Abstract
The fusion gene echinoderm microtubule-associated protein-like 4 (EML4)–anaplastic lymphoma kinase (ALK) is identified in approximately 5% of non-small-cell lung cancer patients. A rare case of ALK-positive squamous cell carcinoma of the lung is reported. A 60-year-old man, an ex-smoker with a 720-packs-per-year tobacco smoking history, presented with a mass lesion in the upper lobe of the left lung on chest computed tomography. Transbronchial biopsy of the mass confirmed a diagnosis of lung squamous cell carcinoma, and it was proven to have ALK rearrangement by fluorescent in situ hybridization. The patient underwent left upper lobectomy. Hematoxylin and eosin staining of the surgical specimen demonstrated the typical morphology of pure squamous cell carcinoma. The patient has been advised to attend regular check-ups for postoperative recurrence. ALK testing and subsequent ALK-targeted treatment can be a possible option in cases of postoperative recurrence.
Keywords: ALK, FISH, immunohistochemistry, squamous cell carcinoma
Introduction
EML4-ALK fusion genes are identified in approximately 5% of non-small-cell lung cancer (NSCLC) patients, a population that consists mostly of adenocarcinoma [1]. Therefore, ALK-positive histologic types of lung cancer, excluding adenocarcinoma, are rare. These fusion genes are often seen in a younger age group of never or light ex-smokers [1]. A rare case of an older man with a heavy smoking history presenting with ALK-positive squamous cell carcinoma (SqCC) of the lung is described.
Case Report
A 60-year-old male ex-smoker with a 720-packs-per-year tobacco smoking history presented to our hospital because of an abnormal chest X-ray. Chest computed tomography (CT) revealed a mass lesion measuring 32 mm × 28 mm in the upper lobe of the left lung. The mass had a microlobulated margin and was accompanied by cavitation (Fig. 1A). Transbronchial biopsy (TBB) of the mass strongly suggested primary lung cancer, but the histologic type was not confirmed due to the small specimen. The biopsy specimen was externally reviewed for immunohistochemistry (IHC) and molecular analysis. This confirmed a diagnosis of lung SqCC, supported by positive staining for P63, P40, and CK5/6 and negative staining for TTF-1 on IHC. Furthermore, the tumor was proven to be positive for ALK rearrangement by fluorescent in situ hybridization (FISH) (Fig. 1B), while it was negative for epidermal growth factor receptor (EGFR) mutation confirmed by the polymerase chain reaction (PCR). The patient underwent left upper lobectomy for lung cancer with a clinical stage of cT2aN0M0. Hematoxylin and eosin (H&E) staining of the specimen obtained at surgery demonstrated the typical morphology of moderately differentiated lung SqCC: eosinophilic foci of intracellular keratinization and intercellular bridges around tumor cells (Fig. 2A). No component of adenocarcinoma or other histologic type was seen. Repeat IHC analysis confirmed that the tumor cells were homogeneously positive for p40 (Fig. 2B), CK5/6 (Fig. 2C), and heterogeneously positive for ALK-protein (Fig. 2D) throughout the whole of the tumor.
Figure 1.
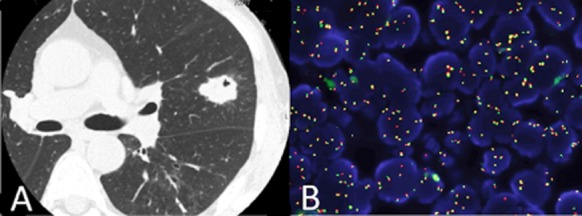
Chest computed tomography shows a mass lesion of 32 mm × 28 mm in the upper lobe of the left lung (A). ALK break apart FISH testing shows separated red and green signals which demonstrate ALK rearrangements (B). ALK, anaplastic lymphoma kinase; EML4, echinoderm microtubule-associated protein-like 4; FISH, fluorescent in situ hybridization.
Figure 2.
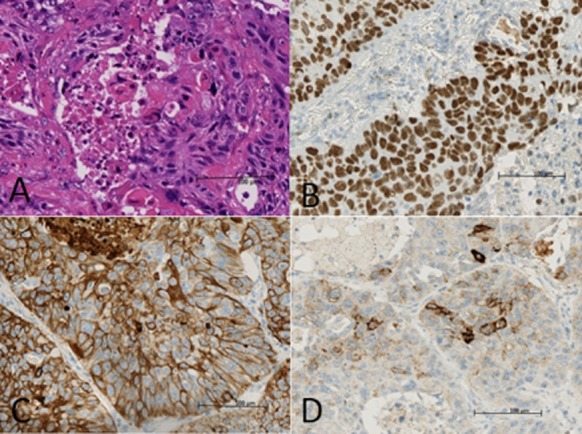
Hematoxylin and eosin staining (original magnification ×400) of the surgical specimen showed the typical morphohistology of squamous cell carcinoma (A). Immunohistochemistry staining (original magnification ×400) of the surgical specimen was positive for p40 (B), CK5/6 (C), and anaplastic lymphoma kinase (ALK)-protein (D).
With a pathological stage of pT2aN0M0, treatment with 250 mg/m2 (400 mg) of uracil-tegafur was started as adjuvant chemotherapy. Currently, the patient is being followed regularly as an outpatient.
Discussion
A rare case of ALK-positive SqCC of the lung was described. The EML4-ALK fusion genes are identified in approximately 5% of NSCLC cases [1], while they are identified with lower frequency in lung SqCC cases. To date, only a few studies have reported the frequency of ALK rearrangement in SqCC of the lung, varying from 1.4% to 2.5% detected by FISH [2], [3]. Accordingly, case reports that describe the details of the clinicopathologic features of lung SqCC with ALK-rearrangements are rare [1], [4]. In addition to the low frequency of ALK rearrangements in lung SqCC, ALK testing is not routinely performed in patients with lung SqCC on the basis of National Comprehensive Cancer Network guidelines [4]. Therefore, we seldom have the opportunity to see lung SqCC with ALK rearrangements in daily clinical practice. In the current case, because the histologic type of the small amount of specimen obtained by TBB was difficult to determine, subsequent IHC and molecular analyses, including ALK testing that unexpectedly resulted in identifying ALK-positive SqCC, were performed.
It was confirmed that the whole specimen obtained by surgery showed the typical morphohistology of SqCC, containing no other histologic type. Furthermore, heterogeneous expression of ALK-protein was seen throughout the entire area of the tumor. Although a certain percentage of ALK-positive NSCLCs are known to have characteristic morphohistological features, such as a solid signet-ring cell pattern or mucinous cribriform pattern, no extraordinary morphohistological features were discovered in the present case. Consequently, it seems impossible, at the moment, to identify cases for ALK testing in SqCC based on morphohistology.
The present patient underwent radical surgery for lung SqCC followed by adjuvant chemotherapy for pathological stage pT2aN0M0 and now requires regular check-ups for postoperative recurrence. Morodomi et al. reported that NSCLC patients with EML4-ALK fusion genes are relatively insensitive to cytotoxic chemotherapy for postoperative recurrent disease or advanced disease, whereas it is now widely recognized that treatment of these patients with the ALK-targeted agent, crizotinib, significantly improves clinical outcomes [5]. Moreover, a case of ALK-positive SqCC showing a marked response to crizotinib after two courses of failed chemotherapy has been reported previously [4]. These facts indicate the presence of patients with ALK-positive SqCC; although they are a rather small population, they do exist with a certain probability, and they may benefit from treatment with ALK-targeted agents for postoperative recurrence or advanced disease. However, considering the low efficacy of gefitinib in non-adenocarcinoma NSCLC patients harboring EGFR mutations, it remains unclear whether ALK-positive non-adenocarcinoma NSCLC patients show a marked response to ALK-targeted therapy that is as good as that of ALK-positive lung adenocarcinoma patients. Since it is of rare occurrence in lung SqCC, routine testing for ALK rearrangement in lung SqCC is not advisable. In the situation described in this report, however, repeat ALK testing and subsequent ALK-targeted treatment should be considered with postoperative recurrence or advanced disease.
The heavy smoking history of the present case is not concordant with one of the clinical features of ALK-positive NSCLC, in that ALK rearrangements are often seen in never or light ex-smokers. However, because tobacco smoking is the most important risk factor for lung SqCC, the inconsistency mentioned above is inevitable.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Acknowledgments
We appreciate the technical assistance provided by Toyoko Mizuno.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
References
- Alrifai D, Popat S, Ahmed M, et al. A rare case of squamous cell carcinoma of the lung harbouring ALK and BRAF activating mutations. Lung Cancer. 2013;80:339–340. doi: 10.1016/j.lungcan.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Wang J, Shen Q, Shi Q, et al. Detection of ALK protein expression in lung squamous cell carcinomas by immunohistochemistry. J. Exp. Clin. Cancer Res. 2014;33:109. doi: 10.1186/s13046-014-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliò A, Nottegar A, Gilioli E, et al. ALK/EML4 fusion gene may be found in pure squamous carcinoma of the lung. J. Thorac. Oncol. 2014;9:729–732. doi: 10.1097/JTO.0000000000000109. [DOI] [PubMed] [Google Scholar]
- Wang Q, He Y, Yang X, et al. Extraordinary response to crizotinib in a woman with squamous cell lung cancer after two courses of failed chemotherapy. BMC Pulm. Med. 2014;14:83. doi: 10.1186/1471-2466-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morodomi Y, Takenoyama M, Inamasu E, et al. Non-small cell lung cancer patients with EML4-ALK fusion gene are insensitive to cytotoxic chemotherapy. Anticancer Res. 2014;34:3825–3830. [PubMed] [Google Scholar]


