reproduction and survival in snail kites [34]. We expected that if drought effects impacted natal habitat preferences, it would do so for birds born in palustrine wetlands, because snail kite survival decreases in palustrine, but not lacustrine, wetlands during droughts [23]. We quantified drought conditions using the palmer hydrological drought index (PHDI), an index derived by the National Oceanic and Atmospheric Administration to capture hydrological drought and wet conditions regarding reservoirs and groundwater, which has been linked to snail kite reproduction previously [24]. We calculated average PHDI for the two regions captured in the geographical range of snail kites for the period of 15 April–15 August each year, a time considered to be limiting for breeding and recently fledged juveniles [35]. We then used a binary measure where we considered values of PHDI of −2 to indicate moderately dry years for birds born in palustrine wetlands; using a categorical measure reflecting more extreme drought conditions (e.g. −3) provided similar results. Note that we could not include lag effects, because we observed only a sample of all individual nesting attempts and individual snail kites may not breed every year [24].
(iii). Natal habitat preference and reproductive success
We determined whether reproductive success of dispersing kites, specifically nest success (i.e. daily survival rates, DSRs), the number of young fledged per successful nest and nest productivity (i.e. the number of young fledged per nest, which is the product of nest success and young per successful nest), differed for those selecting natal-like habitats to those that did not. For DSRs, we fitted mixed effects logistic-exposure models that account for variation in exposure days of nests [36] to determine if breeding in natal-like wetlands improved DSRs. For the number of young fledged per successful nest, we fitted mixed effects Poisson models with generally similar structure as the DSR model (except for the response variable). For each model considered, we included individual and site as random effects to account for non-independence of repeated nesting events for individuals and to control for general site-level variation in assessing the relative effects of natal habitat preferences. We also included nest date (1 January = 1; 31 December = 365) in all models as a nuisance covariate, given the consistent effects of nest date on reproductive success in birds [36]. To estimate nest productivity, we used estimates from the best models explaining DSR and the number of young fledged per successful nest, to calculate nest productivity as: (DSR56) × young per successful nest. We used this estimator rather than the raw number of young fledged per nest because it accounts for variation in nest exposure days (note that using the raw number of young fledged per nest provided similar results). Confidence intervals were estimated using a non-parametric bootstrap (n = 1000 samples).
(iv). Natal habitat preference and the spatial structure of the kite population network
Recently, emergent spatial structure in the movements of snail kites has been observed, which was revealed through spatial modularity analysis [18,20]. Spatial modularity occurs where habitat patches (or local populations) are tightly connected to other patches through movement of individuals or their alleles but only weakly connected to the remaining patches in the landscape [18]. This approach provides an objective means to identify clusters of movement across local sites or populations and thus potential spatial structure in populations, such as the occurrence of ‘subpopulations’ [18,37]. See the electronic supplementary material for more details. Previously, we found evidence of strong modular structure from mark–resight data, with two modules occurring: one small module in the northern portion of their geographical range and a second, larger module in the rest of their range [18]. Here, we ask whether such patterns could be explained by natal habitat preferences. To do so, we focus on modules identified at the annual time-step between 1997 and 2013, but note that the modules identified are not sensitive to the time scale and type of movement considered [20]. We used mixed logit models where the response variable was a binary matrix that described whether wetland i and j belong to the same module, whereas fixed effects included whether wetlands were the same wetland type, distance between wetlands, or both, and random effects were wetland i and j [10].
3. Results
Between 1996 and 2013, 428 banded birds with known natal locations were observed at 947 nests in 19 different wetlands. We observed an average of 2.5 nests individual−1 (s.d. 2.21; range: 1–13), with ages of parents ranging from 1–24 years old (mean = 5.4; s.d. = 4.41). Of the 428 birds with known natal origin, 287 (67%, 524 nests) were observed breeding outside of their natal wetland and 216 (50%, 423 nests) were observed breeding in their natal wetland. For those individuals that dispersed, mean distance from the natal site was 72.6 km (median = 42.0 km; see the electronic supplementary material, figure S3).
For 1 year old kites, we found nests for 72 individuals that bred, 41 of which dispersed to another site. When contrasting models to explain wetland-site selection of dispersing 1 year old kites, we found strong support for natal habitat preference on the basis of natal wetland type (lacustrine or palustrine wetland; see the electronic supplementary material, tables S1–S4, for all model selection comparisons). On the basis of the natal wetland-type univariate model, 1 year old birds were 10.9 times more likely to select the same wetland type than a different wetland type. Yet distance was also important in explaining wetland selection, with the most supported model including both distance and natal-type similarity. When distance was controlled for in this model, we estimated that 1 year old birds were 7.7 times more likely to select the same type of wetland from which they were born (figure 1a; p < 0.0001; electronic supplementary material, table S5). Other factors, including only preference for large wetlands, palustrine wetlands, and nearby wetlands showed little support, based on model selection criteria (electronic supplementary material, table S1).
Figure 1.
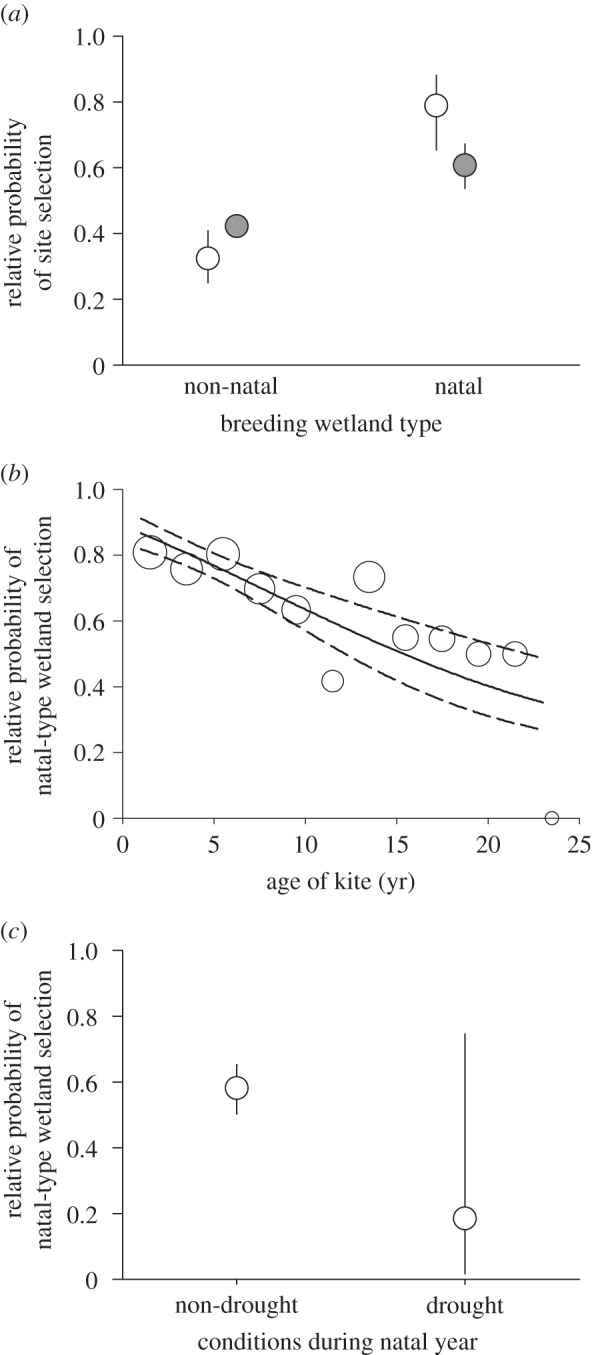
Preference for natal-like habitats by dispersing snail kites. (a) Both 1 year old dispersing individuals (natal dispersal; white) and all ages combined (grey) prefer wetlands of the same type (palustrine or lacustrine) as their natal origin. Shown are relative probabilities, controlling for distance effects, from best models for 1 year old birds and all birds combined. (b) Yet as individuals age, natal habitat preference declines. (c) Birds born in drought years are less likely to show natal habitat preferences based on wetland type. In (b,c), shown are relative probabilities of selection based on best conditional logit models (i.e. the probability of selection conditional on one selection among all possible wetlands). For (b), dots show raw frequencies (summarized in 2 year intervals), with dot size proportional to sample size.
For all non-natal nests (n = 524), we found that natal habitat preferences for wetland type declined with age (p = 0.003) and tended to decline for birds born in palustrine wetlands during drought years (figure 1b,c and electronic supplementary material, table S2). However, few birds were born in palustrine wetlands during drought years, such that while the most supported model included this effect, uncertainty in estimates was large (figure 1c). Based on this most supported model, dispersing birds were 4.2 times more likely to select the same wetland type as their natal wetland (electronic supplementary material, table S5). Overall, 385 of 524 nests from dispersing birds occurred in the natal wetland type (73.5%). Including natal philopatry, 85% of the observed nests (808 of 947) occurred in the natal wetland type. This pattern led to assortative breeding by wetland type: for nests where both parents were known (n = 120), 89% of pairs were born in the same wetland type (randomization test: random mean = 75%; p < 0.0001) and for nests where both parents had dispersed from their natal wetland (n = 41 nests), 83% of pairs came from the same type (random mean = 72%; p = 0.006). Note that there was no evidence for spatial autocorrelation in the residuals of the best conditional logit models using all nests or 1 year old birds only (electronic supplementary material, figure S4).
We monitored 327 nests from banded parents that had dispersed from their natal site and for which we had nest exposure information (11 967 exposure days). Birds that dispersed to natal-like wetlands for nesting (based on wetland type) suffered lower daily nest survival rates (z = −2.285, p = 0.022) than those that switched wetland types for breeding (figure 2; electronic supplementary material, table S3). Age was also included in the most supported model, with older birds having lower nest survival (z = −1.94, p = 0.052), but age did not interact with the effect of natal site similarity. There was no effect of natal wetland type on the number of young fledged per successful nest (electronic supplementary material, table S3). Effects of nest survival impacted nest productivity of birds that switched wetland types for breeding, where individuals that switched wetland types had two times greater nest productivity (figure 2).
Figure 2.
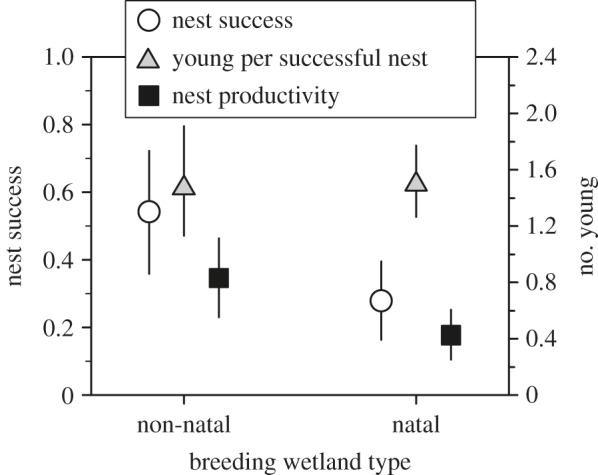
Reproductive consequences of natal habitat preferences. Nest success (daily survival rate56) is lower for birds that disperse to a similar wetland type as their natal wetland than if they switch wetland types, whereas the number of young fledged per successful nest does not vary. Nest productivity, or the number of young fledged per nest (i.e. the product of nest success and the number of young fledged/successful nest), is lower for birds that disperse to a similar wetland type as their natal wetland. Estimates taken from mixed effects models (±95 CI, taken from bootstrapping), with individual and site as random effects.
Modules (i.e. subpopulations delineated via movement) previously identified for adult snail kites revealed two regions [18,20], one concentrated in the northern portion of the range and a second in the remainder of the range (figure 3a). About 76.7% of adult dispersal events from natal locations occurred within modules (figure 3a). Using wetland type to explain module structure resulted in a 90% correct classification rate for modules (2 of 20 wetlands were misclassified). The best model to explain module structure included both wetland type similarity (figure 3b) and distance between wetlands, with wetland type explaining more deviance than distance (electronic supplementary material, table S4).
Figure 3.
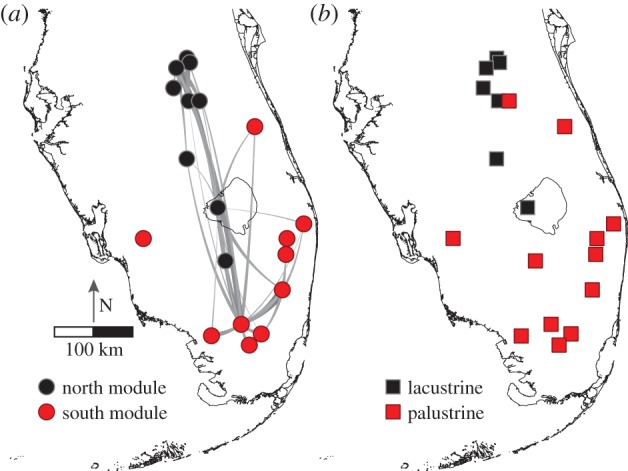
Natal habitat preference helps explain spatial structure in the kite population network in peninsular Florida, USA. (a) Modules identified from annual movements of kites from mark–resight data illustrate a general cluster of movements (i.e. module) in the northern portion of the kite range and in the central southern portion. Colours show modules, tapered lines show observed dispersal events (natal location to breeding location; only two or more dispersal events per location shown). (b) Map of lacustrine and palustrine wetlands identified through cluster analysis. (Online version in colour.)
4. Discussion
Despite the fact that snail kites are a wide-ranging species capable of moving across the entire extent of their geographical range over short periods of time [19], we found that nesting kites showed natal-site philopatry for breeding [17]. For those that dispersed, individuals were more likely to breed in wetland habitats similar to their natal location, independent of effects of distances among wetlands. This preference declined with age and when birds were born in drought years, consistent with recent hypotheses on variation in natal habitat preferences [15]. Yet dispersing to natal-like wetlands resulted in lower nest success and productivity, counter to hypotheses for the evolution of this behaviour [3]. These results provide new insights into habitat selection and its implications for population structure across geographical ranges.
(a). Mechanisms of natal habitat preference
Preference for wetland habitat types (lacustrine versus palustrine) could arise for several reasons. First, these habitat types tend to offer different nesting substrates for kites, where kites frequently nest in bulrush or cattail in lacustrine wetlands, whereas individuals frequently nest in woody substrates in palustrine wetlands. Yet we found no evidence that natal habitat preferences were driven by nesting substrate (electronic supplementary material, table S1). Second, lacustrine and palustrine wetlands offer different foraging substrates for kites and potentially different prey resources. Kites use two different foraging strategies, course hunting and perch hunting, which tend to vary across wetlands. It is possible that kites learn different strategies in their natal wetland, which influences decisions later in life, but this hypothesis has not been tested. In addition, in recent years, many of the lacustrine wetlands have been invaded by an exotic apple snail (Pomacea maculata), which kites readily consume [38], and kites appear to have tracked the invasion sequence of the exotic snail across wetlands over time [21]. Nonetheless, we found no support for this new prey resource driving these dispersal patterns when using the invasion sequence of the exotic snail establishment in each site as a covariate in our models, described either by its effect on current nest site selection or relative to the natal site (p = 0.42 and p = 0.34, respectively). Third, these habitat types generally have different landscape contexts, where lacustrine wetlands are often smaller and surrounded by more urban and agricultural areas than palustrine wetlands [23]. Indeed, Selonen et al. [29] found evidence for natal habitat preferences in Siberian squirrels driven by variation in landscape context. Further research on landscape context and its potential effects on kite habitat selection and reproduction would help untangle these alternative potential mechanisms.
(b). Reproductive consequences
It is frequently hypothesized that natal habitat preferences should be adaptive, because such preference may provide fitness benefits through a variety of mechanisms [4,39]. Yet, we found that nest success and productivity were decoupled from preference, where individuals that dispersed to non-natal-like wetlands had approximately two times greater nest success and productivity, reminiscent of the concepts of ecological and perceptual traps [40–42]. Nonetheless, this decoupling was subtly different than those existing concepts in the sense that reproductive effects observed were related to switching habitats and were above and beyond site-level habitat effects, which were captured as random effects in models. It is possible that some components of fitness over an individual's lifetime might compensate for this reproductive cost (e.g. increased survival for individuals dispersing to natal-like habitats), such that lifetime fitness might not vary [8]. Unfortunately, given the distances snail kites can disperse and their longevity, estimates of lifetime fitness are not possible to obtain. We found no evidence, however, for an interactive effect of individual age and nest success for individuals breeding in non-natal wetlands (electronic supplementary material, table S3), such that this reproductive cost did not dissipate with age. We did find that natal habitat preferences declined with age, yet even old individuals showed some preference for natal-like wetlands (figure 1b). Taken together, these results suggest a non-trivial reproductive cost to natal habitat preferences (see also [8]).
The mechanisms for why this reproductive cost occurred are still unclear. We note that the intensity of this reproductive effect has increased in recent years (adding a year interaction to the best model for DSR: natal similarity: −0.54 ± 0.23; p = 0.018; natal similarity × year: −0.16 ± 0.08; p = 0.037). One hypothesis is that this impact could be driven by the ‘ghost of habitat past’ [43], i.e. individuals show fidelity to habitats where prior success occurred to the detriment of other, better opportunities. Snail kite habitats have been altered considerably in recent years, through changes in prey availability, vegetation and hydrology [44]. Because natal habitat preferences can magnify the effects of potential ecological traps across landscapes [45], better understanding of the large-scale consequences of natal habitat preferences is needed.
(c). The emergence of population structure
Population structure arising from limited movement and/or gene flow is common in nature. A prominent hypothesis for such patterns is distance-limited dispersal. Here, we show that while individual nesting decisions are influenced by geographical distance, natal habitat type played a strong effect independent of distance, correctly classifying modules for adults in all but two wetlands. In addition, the two misclassified wetlands for modularity, Lake Jackson and Lake Okeechobee, were relatively weakly associated with other wetlands in our cluster analysis and contain a mix of lacustrine and palustrine habitats (electronic supplementary material, figures S1 and S2). Overall, this simple behaviour, along with distance between wetlands, can parsimoniously explain patterns of connectivity and population structure in snail kites [18]. These results emphasize that, even in highly mobile animals, simple behaviours can reduce connectivity [46]. Given these results and evidence for assortative breeding by wetland type, further work on genetic structure in this endangered bird would be beneficial for interpreting whether these behaviours operating on ecological time have evolutionary implications [41]. Nonetheless, given that this preference declines with age (figure 1b) and individuals have been observed breeding up to 24 years old, impacts of natal habitat preference on genetic structure could be relatively weak.
While it has long been hypothesized that natal habitat preferences may generate population structure leading to the potential for sympatric speciation [47], empirical evidence in wild populations has been slow to accumulate. Porlier et al. [48] identified habitat-driven population differentiation in the blue tit (Cyanistes caeruleus), where genetic population structure was not explained by geographical distance among populations or by the presence of physical barriers but was instead related to local habitat types (deciduous or evergreen oaks). Similarly, Bolnick et al. [49] found strong evidence for habitat preference leading to genetic divergence through assortative mating in the three-spine stickleback (Gasterosteus aculeatus), although this effect appeared to be largely driven by dispersal distances. Our work complements this growing evidence by illustrating how such structure can occur in ecological time with dispersal movements and how factors such as age and natal environmental conditions may potentially reduce the impact of such preferences over longer time scales.
(d). Conservation implications
Natal preferences by dispersers can impact conservation strategies [7], reduce colonization potential in the light of rapid environmental change [50] and alter the interpretation of restoration successes and failures. Ongoing restoration efforts for the Everglades emphasize multi-species recovery, including recovery of the endangered snail kite, which was previously documented to occur in high abundance in the Everglades during the twentieth century [21]. However, this preference for natal-like habitats may slow the re-establishment of large populations of breeding snail kites in the Everglades, particularly of young snail kites, even if successful habitat restoration occurs. Incorporating knowledge of behavioural processes into conservation and restoration strategies will improve assessments and can provide novel approaches for conserving imperilled species [46].
Supplementary Material
Acknowledgements
We thank all of those who helped collect and manage data over the years, including many individuals who have banded kites (S. Beissinger, R. Bennetts, V. Drietz and K. Meyer). We are especially grateful to A. Charmantier, M. Patten, N. Burrell, C. W. Miller and two anonymous reviewers for insightful comments on earlier versions of the manuscript.
Ethics
The study was approved and conducted under IACUC no. 201005469.
Data accessibility
Snail kites are a listed federally endangered species, the specific location data are sensitive and have not been made available. However, the general data for these analyses are available at Dryad.
Authors' contributions
R.F. conceived the study, carried out the data analysis and wrote the manuscript. E.R. contributed to conception and design of the study, data analysis and interpretation, and edited the manuscript. R.W. and B.R. collected data and edited the manuscript. W.K. helped conceive and design the band–resight study, supervised data collection, and edited the manuscript. J.A. contributed to the data analysis and edited the manuscript.
Competing interests
We have no competing interests.
Funding
Financial support was provided by the US Army Corps of Engineers, US Fish and Wildlife Service, St Johns River Water Management District and US Geological Survey.
References
- 1.Benard MF, McCauley SJ. 2008. Integrating across life-history stages: consequences of natal habitat effects on dispersal. Am. Nat. 171, 553–567. ( 10.1086/587072) [DOI] [PubMed] [Google Scholar]
- 2.Miller CW, Fletcher RJ Jr, Anderson BD, Nguyen LD. 2012. Natal social environment alters habitat selection later in life. Anim. Behav. 83, 473–477. ( 10.1016/j.anbehav.2011.11.022) [DOI] [Google Scholar]
- 3.Davis JM, Stamps JA. 2004. The effect of natal experience on habitat preferences. Trends Ecol. Evol. 19, 411–416. ( 10.1016/j.tree.2004.04.006) [DOI] [PubMed] [Google Scholar]
- 4.Piper WH. 2011. Making habitat selection more ‘familiar’: a review. Behav. Ecol. Sociobiol. 65, 1329–1351. ( 10.1007/s00265-011-1195-1) [DOI] [Google Scholar]
- 5.Doligez B, Cadet C, Danchin E, Boulinier T. 2003. When to use public information for breeding habitat selection? The role of environmental predictability and density dependence. Anim. Behav. 66, 973–988. ( 10.1006/anbe.2002.2270) [DOI] [Google Scholar]
- 6.Davis JM. 2008. Patterns of variation in the influence of natal experience on habitat choice. Quart. Rev. Biol. 83, 363–380. ( 10.1086/592851) [DOI] [PubMed] [Google Scholar]
- 7.Stamps JA, Swaisgood RR. 2007. Someplace like home: experience, habitat selection and conservation biology. Appl. Anim. Behav. Sci. 102, 392–409. ( 10.1016/j.applanim.2006.05.038) [DOI] [Google Scholar]
- 8.Piper WH, Palmer MW, Banfield N, Meyer MW. 2013. Can settlement in natal-like habitat explain maladaptive habitat selection? Proc. R. Soc. B 280, 20130979 ( 10.1098/rspb.2013.0979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabry KE, Stamps JA. 2008. Dispersing brush mice prefer habitat like home. Proc. R. Soc. B 275, 543–548. ( 10.1098/rspb.2007.1541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher RJ Jr, Acevedo MA, Reichert BE, Pias KE, Kitchens WM. 2011. Social network models predict movement and connectivity in ecological landscapes. Proc. Natl Acad. Sci. USA 108, 19 282–19 287. ( 10.1073/pnas.1107549108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bélisle M. 2005. Measuring landscape connectivity: the challenge of behavioral landscape ecology. Ecology 86, 1988–1995. ( 10.1890/04-0923) [DOI] [Google Scholar]
- 12.Davis JM. 2007. Preference or desperation? Distinguishing between the natal habitat's effects on habitat choice. Anim. Behav. 74, 111–119. ( 10.1016/j.anbehav.2006.11.014). [DOI] [Google Scholar]
- 13.Fletcher RJ Jr, Sieving KE. 2010. Social-information use in heterogeneous landscapes: a prospectus. Condor 112, 225–234. ( 10.1525/cond.2010.090236) [DOI] [Google Scholar]
- 14.Koenig WD, VanVuren D, Hooge PN. 1996. Detectability, philopatry, and the distribution of dispersal distances in vertebrates. Trends Ecol. Evol. 11, 514–517. ( 10.1016/s0169-5347(96)20074-6) [DOI] [PubMed] [Google Scholar]
- 15.Stamps JA, Krishnan VV, Willits NH. 2009. How different types of natal experience affect habitat preference. Am. Nat. 174, 623–630. ( 10.1086/644526) [DOI] [PubMed] [Google Scholar]
- 16.Fletcher RJ Jr, Miller CW. 2008. The type and timing of social information alters offspring production. Biol. Lett. 4, 482–485. ( 10.1098/rsbl.2008.0306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin J, Kitchens WM, Hines JE. 2007. Natal location influences movement and survival of a spatially structured population of snail kites. Oecologia 153, 291–301. ( 10.1007/s00442-007-0729-8) [DOI] [PubMed] [Google Scholar]
- 18.Fletcher RJ Jr, Revell A, Reichert BE, Kitchens WM, Dixon JD, Austin JD. 2013. Network modularity reveals critical scales for connectivity in ecology and evolution. Nat. Commun. 4, 2572 ( 10.1038/ncomms3572) [DOI] [PubMed] [Google Scholar]
- 19.Meyer KD, Kent GM, Hart K, Fujisaki I. 2011. Seasonal movements and habitat use of snail kites (Rostrhamus sociabilis) in Florida: a comparative study of satellite and VHF tracking methodologies. (p. 44. Final Report for U.S. Fish and Wildlife Service, Vero Beach, Florida.
- 20.Reichert BE. 2014. Spatial structure in demography and movements of the endangered snail kite: revealing multi-scale patterns and their implications for conservation. Dissertation, University of Florida, Gainesville, FL.
- 21.Reichert BE, Cattau CE, Fletcher JRJ, Sykes JPW, Rodgers JJA, Bennetts RE. 2015. Snail kite (Rostrhamus sociabilis). In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Laboratory of Ornithology. [Google Scholar]
- 22.Cowardin LM, Carter V, Golet FC, LaRoe ET. 1979. Classification of wetlands and deepwater habitats of the United States. Washington, DC: U.S.F.A.W. Service. [Google Scholar]
- 23.Martin J, Nichols JD, Kitchens WM, Hines JE. 2006. Multiscale patterns of movement in fragmented landscapes and consequences on demography of the snail kite in Florida. J. Anim. Ecol. 75, 527–539. ( 10.1111/j.1365-2656.2006.01073.x) [DOI] [PubMed] [Google Scholar]
- 24.Reichert BE, Cattau CE, Fletcher RJ Jr, Kendall WL, Kitchens WM. 2012. Extreme weather and experience influence reproduction in an endangered bird. Ecology 93, 2580–2589. ( 10.1890/12-0233.1) [DOI] [PubMed] [Google Scholar]
- 25.Cattau CE, Darby PC, Fletcher RJ Jr, Kitchens WM. 2014. Reproductive responses of the endangered snail kite to variations in prey density. J. Wildl. Manage. 78, 620–631. ( 10.1002/jwmg.706) [DOI] [Google Scholar]
- 26.Greenwood PJ, Harvey PH. 1982. The natal and breeding dispersal of birds. Annu. Rev. Ecol. Syst. 13, 1–21. ( 10.1146/annurev.es.13.110182.000245) [DOI] [Google Scholar]
- 27.Takekawa JE, Beissinger SR. 1989. Cyclic drought, dispersal, and the conservation of the snail kite in Florida: lessons in critical habitat. Conserv. Biol. 3, 302–311. ( 10.1111/j.1523-1739.1989.tb00090.x) [DOI] [Google Scholar]
- 28.Chalfoun AD, Martin TE. 2010. Facultative nest patch shifts in response to nest predation risk in the Brewer's sparrow: a ‘win-stay, lose-switch’ strategy? Oecologia 163, 885–892. ( 10.1007/s00442-010-1679-0) [DOI] [PubMed] [Google Scholar]
- 29.Selonen V, Hanski IK, Desrochers A. 2007. Natal habitat-biased dispersal in the Siberian flying squirrel. Proc. R. Soc. B 274, 2063–2068. ( 10.1098/rspb.2007.0570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufman L, Rousseeuw PJ. 1990. Finding groups in data: an introduction to cluster analysis. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 31.Vardakis M, Goos P, Adriaensen F, Matthysen E. In press. Discrete choice modeling of natal dispersal: ‘choosing’ where to breed from a finite set of available areas. Methods Ecol. Evol. ( 10.1111/2041-210X.12404) [DOI] [Google Scholar]
- 32.Fortin D, Beyer HL, Boyce MS, Smith DW, Duchesne T, Mao JS. 2005. Wolves influence elk movements: behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86, 1320–1330. ( 10.1890/04-0953) [DOI] [Google Scholar]
- 33.McCarthy KP, Fletcher RJ Jr. 2015. Does hunting activity for game species have indirect effects on resource selection by the endangered Florida Panther? Anim. Conserv. 18, 138–145. ( 10.1111/acv.12142) [DOI] [Google Scholar]
- 34.Darby PC, Bennetts RE, Percival HF. 2008. Dry down impacts on apple snail (Pomacea paludosa) demography: implications for wetland water management. Wetlands 28, 204–214. ( 10.1672/07-115.1) [DOI] [Google Scholar]
- 35.Bennetts RE, Kitchens WM. 1999. Within-year survival patterns of snail kites in Florida. J. Field Ornithol. 70, 268–275. [Google Scholar]
- 36.Shaffer TL. 2004. A unified approach to analyzing nest success. Auk 121, 526–540. ( 10.1642/0004-8038%282004%29121%5B0526%3AAUATAN%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 37.Fortuna MA, Albaladejo RG, Fernandez L, Aparicio A, Bascompte J. 2009. Networks of spatial genetic variation across species. Proc. Natl Acad. Sci. USA 106, 19 044–19 049. ( 10.1073/pnas.0907704106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cattau CE, Martin J, Kitchens WM. 2010. Effects of an exotic prey species on a native specialist: example of the snail kite. Biol. Conserv. 143, 513–520. ( 10.1016/j.biocon.2009.11.022). [DOI] [Google Scholar]
- 39.Stamps JA, Davis JM. 2006. Adaptive effects of natal experience on habitat selection by dispersers. Anim. Behav. 72, 1279–1289. ( 10.1016/j.anbehav.2006.03.010) [DOI] [Google Scholar]
- 40.Schlaepfer MA, Runge MC, Sherman PW. 2002. Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480. ( 10.1016/S0169-5347(02)02580-6) [DOI] [Google Scholar]
- 41.Fletcher RJ Jr, Orrock JL, Robertson BA. 2012. How the type of anthropogenic change alters the consequences of ecological traps. Proc. R. Soc. B 279, 2546–2552. ( 10.1098/rspb.2012.0139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patten MA, Kelly JF. 2010. Habitat selection and the perceptual trap. Ecol. Appl. 20, 2148–2156. ( 10.1890/09-2370.1) [DOI] [PubMed] [Google Scholar]
- 43.Knick ST, Rotenberry JT. 2000. Ghosts of habitats past: contribution of landscape change to current habitats used by shrubland birds. Ecology 81, 220–227. ( 10.1890/0012-9658%282000%29081%5B0220%3AGOHPCO%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 44.Zweig CL, Kitchens WM. 2014. Reconstructing historical habitat data with predictive models. Ecol. Appl. 24, 196–203. ( 10.1890/13-0327.1) [DOI] [PubMed] [Google Scholar]
- 45.Hale R, Treml EA, Swearer SE. 2015. Evaluating the metapopulation consequences of ecological traps. Proc. R. Soc. B 282, 20142930 ( 10.1098/rspb.2014.2930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasudev D, Fletcher RJ Jr, Goswami VR, Krishnadas M. In press. From dispersal constraints to landscape connectivity: lessons from species distribution modeling. Ecography 38 ( 10.1111/ecog.01306) [DOI] [Google Scholar]
- 47.Maynard Smith J. 1966. Sympatric speciation. Am. Nat. 100, 637–650. ( 10.1086/282457) [DOI] [Google Scholar]
- 48.Porlier M, Garant D, Perret P, Charmantier A. 2012. Habitat-linked population genetic differentiation in the blue tit Cyanistes caeruleus. J. Hered. 103, 781–791. ( 10.1093/jhered/ess064) [DOI] [PubMed] [Google Scholar]
- 49.Bolnick DI, Snowberg LK, Patenia C, Stutz WE, Ingram T, Lau OL. 2009. Phenotype-dependent native habitat preference facilitates divergence between parapatric lake and stream stickleback. Evolution 63, 2004–2016. ( 10.1111/j.1558-5646.2009.00699.x) [DOI] [PubMed] [Google Scholar]
- 50.Reed JM, Levine SH. 2005. A model for behavioral regulation of metapopulation dynamics. Ecol. Model. 183, 411–423. ( 10.1016/j.ecolmodel.2004.02.025) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Snail kites are a listed federally endangered species, the specific location data are sensitive and have not been made available. However, the general data for these analyses are available at Dryad.


