Abstract
Background
A delayed post-pancreatoduodenectomy haemorrhage is associated with a significant increase in peri-operative mortality. Endovascular techniques are frequently used for a delayed haemorrhage. However, limited data exists on the short- and long-term outcomes of this approach. A retrospective review over a 10-year period at a quaternary-referral pancreatic centre was performed.
Methods
Between 2002–2012, 1430 pancreatoduodenectomies were performed, and 32 patients had a delayed haemorrhage (occurring >24 h post-operatively) managed by endovascular techniques. The clinicopathological variables related to a haemorrhage were investigated.
Results
A total of 42 endovascular procedures were performed at a median of 25 days, with the majority of delayed haemorrhages occurring after 7 days. There were four deaths (13%) with three occurring in patients with a grade C haemorrhage. Seven patients (22%) experienced rebleeding, and two patients developed hepatic abscesses.
Conclusion
A delayed haemorrhage post-pancreaticoduodenectomy can be managed by endovascular techniques with acceptable morbidity and mortality. Rebleeding and hepatic abscesses may occur and can be managed non-operatively in most cases. The association of a delayed haemorrhage with a pancreatic fistula makes this a challenging clinical problem.
Introduction
The peri-operative mortality after a pancreatoduodenectomy (PD) at high-volume centres in contemporary series is < 3%.1,2 While mortality has decreased, peri-operative complications including delayed gastric emptying, a pancreatic fistula and abscesses remain common. In contrast, a post-pancreatectomy hemorrhage (PPH) occurs less frequently but has been associated with a significant increase in mortality.3 To facilitate standardized reporting and study of this complication, the International Study Group for Pancreatic Surgery (ISGPS) has proposed a classification system for PPH.4 The definitions distinguish an early haemorrhage as occurring within 24 h of the index operation and a late haemorrhage beyond 24 h. Several recent studies have evaluated the classification system.5–7 These studies indicate that haemorrhage after a PD may occur in as many as 6–8% of patients. Importantly, there appear to be clear differences in patient outcomes depending on the timing and severity of bleeding. For example, in one study, patients with delayed extraluminal grade C PPH had a mortality of 41%.6 The studies to date underscore the fact that delayed bleeding after a PD represents one of the most challenging and potentially life-threatening post-operative complications.
In recent years, a delayed post-operative haemorrhage has been preferentially treated with endovascular techniques. The use of coil embolization for a gastroduodenal artery stump haemorrhage and the use of hepatic artery stents are well described. While these methods are considered highly effective and avoid the morbidity of a laparotomy, there are limited data evaluating the potential complications and long-term outcomes. Given the paucity of data on patients managed by endovascular techniques for a delayed PPH, we focused specifically on this population. The aims of this study were two-fold. First, we sought to identify patients with a delayed PPH managed with the endovascular approach to assess short- and long-term outcomes. Second, given the significant increase in morbidity and mortality, we evaluated potential risk factors for Grade B and C PPH.
Patients and methods
A retrospective review of a prospectively maintained database of vascular interventional procedures after pancreatoduodenectomies between January 2002 and November 2012 was performed at a quaternary-referral pancreatic surgery centre. Patients undergoing other pancreatic operations including enucleation, a total pancreatectomy and a distal pancreatectomy were excluded. ISGPS definitions and grading system for PPH were used. A delayed PPH was defined as a haemorrhage occurring beyond 24 h post-operatively. Patients with grade A PPH were clinically well and did not require therapeutic intervention. Patients with a grade B PPH had a mild, late intra- or extraluminal haemorrhage requiring intervention that may include transfusion of blood, intermediate care or intensive care unit, endoscopy, embolization or relaparotomy for early PPH. Patients with grade C PPH had severe bleeding, were critically ill requiring embolization, endoscopy, or a laparotomy and intensive care unit care. All identified cases of post-PD haemorrhage managed by interventional radiology (IR) occurred after 24 h, therefore, by ISGPS definition would be considered a delayed haemorrhage. Pancreatic fistula was defined and graded per ISGPS. Cases in which drain amylase was not available but clinical notes documented a pancreatic leak and/or management of a leak was described were deemed grade B or C leaks as appropriate. Clinical variables associated with bleeding and postoperative management were reviewed. Operative reports were reviewed; estimated blood loss and operative time were extracted from the anaesthetic record. Pathology reports were reviewed to determine the final pathologic diagnosis.
This study was approved by the Mayo Clinic Institutional Review Board.
Results
Between 2002 and 2012, 1430 pancreatoduodenectomies were performed. Thirty-two patients (2%) required endovascular management of a delayed haemorrhage. The median age of patients undergoing endovascular management for bleeding was 62.5 years, and 66% were males (Table1). The median body mass index (BMI) was 27.9. Sixty-nine per cent of the patients underwent an open PD with the remaining undergoing a laparoscopic or robot-assisted PD. All patients underwent a pancreaticojejunostomy with 77% of these being performed as a duct-to-mucosa reconstruction. The remaining patients underwent an end-to-side invaginating or dunking pancreaticojejunostomy. The mean operative time was 389 min. Most patients had a drain placed at the time of operation (81%). Nine per cent of patients underwent concomitant vein resection. The pancreatic gland texture was soft in 56% of the patients. Only 28% of the patients presented with pancreatic ductal adenocarcinoma and the majority of the pancreatic ducts were noted to be small (<5 mm). Half of the patients presented with tumours located within the duodenum, ampulla or common bile duct.
Table 1.
Patient and operative factors
| Variable | n (%) |
|---|---|
| Patients | 32 |
| Demographics | |
| Median age | 63 (36–82) |
| Male | 21 (66%) |
| Body mass index | 28 (21–36) |
| Operation, n (%) | |
| Open PD | 22 (69%) 22/1197 (1.8%)a |
| Laparoscopic/Robot assist PD | 10 (31%) 10/233 (4.3%)b |
| Operative time (minutes), mean | 389 (218–696) |
| Vein resection | 3 (9%) |
| Duct to mucosa anastomosis | 24 (77%) |
| Gland texture, n (%) | |
| Soft | 18 (56%) |
| Firm | 8 (25%) |
| Unknown | 6 (19%) |
| Duct | |
| Small (<5 mm) | 24 (75%) |
| Dilated (>=5 mm) | 3 (9%) |
| Unknown | 5 (16%) |
| Median duct size, mm (range) | 3 (1–10) |
| Intra-operative blood loss, median | 500 (100–3100) |
| Pathology (%) | |
| Adenocarcinoma other (ampullary, duodenum and CBD) | 10 (28%) |
| PDAC | 10 (28%) |
| Other | 12 (38%) |
| Grade B/C Pancreatic fistula | 23 (72%) |
| Fistula risk score (mean) | 5 (2–9) |
| Use of pancreatic stent | 14 (45%) |
| Use of operative drain | 25 (81%) |
PD, pancreatoduodenectomy; PDAC, pancreatic ductal adenocarcinoma; CBD, common bile duct.
Number (%) of open PD with a post-pancreatectomy haemorrhage managed by endovascular techniques.
Number (%) of laparoscopic/robotic-assisted PD with a post-pancreatectomy haemorrhage managed with endovascular techniques.
Forty-two endovascular procedures were performed in the 32 patients including 27 embolizations and 14 covered endovascular stents to manage pseudoaneurysms (Table2). One patient underwent balloon angioplasty to manage an endoleak from a previously placed stent. Eight patients (25%) required repeat intervention. One patient underwent angiography without active bleeding identified. A bleeding source was identified on repeat angiography 8 days later. The presentation was sanguineous drain output 31% of the time, gastrointestinal (GI) bleeding in 48%, and 12% of the time both intraluminal and extraluminal bleeding was identified. Eleven patients with suspected intraluminal bleeding underwent endoscopy prior to angiography; however, not all patients with luminal bleeding underwent endoscopy. Patients with both intra-luminal and extra-luminal bleeding more consistent with pseudoaneurysmal bleeding were managed by interventional angiography. One of the patients had a delayed haemorrhage from the pancreaticojejunal anastomosis, specifically the cut surface of the pancreas. Interestingly, 17% of the procedures were performed in the absence of overt bleeding from the drain or GI tract. These were noted incidentally when cross-sectional imaging was performed to assess for intra-abdominal abscesses or during routine oncological follow-up (Fig.1).
Table 2.
Presentation, endovascular management and outcomes of a delayed post-pancreatectomy haemorrhage
| Variable | n (%) |
|---|---|
| Presentation of bleed | |
| Gastrointestinal bleed | 20 (48) |
| Blood in drain | 13 (31) |
| Both intraluminal/extraluminal | 5 (12) |
| Imaging | 4 (10) |
| Embolization | 27 (64) |
| Stent | 14 (33) |
| Balloon | 1 (2) |
| Involved vessel | |
| CHA/GDA | 18 (43) |
| 10 coils (3 recurrent bleed, 1 liver abscess) | |
| 8 stent (2 recurrent bleed, 1 liver abscess) | |
| SMA/IPD | 14 (33) |
| 8 coils | |
| 5 stents (2 recurrent bleed) | |
| 1 balloon | |
| Other | 10 (24) |
| Aberrant arterial anatomy | 13 (41) |
| PRBC units transfused | 5 (1–25) range |
| Higher level of care | 21 (65) |
| Recurrent bleed | 7 (21) |
| Recurrent pseudoaneurysm (CHA, SMA, rRHA) | 3 |
| Rebleed from coiled vessel (GDAx2, rRHA) | 2 |
| Blocked CHA stent | 1 |
| Endoleak from SMA stent | 1 |
| Recurrent bleed interval | 10 (2–152) days |
| Hepatic abscess | 2 (6) |
| In-hospital mortality | 4 (13) |
| Concomitant endoscopy | 11 (34) |
| Intraluminal blood | 9 |
| Reoperation | 11 (34) |
| For bleeding | 2(6) |
| Total number of interventional procedures (mean) | 7.7 |
| Length of stay (days) | 35 (10–119) |
CHA, common hepatic artery; rRHA, replaced right hepatic artery; GDA, gastroduodenal artery; SMA, superior mesenteric artery; IPD, inferior pancreatoduodenal artery; PRBC, packed red blood cells.
Figure 1.
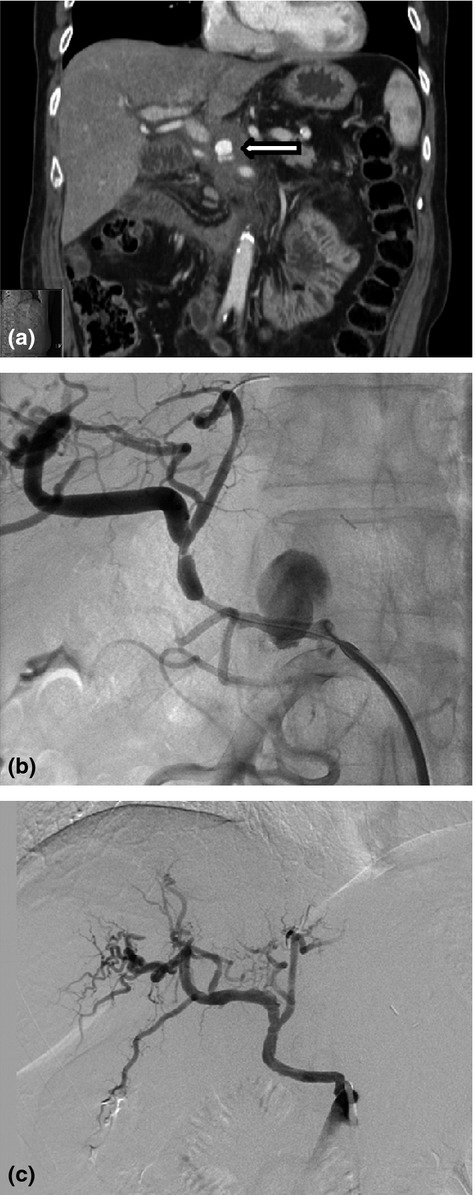
Stenting of incidentally discovered a hepatic artery pseudoaneurysm. (a) Hepatic artery pseudoaneurysm identified incidentally on follow-up imaging 6 weeks post-operatively. (b) Interventional angiography reveals a large pseudoaneurysm from a replaced hepatic artery. (c) Placement of covered stent in a replaced hepatic artery
Endovascular procedures were performed at a median of 25 days after a PD (range 4–68) with 94% occurring after 7 days and 31% occurring after 30 days. Forty-one per cent of patients had an interventional procedure performed after hospital discharge after the index operation. Three patients were transferred from outside institutions where they presented with anaemia, blood from an operative drain, or gastrointestinal bleeding. The median units of blood transfused were 5 (range 1–25), and 65% of the patients required admission to a higher level of care (53% intensive care unit). Patients undergoing endovascular management had a concomitant pancreatic leak documented in 72% of cases and a median hospital length of stay of 35 days (range 10–119).
After a median follow-up of 356 days, recurrent bleeding occurred in 21% of patients after endovascular management. These were treated with embolization and two patients ultimately required reoperation. Twenty-one patients had imaging follow-up beyond 30 days with a median of 329 days (range 42–2030). Two hepatic abscesses were identified after embolization or stenting of the hepatic artery, and both were managed with percutaneous drainage. One focal hepatic infarction managed without drainage was also identified. Patients were not anticoagulated after stent placement owing to concern for re-bleeding and because the majority had bleeding in the context of other post-operative complications. We did not identify a delayed haemorrhage attributable to a drainage procedure for an abscess or fistula. Fifteen patients (47%) had follow-up beyond 1 year, with a median of 1377 days (range 448–2398). Overall, reoperation was required in 11 (34%) of which nine patients (82%) underwent reoperation for septic complications secondary to pancreatic leaks. 72% of the reoperations occurred prior to the interventional procedures for PPH. In the two patients who returned to the operating room for a recurrent haemorrhage, one had ligation of a bleeding vessel on the cut surface of the pancreas and in the other the gastroduodenal artery stump was ligated. Overall, there was a 12.5% mortality rate with four deaths. One death occurred within 30 days, two occurred within 90-days, and one occurred beyond 90 days. One patient with a grade B PPH died of causes unrelated to a pancreatic fistula or haemorrhage. The remaining three deaths were in patients with grade C PPH. Two of the deaths were directly related to a haemorrhage whereas overwhelming sepsis was the cause of death in the third case. One patient developed a recurrent haemorrhage, suffered aspiration and after discussion regarding goals of care, the patient died over 2 months after the index operation. The other death secondary to a haemorrhage was a prolonged and complicated post-operative course. The patient had multiple interventional angiography procedures in the setting of a pancreatic fistula, portal vein thrombosis and recurrent haemorrhage. The patient developed a massive upper GI bleed and after discussion regarding the philosophy of care the patient died over 4 months after the initial PD.
Thirty of the thirty-two patients had grade B or C PPH. Univariate analyses evaluated potential associations between patient-related and technical factors and grade of PPH. There were no significant differences in age, BMI, ASA classification or type of operation (all P ≥ 0.565). There were no associations between pancreas-specific factors, such as gland texture, duct diameter, pathology, estimated blood loss, transfusion requirement and the development of a grade B versus C PPH (all P ≥ 0.123). The proportion of grade B and C pancreatic fistulae did not differ between the two groups (P = 0.657). Data not shown.
Discussion
A delayed PPH is among the most devastating post-operative complications after PD. In one review of a prospective database of 1669 consecutive pancreatic resections, the mortality rate among patients presenting with a delayed haemorrhage was nearly 50%.8 Another study of PPH demonstrated a 17-fold higher mortality in patients with grade C PPH.6 A contemporary report of management of PPH indicated a lower mortality rate of 3%. The present study is among the largest to investigate a haemorrhage managed by interventional techniques post-pancreatoduodenectomy. The data presented support the findings of others that a delayed haemorrhage after a PD is associated with significant morbidity and increased mortality.
In the present study, the most common presentation for a delayed haemorrhage was GI bleeding followed by sanguineous drain output. This finding is consistent with the report of Correa-Gallego in which an intraluminal haemorrhage was the presenting sign in 69% of delayed hemorrhages.5 However, the initial presentation of delayed PPH varies among reports, with some studies identifying an extraluminal haemorrhage as the more common initial presentation. The presentation of PPH is affected by whether an operative drain was placed, as a change in drain output or character is obviously not detectable upon removal or in the absence of a drain. Furthermore, a false intraluminal or extraluminal haemorrhage can occur where, in the presence of anastomotic dehiscence, extraluminal bleeding may present intraluminally and vice versa.8
Others report simultaneous extra- and intra-luminal bleeding in 7% of cases, which was observed in 12% of our bleeds.9,10 Interestingly, in the present study, 17% of the pseudoaneurysms were identified incidentally without overt signs of a haemorrhage. To our knowledge, this is a novel finding that could be missed by retrospective review of databases for PPH as there may be no clinical signs of a haemorrhage documented. Given the potential for pseudoaneurysm rupture, we strongly recommend intervention even in the absence of signs of a haemorrhage. Indeed, three patients subsequently developed clinical signs of haemorrhage including one pseudoaneurysm adjacent to an existing endovascular stent and two patients with GI bleeding. Exclusion of four patients who did not manifest any clinical signs of bleeding during the follow-up yields a PPH incidence of 2%, a re-bleed rate of 25% and an overall mortality rate of 14%.
Regardless of the mode of presentation, a delayed haemorrhage occurred many days and even weeks after the PD. We concur with other groups that the definition of an early haemorrhage as occurring within 24 h may be too strict as the pathophysiology and management of bleeding within the first several postoperative days is unlikely to differ.5,8 In the present study, the endovascular interventions were performed a median of 3 weeks after a PD. Almost half of the patients had been discharged after their index operation. This finding is consistent with a systematic review that showed a delayed haemorrhage presenting at a median of 13–27 days post-operatively.10 Thus, a delayed haemorrhage should be an important consideration when patients discharged from the hospital report GI bleeding or sanguineous drainage. Three patients were transferred from an outside institution after presenting with signs of bleeding. This study is limited to patients undergoing IR procedures at our institution. Patients are routinely followed post-operatively in person or by phone, and surgical staff are available at all times to respond to clinical questions from outside institutions. Therefore, the anticipated number of patients who develop a PPH at an outside institution that is undocumented would be low. However, we acknowledge that this study may underestimate the true incidence of a delayed PPH.
When a delayed haemorrhage is suspected, the subsequent management remains controversial. This is in contrast to an early PPH, where most surgeons would advocate for reoperation. An early haemorrhage is most commonly as a result of inadequate or incomplete haemostasis and can be effectively treated by reoperation. In the setting of a delayed haemorrhage, some groups advocate for operative intervention, whereas others support an interventional or endoscopic approach whenever possible.3,5,8 This is because the operative field after a PD, particularly when a pancreatic leak is present, may be hostile which may render access to and control of arterial haemorrhage extremely difficult. This study supports the findings of others that the majority of delayed haemorrhages are arterial in origin.8 One patient underwent stenting of a portal vein pseudoaneurysm. In the present study, 72% of the patients had a concomitant grade B/C pancreatic fistula and, thus, a non-operative approach to control the haemorrhage was given high priority. However, in the absence of emergent IR services or an unstable patient, some would argue that reoperation remains the preferred management.8 Indeed, in one systematic review, the most common reason for not performing angiography was haemodynamic instability.10 In the present study, only two patients underwent a reoperation after a failed angiography. Our study group was limited to patients undergoing IR; therefore, patients managed exclusively by reoperation would not have been captured. However, we anticipate that this number would be small given our preference to manage a delayed haemorrhage by endovascular techniques. A specific management pathway or algorithm for delayed haemorrhage was not used during the study period. In general, patients presenting with evidence of a delayed extraluminal haemorrhage are preferentially managed by endovascular techniques. In contrast, those that presented with evidence of intraluminal bleeding generally undergo endoscopy. CT angiography is not used for haemodynamically unstable patients. In our prospectively maintained database, a review of over 1000 consecutive Whipple procedures performed over a 7-year period from the present centre indicated a reoperation rate of 0.7% for a haemorrhage (C. Shubert and M. Kendrick, unpublished data). In the present study, both re-operative cases identified the bleeding source that was successfully controlled. A recurrent haemorrhage developed in one of these patients. A completion pancreatectomy has been described in up to 40% of reoperations for haemorrhage; however, it was not performed in either of these cases and is not a preferred approach at our institution.8–10
Complications after endovascular management of PPH have been described. These include rebleeding, stent stenosis or thrombosis and infarction of end organs secondary to thrombosis or embolization.11,12 Choi et al.13 describe two deaths from hepatic failure after hepatic artery embolization for PPH, whereas Heiss et al.14 described hepatic artery stent thrombosis without evidence of sequelae. In the present study, rebleeding was seen in 25% of patients. These were as a result of recurrent pseudoaneurysms, rebleeding from coiled vessels, blocked stents and endoleaks. As discussed above, in most cases these were successfully managed by repeat interventional procedures. Only two patients developed hepatic abscesses, one post-embolization of the hepatic artery and one after common hepatic artery stenting. Both patients were successfully managed with computed tomography-guided drain placement. Overall, the present results would indicate an acceptable rate of morbidity in this complex set of patients. With respect to the long-term outcomes, our median follow-up for all patients in the study was 356 days. This makes it difficult to comment on the long-term durability of this approach, and further study is required. It should be noted, however, that 47% of the patients had follow-up beyond 1 year, with a median of nearly 4 years. Among these patients, we did not identify long-term sequelae from the endovascular management of a haemorrhage.
Given the increase in mortality associated with PPH, it would be valuable to identify risk factors for a haemorrhage. Furthermore, while there has been a limited evaluation of the ISGPS grading system, it appears that worsening grade of haemorrhage correlates with mortality. In one report, the mortality for patients with grade C PPH was 34% compared with 6% for grade B PPH.6 Thus, identification of patients at risk for severe PPH would be clinically relevant. A recent study identified several factors for grade C PPH, including advanced age, increased BMI, an intra-operative transfusion, portal venous resection, multivisceral resection and a post-operative pancreatic fistula on univariable analysis.7 On multivariable analysis, a post-operative pancreatic fistula was most strongly associated with grade C PPH with an odds ratio of 5.6. Several other studies have also investigated the risk factors for PPH and the most uniformly associated risk factor among these studies is a postoperative fistula.15,16 The incidence of a Grade C fistula was 11 times greater in patients with grade C PPH and another study reported 55% of post-pancreatoduodenectomy hemorrhages had concurrent POPF.6–8 We sought to identify if risk factors could be identified distinguishing Grade B and C PPH. As a post-operative pancreatic fistula is strongly associated with PPH, we included elements of the externally validated fistula risk score that predict the development of a post-operative pancreatic fistula.17 We did not find any specific associations between patient specific or technical factors and progression to grade B versus a grade C PPH haemorrhage. This lack of association could be a result of a Type II error owing to the rarity of the events. The application of the fistula risk score to a larger population may permit identification of specific risk factors for a severe haemorrhage.
The pathophysiological relationship between a pancreatic fistula and haemorrhage is unclear. The role of pancreatic enzymes, infection and inflammatory mediators to the process that predisposes to bleeding remains undetermined. It has not been the routine practice at our institution to protect vessels, however, in the absence of routinely identifiable risk factors for a severe haemorrhage, the use of vascularized pedicle flaps to cover exposed vessels may be considered.18,19
Of 1430 pancreatoduodenectomies performed over a recent 10-year period, 32 patients (2%) required endovascular management of a delayed haemorrhage. The incidence of a PPH managed with endovascular techniques was 4.3% after a laparoscopic PD and 1.8% after an open PD (Table1). This difference is noteworthy but beyond the scope of this manuscript and will be the subject of future investigations. A delayed PPH was associated with a pancreatic fistula in 72% and resulted in a mortality rate of 12%. Endovascular procedures were performed a median of 25 days after a PD, and almost one-half of the patients had been discharged from the hospital after their pancreatic resection. A high index of suspicion for PPH should be maintained well beyond discharge for the index procedures. While rebleeding occurred in 22% of the patients, repeat angiographic procedures were successful in managing the majority. It was noteworthy that 17% of the procedures were performed based on abdominal computed tomography findings without clinical evidence of intra- or extraluminal bleeding. While the median follow-up was 1 year, almost half of the patients had a median follow-up of nearly 4 years without long-term sequelae of endovascular management of a haemorrhage.
A delayed haemorrhage after a PD remains one of the most challenging ongoing clinical problems. The patients frequently require coordinated care, multiple procedures and a prolonged stay in the hospital. However, in the majority of cases, a delayed haemorrhage after a PD can be successfully managed with endovascular techniques with low morbidity and acceptable mortality. Further investigation is required to define risk factors for a severe haemorrhage.
Conflicts of interest
None declared.
References
- Lewis R, Drebin JA, Callery MP, Fraker D, Kent TS, Gates J, et al. A contemporary analysis of survival for resected pancreatic ductal adenocarcinoma. HPB. 2013;15:49–60. doi: 10.1111/j.1477-2574.2012.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Ahuja N, Makary MA, Cameron JL, Eckhauser FE, Choti MA, et al. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB. 2014;16:83–90. doi: 10.1111/hpb.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc T, Cortes A, Goere D, Sibert A, Pessaux P, Belghiti J, et al. Hemorrhage after pancreaticoduodenectomy: when is surgery still indicated? Am J Surg. 2007;194:3–9. doi: 10.1016/j.amjsurg.2006.08.088. [DOI] [PubMed] [Google Scholar]
- Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Correa-Gallego C, Brennan MF, D'Angelica MI, DeMatteo RP, Fong Y, Kingham TP, et al. Contemporary experience with postpancreatectomy hemorrhage: results of 1,122 patients resected between 2006 and 2011. J Am Coll Surg. 2012;215:616–621. doi: 10.1016/j.jamcollsurg.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Grutzmann R, Ruckert F, Hippe-Davies N, Distler M, Saeger HD. Evaluation of the International Study Group of Pancreatic Surgery definition of post-pancreatectomy hemorrhage in a high-volume center. Surgery. 2012;151:612–620. doi: 10.1016/j.surg.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Wellner UF, Kulemann B, Lapshyn H, Hoeppner J, Sick O, Makowiec F, et al. Postpancreatectomy hemorrhage–incidence, treatment, and risk factors in over 1,000 pancreatic resections. J Gastrointest Surg. 2014;18:464–475. doi: 10.1007/s11605-013-2437-5. [DOI] [PubMed] [Google Scholar]
- Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski D, Koenig AM, et al. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246:269–280. doi: 10.1097/01.sla.0000262953.77735.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhu X, Chen H, Qian HG, Leng JH, Qiu H, et al. Management of delayed post-pancreaticoduodenectomy arterial bleeding: interventional radiological treatment first. Pancreatology. 2011;11:455–463. doi: 10.1159/000331456. [DOI] [PubMed] [Google Scholar]
- Roulin D, Cerantola Y, Demartines N, Schafer M. Systematic review of delayed postoperative hemorrhage after pancreatic resection. J Gastrointest Surg. 2011;15:1055–1062. doi: 10.1007/s11605-011-1427-8. [DOI] [PubMed] [Google Scholar]
- Lee HG, Heo JS, Choi SH, Choi DW. Management of bleeding from pseudoaneurysms following pancreaticoduodenectomy. World J Gastroenterol. 2010;16:1239–1244. doi: 10.3748/wjg.v16.i10.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Zhu J, Zhu M, Li C, Jian W, Jiang J, et al. Therapeutic management of hemorrhage from visceral artery pseudoaneurysms after pancreatic surgery. J Gastrointest Surg. 2011;15:1417–1425. doi: 10.1007/s11605-011-1561-3. [DOI] [PubMed] [Google Scholar]
- Choi SH, Moon HJ, Heo JS, Joh JW, Kim YI. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg. 2004;199:186–191. doi: 10.1016/j.jamcollsurg.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Heiss P, Bachthaler M, Hamer OW, Piso P, Herold T, Schlitt HJ, et al. Delayed visceral arterial hemorrhage following Whipple's procedure: minimally invasive treatment with covered stents. Ann Surg Oncol. 2008;15:824–832. doi: 10.1245/s10434-007-9715-y. [DOI] [PubMed] [Google Scholar]
- Manas-Gomez MJ, Rodriguez-Revuelto R, Balsells-Valls J, Olsina-Kissler JJ, Caralt-Barba M, Perez-Lafuente M, et al. Post-pancreaticoduodenectomy hemorrhage. Incidence, diagnosis, and treatment. World J Surg. 2011;35:2543–2548. doi: 10.1007/s00268-011-1222-4. [DOI] [PubMed] [Google Scholar]
- Ricci C, Casadei R, Buscemi S, Minni F. Late postpancreatectomy hemorrhage after pancreaticoduodenectomy: is it possible to recognize risk factors? JOP. 2012;13:193–198. [PubMed] [Google Scholar]
- Miller BC, Christein JD, Behrman SW, Drebin JA, Pratt WB, Callery MP, et al. multi-institutional external validation of the fistula risk score for pancreatoduodenectomy. J Gastrointest Surg. 2014;18:172–179. doi: 10.1007/s11605-013-2337-8. ; discussion 9-80. [DOI] [PubMed] [Google Scholar]
- Iannitti DA, Coburn NG, Somberg J, Ryder BA, Monchik J, Cioffi WG. Use of the round ligament of the liver to decrease pancreatic fistulas: a novel technique. J Am Coll Surg. 2006;203:857–864. doi: 10.1016/j.jamcollsurg.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Sadamori H, Umeda Y, Shinoura S, Yoshida R, Satoh D, et al. Preventive effect of omental flap in pancreaticoduodenectomy against postoperative pseudoaneurysm formation. Hepatogastroenterology. 2012;59:578–583. doi: 10.5754/hge11452. [DOI] [PubMed] [Google Scholar]


