Abstract
Background
A liver resection under low central venous pressure (CVP) has become standard practice; however, the benefits beyond a reduction in blood loss are not well reported. Moreover, the precise method to achieve CVP reduction has not been established. A systematic review and meta-analysis of randomized controlled trials (RTCs) was performed to assess the effects of CVP on clinical outcome and to identify the optimum method of CVP reduction.
Methods
EMBASE, Medline, PubMed and the Cochrane database were searched for trials comparing low CVP surgery with controls. The primary outcome was post-operative complications within 30 days. Secondary outcomes included estimated blood loss (EBL), blood transfusion rates and length of stay (LOS). Sub-group analysis was performed to assess the CVP reduction method on the outcome.
Results
Eight trials were identified. No difference was observed in the morbidity rate between the high CVP and control groups [odds ratio (OR) = 0.96 (95% confidence interval (CI) 0.66, 1.40) P = 0.84, I2 = 0%]. EBL [weighted mean difference (WMD) = −308.63 ml (95% CI −474.67, −142.58) P = < 0.001, I2 = 73%] and blood transfusion rates [OR 0.65 (95% CI 0.44, 0.97) P = 0.040, I2 = 37%] were significantly lower in the low CVP groups. Neither anaesthetic nor surgical methods of CVP reduction were associated with a reduced post-operative morbidity.
Conclusion
Low CVP surgery is associated with a reduction in EBL; however, this does not translate into an improvement in post-operative morbidity. The optimum method of CVP reduction has not been identified.
Introduction
Liver resectional surgery is frequently the only opportunity for curative treatment of a number of primary and secondary tumours. While it is complex surgery, mortality rates in high-volume centres should be < 5%.1 Morbidity rates can be as high as 45%.2
Enhanced Recovery After Surgery (ERAS) protocols after a liver resection have increased in popularity in recent years and several studies have highlighted not only the feasibility and safety of fast-track protocols3 but a reduction in the length of stay (LOS) and morbidity rates post-operatively.4 Morbidity rates, however, remain significant, and peri-operative protocols require optimization to minimize complications.4
It has been frequently reported that blood loss during a liver resection is associated with increased post-operative morbidity rates.2,5,6 Methods to reduce intra-operative blood loss have included techniques to reduce the central venous pressure (CVP) during a liver resection. The maintenance of a low CVP is currently routine practice during liver surgery.5 However, an initial review of the available data questioned the outcome benefit of low CVP surgery beyond a reduction in blood loss.7 Moreover, new techniques have been introduced to reduce CVP, the efficacy of which is not well established. This review aims to assess techniques for CVP reduction on clinical outcomes after a liver resection.
Methods
This study was conducted according to the PRISMA guidelines for meta-analysis conduct.8 The protocol was registered prospectively on the PROSPERO database for meta-analyses (registration number CRD42014007651).
A literature search was performed independently by two researchers of EMBASE, Medline, PubMed and the Cochrane databases. The databases were searched from 1966 to 2014 with the following terms: ‘central venous pressure’ or ’CVP‘ and ’liver resection‘ or ’liver surgery‘ or ’hepatic resection‘ or ’hpb’.
All abstracts were reviewed for relevance by two independent investigators. Relevant full-text articles were subsequently reviewed and critiqued.
Inclusion criteria
The inclusion criteria were randomized controlled trials (RCTs) that compared significantly different CVPs or compared a low CVP group with a control group and reported on patient outcomes (morbidity, EBL and LOS) after an elective open liver resection.
Exclusion criteria
Non-randomized trials were excluded. Trials that did not report significantly different CVPs between groups did not compare a low CVP with a control group or did not report outcomes of EBL and/or morbidity rate were excluded. All reviews were excluded. Irrelevant studies, letters, case reviews, paediatric populations and animal studies were excluded. Non-hepatic surgery and trials including transplant recipients were excluded.
Intervention
The intervention investigated was a reduction in intra-operative CVP. This was defined as a statistically significant difference in CVP between groups, or a ’low CVP‘ group compared with a control group. Where multiple recordings of CVP were reported, the CVP during a liver parenchymal transection was used.
Comparator
The comparator group was the study arm where a significantly higher intra-operative CVP was reported or the ’control‘ group. The comparator group was defined as demonstrating a higher CVP (mmHg), regardless of absolute CVP value or technique used to achieve CVP.
Outcomes
Primary outcome
The primary outcome was a composite end-point of the occurrence of one or more systemic complication within 30 days of a liver resection. Specific blood test abnormalities were not regarded as systemic complications.
Secondary outcome
Further comparisons were made between low CVP groups and control groups. The mean CVP (mmHg/cm H20) during the operation (during transection if multiple readings were provided) was compared between groups. EBL in millilitres and LOS (recorded in whole days) were also compared between groups. CVP-lowering protocols were also recorded and compared.
Subgroup analysis
Subgroup analysis was performed according to technique of CVP reduction: anaesthetic methods [intravenous infusion (IVI) restriction, epidural, vasodilators and/or diuretics] and surgical methods (IVC clamping/total or selective vascular hepatic exclusion).
Data extraction
Abstracts were reviewed for relevance and suitability for inclusion by two independent investigators. Full-text articles were reviewed, and data were extracted using pre-designed data extraction forms. If data were not presented in a format conducive to data synthesis, the authors were contacted using the published correspondence details. In the event of no response, an attempt to contact authors was made by repeat email, followed by a letter and/or phone call. Where no author response was received, the medians and ranges were converted to mean/standard deviation using methods described by Hozo et al.9 The Cochrane bias risk assessment tool was used to assess study quality.10
Statistical analysis
Statistical analysis was performed using review manager (RevMan ver 5.2; The Nordic Cochrane Centre, Copenhagen, Denmark). The following outcomes were treated as dichotomous data and were analysed using pooled odds ratios (ORs): primary outcome- major complication rate, intra-operative blood transfusion requirement. The following outcomes were treated as continuous data and were analysed with a weighted mean difference (WMD): EBL, LOS. Statistical significance was set at P < 0.05. Heterogeneity was assessed using I2 and X2 and adjudged to be significant if I2 >50% and/or P < 0.05.
Results
Included trials
The PRISMA diagram of included trials is shown in Fig.1. Eight randomized controlled trials (RCTs) met the inclusion criteria with 339 patients with a significantly lower CVP and 342 control patients. 11–18 Patient demographics and indications for a hepatic resection are displayed in Table1. Bias assessment scores for included trials are presented in Table2.
Figure 1.
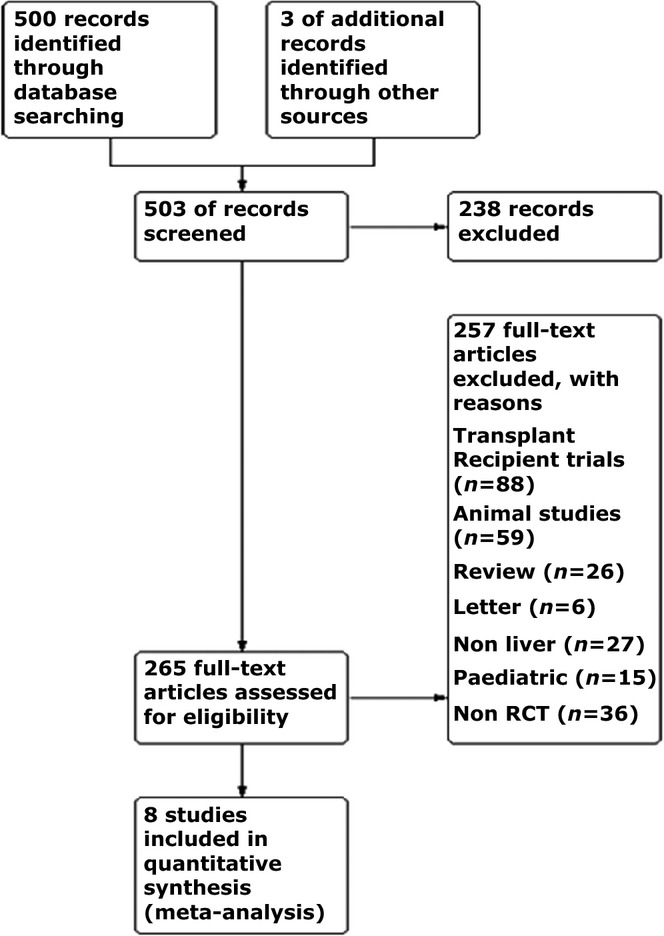
PRISMA diagram. RCT, randomized controlled trials
Table 1.
Patient demographics and baseline characteristics
| Wang et al.17 | Liu et al.14 | Liu et al.15 | El-Khaboutley et al.11 | |||||
|---|---|---|---|---|---|---|---|---|
| Ex (n = 25) | Con (n = 25) | Ex (n = 30) | Con (n = 30) | Ex (n = 23) | Con (n = 23) | Ex (n = 20) | Con (n = 20) | |
| Male | 19 (76) | 21 (84) | NA | NA | 14 (61) | 16 (70) | 11 (55) | 12 (60) |
| Age (years) | 45 ± 15 | 46 ± 12 | NA | NA | 45 ± 13 | 43 ± 13 | 50 ± 10 | 52 ± 7 |
| Indication | ||||||||
| CLM | – | – | NA | NA | – | – | – | – |
| HCC | 25 (100) | 25 (100) | 23 (100) | 23 (100) | 19 (95) | 18 (90) | ||
| Cholangiocarcinoma | – | – | – | – | – | – | ||
| Other | – | – | – | – | 1 (5) | 2 (10) | ||
| Liver pathology | ||||||||
| Cirrhosis | 14 (56) | 15 (60) | NA | NA | NA | NA | 20 (100) | 20 (100) |
| Steatosis | NA | NA | NA | NA | NA | NA | ||
| Resection extent | ||||||||
| ≥2 segments | 21 (84) | 19 (76) | NA | NA | NA | NA | 13 (65) | 12 (60)a |
| Operating time (min) | 230 ± 67 | 246 ± 112 | NA | NA | 157 ± 39 | 163 ± 61 | 164 ± 42 | 190 ± 24 |
| Transection time (min) | NA | NA | NA | NA | NA | NA | NA | NA |
| Figueras et al.12 | Zhu et al.18 | Rahbari et al.16 | Kato et al.13 | |||||
|---|---|---|---|---|---|---|---|---|
| Ex (n = 41) | Con (n = 39) | Ex (n = 96) | Con (n = 96) | Ex (n = 65) | Con (n = 63) | Ex (n = 43) | Con (n = 42) | |
| Male | 28 (68) | 31 (79) | ns | 37 (57) | 42 (67) | NA | ||
| Age (years) | 62 ± 11 | 61.8 ± 13 | ns | 57 ± 11 | 59 ± 12. | 65 (28–82) | 67 (38–79) | |
| Indication | ||||||||
| CLM | 16 (39) | 15 (39) | ns | 35 (54) | 20 (32) | 6 (14) | 7 (42) | |
| HCC | 17 (41) | 16 (41) | 19 (29) | 37 (59) | 35 (81) | 34 (81) | ||
| Cholangiocarcinoma | – | – | – | – | 1 (2) | 0 (0) | ||
| Other | 8 (19) | 8 (21) | 11 (17) | 6 (9) | 1 (2) | 1 (2) | ||
| Liver pathology | ||||||||
| Cirrhosis | 21 (51) | 18 (46) | ns | NA | NA | NA | NA | |
| Steatosis | 15%±19 | 21%±23 | 40 (62) | 39 (62) | NA | NA | ||
| Resection extent | ||||||||
| ≥2 segments | 12 (29) b | 14 (36) | ns | 38 (58) | 37 (59) c | 19 (44) | 18 (43) d | |
| Operating time (min) | 219 ± 45 | 207 ± 48 | 162 (36) e | 172 (46) e | 145 (112–212) f | 155 (120–221) f | NA | NA |
| Transection time (min) | 65 ± 25 | 60 ± 26 | NA | NA | 7 (4–19) | 9 (5–19) | 55 (15–108) | 49 (7–157) |
Significantly different results (P < 0.05) are highlighted in bold.
Major resection
>1 segment
>2 segments
>1 segment
Mean (SEM)
Median [interquartile range (IQR)] data are otherwise presented as n(%) and the mean ± standard deviation (SD) or median (range).
Ex, experimental group; Con, Control groups; NA, not reported; ns, no significant difference; CLM, colorectal liver metastases; HCC, hepatocellular carcinoma.
Table 2.
Bias assessment table
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other Bias | |
|---|---|---|---|---|---|---|---|
| Wang et al.17 | + | + | + | − | + | + | + |
| Liu et al.14 | + | + | − | − | + | ? | + |
| Liu et al.15 | + | + | + | ? | + | + | + |
| El-Khaboutley et al.11 | + | ? | ? | ? | + | + | + |
| Rahbari et al.16 | + | + | + | − | + | + | + |
| Zhu et al.18 | + | + | ? | ? | + | + | + |
| Kato et al. 13 | + | ? | ? | ? | + | + | + |
| Figueras et al.12 | + | ? | ? | ? | + | + | + |
+=present; −=absent; ?=unclear.
Three RCTs were excluded. Ryu et al.19 maintained a low CVP in both groups and observed the effect of milrinone on the operative field without reporting EBL or the complication rate; Sand et al.20 assessed the effect of patient position and peak-end expiratory pressure (PEEP) on CVP and did not assess EBL, morbidity or LOS; and Lin et al.21 incorporated five randomized groups and assessed EBL as volume per transection area and did not report morbidity or LOS. It was felt that this was not meaningfully comparable to the other included studies and so excluded from the quantitative analysis.
Evaluation of intervention
Exact CVP was not reported in two trials.15,17 In the six trials that did report CVP,11–14,16,18 the CVP was significantly reduced in the low CVP group (n = 291) compared with the control groups (n = 294) [WMD −2.37 mmHg (95% CI −4.11, −0.63) P = 0.008, I2 = 92%]. No difference in in-flow occlusion time was observed in the low CVP group (n = 223) compared with the control group (n = 227) in the trials that reported using it [WMD 0.21 min (95%CI −1.47, 1.88) P = 0.810, I2 = 23%].12,16–18
Details of the trial protocols used are shown in Table3. Four trials utilized anaesthetic methods to reduce CVP,11,14,15,17 three trials13,16,18 used IVC clamping to reduce CVP and one trial12 performed an RCT comparing complete in-flow occlusion with selective in-flow occlusion and observed a significant difference in CVP between the two groups
Table 3.
Trial protocol details
| Author | Experimental protocol | In flow occlusion | CVP (mmHg) | Control Protocol | In flow occlusion | CVP (mmHg) |
|---|---|---|---|---|---|---|
| Wang et al.17 | IVI, Head tilt, GTN, Furosemide, | Y | 2–4 | IVI | Y | NA |
| Liu et al.14 | IVI, head tilt, GTN, isoflurane, fentanyl | N | 3.6 ± 0.4 | IVI | N | 8.9 ± 2.1 |
| Liu et al.15 | IVI, head tilt, GTN, furosemide, transfusion Hb <80 g/l | N | 2–4 | IVI | N | NA |
| El-Khaboutley et al.11 | GTN, IVI | Y | 3.0 ± 0.1 | IVI | Y | 6.9 ± 2.8 |
| Rahbari et al.16 | IVC clamp, epidural | Y | 4.0 ± 3.2 | Epidural, IVI, opioids, GTN, furosemide, reduced PEEP, epidural fentanyl | Y | 2.6 ± 1.8 |
| Zhu et al.18 | IVC clamp | Y | 4.3 (0.9)* | GTN, head tilt, furosemide, IVI | Y | 4.7 (0.5)* |
| Kato et al.13 | IVC clamp, IVI, | N | 4 (0–13) | IVI | N | 6 (1–14) |
| Figueras et al.12 | NA | Y (Complete occlusion) | 6.4 ± 3 | NA | Y (Selective occlusion) | 7.2 ± 3.6 |
Statistically significant (P < 0.05) CVP differences highlighted in bold.
The mean (SEM) data otherwise presented as the mean ± SD or median (range).
IVI, intravenous infusion; GTN, glycerine trinitrate; PEEP, peak-end expiratory pressure; NA, Not reported.
Primary outcome: morbidity rate
Five studies11,12,16–18 with a total of 490 patients (low CVP n = 243, control n = 247) reported overall systemic complication rates between the groups. There was no difference in the overall morbidity rate between low and high CVP surgery [OR=0.96 (95% CI 0.66,1.40) P = 0.840, I2 = 0%, Fig.2].
Figure 2.
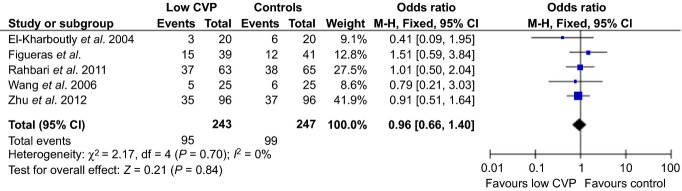
Post-operative morbidity rates. CVP, central venous pressure; CI, confidence interval
Primary outcome: subgroup analysis
Subgroup analysis was performed according to the method of CVP reduction (i.e. anaesthetic or surgical). The two trials that used anaesthetic techniques to reduce CVP11,17 demonstrated no difference in complication rates [OR=0.6 (95% CI 0.22, 1.63) P = 0.310, I2 = 0%]. The two trials14,15 not included in the quantitative analysis did not report any significant differences in post-operative renal functioning between the two groups. Sub-group analysis of the two trials comparing IVC clamping (n = 161) versus no IVC clamping (n = 159)16,18 demonstrated no difference in the morbidity rate [OR 1.06 (95% CI 0.68, 1.66) P = 0.800, I2 = 0%].
Secondary outcomes
Estimated blood loss
Seven trials11–15,17,18 comprising 553 patients (low CVP n = 276, control n = 277) reported EBL. There was a significant reduction in EBL in the low CVP group compared with the control group [WMD = −308.63 ml (95% CI −474.67, −142.58) P < 0.001, I2 = 73%]. Subgroup analysis11,14,15,17 demonstrated that anaesthetic measures to reduce CVP (n = 98) led to a significantly reduced EBL compared with the control (n = 98) (WMD = −406.26 ml, CI −499.77, −321.76, P = <0.001, I2 = 52). Sub-group analysis of two trials comparing IVC clamping (n = 139) with no IVC clamping (n = 138)13,18 showed no significant difference in EBL between the intervention and control groups (WMD = −88.7 ml, CI −268.02, 90.7, P = 0.330, I2 = 0%).
Intra-operative transfusion requirement
Intra-operative blood transfusion requirements were reported in seven trials11,12,14–18 including 681 patients (low CVP n = 339, control n = 342). Significantly fewer blood transfusions were required in patients in the low CVP group compared with the control group [OR 0.65 (95%CI 0.44, 0.97) P = 0.040, I2 = 37%].
Length of stay
Length of stay was reported in 4 of the 8 trials12,13,17,18 with 407 patients (low CVP n = 203, control n = 204). No significant difference was observed between the low CVP group and the control group [WMD −1.75 days (95% CI −5.84, 2.34), P = 0.400 I2 = 64%].
Discussion
This review demonstrates that low CVP surgery reduces EBL and blood transfusion rates after a liver resection. However, this does not correspond to improved outcomes in terms of morbidity or hospital stay.
Two other reviews have assessed the effect of CVP reduction on EBL.7,22 Gurusamy et al.7 and Li et al.22 meta-analysed three and five studies, respectively, comparing low CVP with high CVP and found a significant reduction in blood loss during the low CVP surgery. This present review includes a further three RCTs assessing high and low CVP,12,16,18 discriminates between the anaesthetic and surgical methodology and investigates quantitatively the effect of CVP reduction not only on EBL but also morbidity. This review further demonstrates the beneficial effects of a lower CVP during a transection when compared with higher CVPs in terms of intra-operative blood loss and blood transfusion rates. This consolidates the data from the previous reviews.
As well as the previous reviews of RCTs, retrospective series exist which have shown low CVP to be safe and associated with satisfactory EBL and outcomes23–26 and the few comparative studies comparing a CVP of >5 mmHg with a CVP of <5 mmHg during a liver transection24,27,28 retrospectively concluded that those patients undergoing a resection with CVP >5 mmHg suffered a higher EBL. These published series have often guided practice, and low CVP surgery is regarded as a standard practice.
Controversy remains regarding the evidence base for this issue with several trials29–31 reporting that CVP is not associated with a reduced EBL or predictive of EBL after regression analysis. Chibber et al.29 performed a sub-group analysis of CVP greater and less than 5 mmHg from their cohort (all using the same protocol) and did not observe a difference in blood loss in living donor patients.
Moreover, two of the included trials in the current analysis12,13 did not show a significant reduction in EBL and Zhu et al.18 showed a mean reduction by only 150 ml despite all achieving significant reductions in CVP. The reason for the modest reduction in EBL seen in these three included trials is potentially explained by the methodology. The overall difference in CVP between these groups was low and despite statistical significance in difference observed, a clinically significant difference is less obvious. Zhu et al.18 and Rahbari et al.13 had a control group as a low CVP group achieved by standard techniques compared with the IVC clamping group. Therefore, the comparisons in the meta-analysis were not all uncontrolled high CVP versus low CVP (although the difference between mean CVPs in these groups was statistically significant). When CVP difference was larger, a more significant difference in EBL was observed.11,14 This finding is suggestive of the importance of well-controlled CVP and does not identify benefits associated with ultra-low CVP.
It is well established that a high blood loss and blood transfusion intra-operatively has a negative effect on peri-operative complication rates and a reduction in EBL leads to improved post-operative outcomes.2,5 The overall meta-analysis, however, revealed no significant difference in complication rates between the low CVP and control groups.
An explanation for this might be the modest reduction in EBL reported by several of the trials12,13,18 that could have potentially influenced the analysis. The reported difference in EBL in these trials was around 100–200 ml less than the control group. This is much less than compared with the other included trials.11,16,17 Intuitively it can be appreciated why a large drop in EBL could contribute to an improved outcome, and a modest reduction would not necessarily translate into an enhanced post-operative course.
However, it is also important to consider the methodology of the techniques used to reduce CVP and their impact on the outcome. Three of the trials compared IVC clamping to reduce CVP compared with no IVC clamping. The potential hazards of IVC clamping have not fully been investigated. A negative effect on hepatic and renal function has not been routinely observed;16,32 however, significantly higher rates of thromboembolic events16 were reported. Such complications could negate the benefit of low CVP and low blood loss surgery. Considering the lack of benefit gained by this technique in terms of EBL and outcome when compared with standard practice, the routine performance of IVC clamping during transection cannot be supported.
A potential explanation for the negative result in morbidity rates from the anaesthetic studies is the small numbers of participants in the included RCTs. Only two of the anaesthetic trials reported systemic complication rates. These both showed improved outcomes (although not statistically significant) in the low CVP group. These trials are likely to have been under-powered to detect a difference in systemic complications. The subgroup analysis in the present meta-analysis also failed to detect a significant difference in systemic complications, and a type II error may be present. However, the two trials included in the meta-analysis11,17 did not report significant differences in complication rates and the two excluded trials, not reporting overall complication rates,14,15 did not report significant differences in renal or hepatic function despite significant reductions in EBL. Therefore, a clinical benefit to low CVP surgery is not unequivocally presented by the evidence.
Another explanation for similar morbidity rates between the groups is the potentially detrimental impact of the individual techniques used to reduce CVP by anaesthetic methods.11,14,15,17 Intravenous (IV) fluid restriction during the transection phase, glycerine trinitrate (GTN) and furosemide were frequently utilized by the included studies. These simple methods help to maintain a state of hypovolaemia and vasodilation reduces hepatic vein backpressure, which in turn reduces venous bleeding during hepatic transection. The potential for renal dysfunction has not been established.25 However, there is a lack of prospective evidence for the efficacy and/or safety of each individual component of such CVP lowering protocols.
Only, Ryu et al.19, and Sand et al.20 have performed assessments of single anaesthetic techniques in low CVP surgery. These studies did not fulfil the criteria for inclusion into the meta-analysis. Sand et al.20 investigated the benefit of positional change with or without PEEP on CVP during liver resection, finding a head-up tilt to be successful in reducing CVP. Several studies in this review performed a positional change to reduce CVP.14,15,17,18 Sand et al.20 found that the CVP rose in the head-down position but fell in the head-up position. Given that the hepatic vein pressure did not change regardless of position, and that head-up tilt is associated with gas embolism and haemodynamic instability,33 a positional change is advised against.
Ryu et al.19 assessed the effect of milrinone on CVP and the operative field. Milirone was suggested as beneficial owing to its inotropic as well as vasodilatory effect that would prevent the haemodynamic instability of fluid restriction or vasodilation with nitrates. The results were favourable although the study only assessed living donors, and so its effect on patients with significant co-morbidities is not yet established.
Another technique that was utilized sparingly by the included trials was an epidural blockade. In a study by Rahbari et al.,16 epidural anaesthesia used in the control group was associated with a significantly reduced CVP compared with IVC clamping. There is a lack of consensus among non-randomized trials with two trials not demonstrating an effect on blood loss when using an epidural in the CVP-lowering protocol29,34 and several others finding its absence associated with improvements in EBL and morbidity.27,28,35 This is a particularly pertinent issue relating to a liver resection as the opinion regarding routine epidural use remains divided. The advocates of an epidural would welcome its inclusion peri-operatively owing to the perceived improvement in post-operative pain, the attenuation of the inflammatory response and a reduction in morbidity rates.36 However, others have voiced concerns of coagulopathy secondary to a liver resection affecting epidural removal,37 increased IV fluids owing to epidural-related hypotension34 and increased post-operative transfusions of red cells.34 Such disadvantages are often accepted owing to perceptions of improved overall outcomes. However, this concept has been increasingly challenged as alternatives to epidurals are becoming more widespread, and enhanced recovery protocols improve the speed of recovery often without the need for an epidural.38,39 This is, therefore, an area that warrants prospective investigation to clarify the effect of an epidural on CVP and outcome after a liver resection.
An additional point to consider is the effect of in-flow occlusion. All but Kato et al.13 combined IVC clamping within flow occlusion. This could potentially result in a deleterious effect on outcome owing to a potential ischaemia-associated liver injury.40 This suspicion is further supported by three RCTs12,41,42 whereby selective inflow occlusion rather than complete in-flow occlusion was associated with a significantly reduced post-operative morbidity. All maintained a CVP of less than 5 mmHg, there was no difference in blood loss, but Ni et al.42 and Fu et al.41 reported a reduction in morbidity in the selective Pringle groups when compared with the Pringle groups. This, therefore, points to other areas of protocol optimization to consider when CVP has been optimized below 5 mmHg.
Whie the results of the present meta-analysis did not demonstrate a significant reduction in morbidity in the low CVP group, other advantages of low CVP surgery merit discussion. A reduction in bleeding during transection has practical implications that warrant consideration. Patient preference to avoid a blood transfusion is important. Furthermore, surgeons' assessment of the operative field and ease of surgery are important factors when attempting to improve short- and long-term outcomes. Such qualitative metrics was not assessed in the included trials, and these outcomes may be the subject of future research.
The main limitation of this review is the heterogeneity of the included studies. The anaesthetic studies assessed a CVP of over five with a CVP of <5 mmHg. The IVC clamping studies, despite significant differences in CVP, assessed a CVP of <5 mmHg in both groups. It is, therefore, unsurprising that little difference is seen in the outcome. However, the primary aim of all the included trials was to assess the effect of CVP lowering. Therefore, the included trials provide an accurate reflection of current practice in hepatic surgery.
In summary, this review and meta-analysis shows that low CVP surgery effectively results in reduced blood loss and transfusion requirement during a liver resection and its practice is supported. However, no improvement in clinical outcomes is associated with this, and this may be affected by methodology. IVC clamping does not improve outcome over low CVP surgery achieved by standard CVP lowering techniques and is not advised. The optimum technique to achieve a CVP reduction is not known with controversies existing regarding the correct anaesthetic technique and potential disadvantages of using the Pringle manoeuvre. Prospective, randomized trials are required to establish precise protocol components when attempting to optimize outcomes after a liver resection.
Acknowledgments
No funding was obtained for this study. This article is being submitted as a review and has not been presented elsewhere.
Conflicts of interest
None to declare.
References
- Cescon M, Vetrone G, Grazi GL, Ramacciato G, Ercolani G, Ravaioli M, et al. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249:995–1002. doi: 10.1097/SLA.0b013e3181a63c74. [DOI] [PubMed] [Google Scholar]
- Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. ; discussion -7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolsen MM, Wong-Lun-Hing EM, van Dam RM, van der Wilt AA, Slim K, Lassen K, et al. A systematic review of outcomes in patients undergoing liver surgery in an enhanced recovery after surgery pathways. HPB. 2013;15:245–251. doi: 10.1111/j.1477-2574.2012.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MJ, McNally S, Wigmore SJ. Enhanced recovery following liver surgery: a systematic review and meta-analysis. HPB. 2014;16:699–706. doi: 10.1111/hpb.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. doi: 10.1097/01.sla.0000141195.66155.0c. ; discussion -10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MT, Molenaar IQ, Porte RJ. Impact of blood loss on outcome after liver resection. Dig Surg. 2007;24:259–264. doi: 10.1159/000103656. [DOI] [PubMed] [Google Scholar]
- Gurusamy KS, Li J, Vaughan J, Sharma D, Davidson BR. Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database Syst Rev. 2012;5:Cd007338. doi: 10.1002/14651858.CD007338.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. 2011. (eds.). ( Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated arch 2011]. The Cochrane Collaboration. Available at www.cochrane-handbook.org (last accessed 1 May 2014)
- El-Kharboutly WS, El-Wahab M. The role of adoption of low central venous pressure in hepatic resection with pringle manoeuvre in reducing blood loss and improving operative outcome. Egypt J Anaesth. 2004;20:369–376. [Google Scholar]
- Figueras J, Llado L, Ruiz D, Ramos E, Busquets J, Rafecas A, et al. Complete versus selective portal triad clamping for minor liver resections: a prospective randomized trial. Ann Surg. 2005;241:582–590. doi: 10.1097/01.sla.0000157168.26021.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Kubota K, Kita J, Shimoda M, Rokkaku K, Sawada T. Effect of infra-hepatic inferior vena cava clamping on bleeding during hepatic dissection: a prospective, randomized, controlled study. World J Surg. 2008;32:1082–1087. doi: 10.1007/s00268-007-9445-0. [DOI] [PubMed] [Google Scholar]
- Liu HZZQ, Wang XH, Yang CX, Xu YH. Application of low central venous pressure in liver resection. Zhonghua Gandan Waike Zazhi. 2005;11:461–463. [Google Scholar]
- Liu YCM, Duan S, Peng X, Lai Y, Li Y. Effect of controlled low central venous pressure on renal function in major liver resection. Chin-Germ J Clin Oncol. 2008;7:7–9. [Google Scholar]
- Rahbari NN, Koch M, Zimmermann JB, Elbers H, Bruckner T, Contin P, et al. Infrahepatic inferior vena cava clamping for reduction of central venous pressure and blood loss during hepatic resection: a randomized controlled trial. Ann Surg. 2011;253:1102–1110. doi: 10.1097/SLA.0b013e318214bee5. [DOI] [PubMed] [Google Scholar]
- Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12:935–939. doi: 10.3748/wjg.v12.i6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Lau WY, Chen YF, Zhang BX, Huang ZY, Zhang ZW, et al. Randomized clinical trial comparing infrahepatic inferior vena cava clamping with low central venous pressure in complex liver resections involving the Pringle manoeuvre. The British journal of surgery. 2012;99:781–788. doi: 10.1002/bjs.8714. [DOI] [PubMed] [Google Scholar]
- Ryu HG, Nahm FS, Sohn HM, Jeong EJ, Jung CW. Low central venous pressure with milrinone during living donor hepatectomy. Am J Transplant. 2010;10:877–882. doi: 10.1111/j.1600-6143.2010.03051.x. [DOI] [PubMed] [Google Scholar]
- Sand L, Rizell M, Houltz E, Karlsen K, Wiklund J, Odenstedt Herges H, et al. Effect of patient position and PEEP on hepatic, portal and central venous pressures during liver resection. Acta Anaesthesiol Scand. 2011;55:1106–1112. doi: 10.1111/j.1399-6576.2011.02502.x. [DOI] [PubMed] [Google Scholar]
- Lin CX, Guo Y, Lau WY, Zhang GY, Huang YT, He WZ, et al. Optimal central venous pressure during partial hepatectomy for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:520–524. doi: 10.1016/s1499-3872(13)60082-x. [DOI] [PubMed] [Google Scholar]
- Li Z, Sun YM, Wu FX, Yang LQ, Lu ZJ, Yu WF. Controlled low central venous pressure reduces blood loss and transfusion requirements in hepatectomy. World J Gastroenterol. 2014;20:303–309. doi: 10.3748/wjg.v20.i1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayanoglu HO, Ulukaya S, Yuzer Y, Tokat Y. Anesthetic management and complications in living donor hepatectomy. Transpl Proc. 2003;35:2970–2973. doi: 10.1016/j.transproceed.2003.10.090. [DOI] [PubMed] [Google Scholar]
- Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058–1060. doi: 10.1046/j.1365-2168.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- Moug SJ, Smith D, Leen E, Angerson WJ, Horgan PG. Selective continuous vascular occlusion and perioperative fluid restriction in partial hepatectomy. Outcomes in 101 consecutive patients. Eur J Surg Oncol. 2007;33:1036–1041. doi: 10.1016/j.ejso.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Bui LL, Smith AJ, Bercovici M, Szalai JP, Hanna SS. Minimising blood loss and transfusion requirements in hepatic resection. HPB. 2002;4:5–10. doi: 10.1080/136518202753598672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Merchant NB, Didolkar MS. Hepatic resection using intermittent vascular inflow occlusion and low central venous pressure anesthesia improves morbidity and mortality. J Gastrointest Surg. 2000;4:162–167. doi: 10.1016/s1091-255x(00)80052-9. [DOI] [PubMed] [Google Scholar]
- Chhibber A, Dziak J, Kolano J, Norton JR, Lustik S. Anesthesia care for adult live donor hepatectomy: our experiences with 100 cases. Liver Transpl. 2007;13:537–542. doi: 10.1002/lt.21074. [DOI] [PubMed] [Google Scholar]
- Kim YK, Chin JH, Kang SJ, Jun IG, Song JG, Jeong SM, et al. Association between central venous pressure and blood loss during hepatic resection in 984 living donors. Acta Anaesthesiol Scand. 2009;53:601–606. doi: 10.1111/j.1399-6576.2009.01920.x. [DOI] [PubMed] [Google Scholar]
- Leelanukrom R, Songthamwat B, Thonnagith A, Narkburin S. Factors affecting intraoperative blood loss during liver resection. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2013;96:58–63. [PubMed] [Google Scholar]
- Otsubo T, Takasaki K, Yamamoto M, Katsuragawa H, Katagiri S, Yoshitoshi K, et al. Bleeding during hepatectomy can be reduced by clamping the inferior vena cava below the liver. Surgery. 2004;135:67–73. doi: 10.1016/s0039-6060(03)00343-x. [DOI] [PubMed] [Google Scholar]
- Palmon SC, Moore LE, Lundberg J, Toung T. Venous air embolism: a review. J Clin Anesth. 1997;9:251–257. doi: 10.1016/s0952-8180(97)00024-x. [DOI] [PubMed] [Google Scholar]
- Page A, Rostad B, Staley CA, Levy JH, Park J, Goodman M, et al. Epidural analgesia in hepatic resection. J Am Coll Surg. 2008;206:1184–1192. doi: 10.1016/j.jamcollsurg.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Eid EA, Sheta SA, Mansour E. Low central venous pressure anesthesia in major hepatic resection. Middle East J Anaesthesiol. 2005;18:367–377. [PubMed] [Google Scholar]
- Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr. 2012;31:783–800. doi: 10.1016/j.clnu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Takasita M, Matsumoto H, Sonoda H, Tsumura H, Torisu T. Spinal epidural hematoma after removal of an epidural catheter: case report and review of the literature. J Spinal Disord Tech. 2005;18:547–551. doi: 10.1097/01.bsd.0000128692.44276.cf. [DOI] [PubMed] [Google Scholar]
- Koea JB, Young Y, Gunn K. Fast track liver resection: the effect of a comprehensive care package and analgesia with single dose intrathecal morphine with gabapentin or continuous epidural analgesia. HPB Surg. 2009;2009:271986. doi: 10.1155/2009/271986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revie EJ, McKeown DW, Wilson JA, Garden OJ, Wigmore SJ. Randomized clinical trial of local infiltration plus patient-controlled opiate analgesia vs. epidural analgesia following liver resection surgery. HPB. 2012;14:611–618. doi: 10.1111/j.1477-2574.2012.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Hwang CR, Liu TJ, P’Eng FK. Effects and limitations of prolonged intermittent ischaemia for hepatic resection of the cirrhotic liver. The British journal of surgery. 1996;83:121–124. doi: 10.1002/bjs.1800830139. [DOI] [PubMed] [Google Scholar]
- Fu SY, Lau WY, Li GG, Tang QH, Li AJ, Pan ZY, et al. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. Am J Surg. 2011;201:62–69. doi: 10.1016/j.amjsurg.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Ni JS, Lau WY, Yang Y, Pan ZY, Wang ZG, Liu H, et al. A prospective randomized controlled trial to compare pringle manoeuvre with hemi-hepatic vascular inflow occlusion in liver resection for hepatocellular carcinoma with cirrhosis. J Gastrointest Surg. 2013;17:1414–1421. doi: 10.1007/s11605-013-2236-z. [DOI] [PubMed] [Google Scholar]


