Abstract
Background
Elderly patients undergoing open pancreatoduodenectomy (OPD) are at increased risk for surgical morbidity and mortality. Whether totally laparoscopic pancreatoduodenectomy (TLPD) mitigates these risks has not been evaluated.
Methods
A retrospective review of outcomes in patients submitted to pancreatoduodenectomy during 2007–2014 was conducted (n = 860). Outcomes in elderly patients (aged ≥70 years) were compared with those in non-elderly patients with respect to risk-adjusted postoperative morbidity and mortality. Differences in outcomes between patients submitted to OPD and TLPD, respectively, were evaluated in the elderly subgroup.
Results
In elderly patients, the incidences of cardiac events (odds ratio [OR] 3.21, P < 0.001), respiratory events (OR 1.68, P = 0.04), delayed gastric emptying (DGE) (OR 1.73, P = 0.003), increased length of stay (LoS, 1 additional day) (P < 0.001), discharge disposition other than home (OR 8.14, P < 0.001) and blood transfusion (OR 1.48, P = 0.05) were greater than in non-elderly patients. Morbidity and mortality did not differ between the OPD and TLPD subgroups of elderly patients. In elderly patients, OPD was associated with increased DGE (OR 1.80, P = 0.03), LoS (1 additional day; P < 0.001) and blood transfusion (OR 2.89, P < 0.001) compared with TLPD.
Conclusions
Elderly patients undergoing TLPD experience rates of mortality, morbidity and cardiorespiratory events similar to those in patients submitted to OPD. In elderly patients, TLPD offers benefits by decreasing DGE, LoS and blood transfusion requirements.
Introduction
Pancreatoduodenectomy (PD) has been performed for almost a century and minimally invasive approaches to this procedure have been introduced since the mid-1990s.1–4 Although improvements in overall morbidity and mortality have been observed over time, elderly patients represent one patient group that remains vulnerable to the risks associated with this procedure.5,6 A prior review at the present authors' institution suggested PD could be safely performed in well-selected patients of >70 years of age with acceptable perioperative outcomes.7 Subsequent studies have demonstrated increased postoperative mortality, intensive care unit (ICU) admission and cardiorespiratory morbidity in elderly compared with non-elderly patients.8,9 Despite these findings, PD may be safely performed in octogenarians with malignant diagnoses and offers a survival benefit to these patients.10
Studies evaluating strategies to improve perioperative outcomes in elderly patients undergoing PD are lacking. Minimally invasive approaches to pancreatectomy have demonstrated decreases in postoperative pain, hospital length of stay (LoS) and surgical morbidity.11,12 Totally laparoscopic PD (TLPD) has been shown to be safe and effective in several studies13–15 and is associated with decreases in hospital LoS and operative blood loss compared with open PD (OPD).13 Major venous resection during TLPD has also been reported to have short-term outcomes comparable with those of OPD.16 Importantly, retrospective comparative studies now demonstrate that oncologic outcomes of TLPD are comparable with those of OPD.13,17
Robotic PD has demonstrated perioperative outcomes in elderly patients of >70 years of age equivalent to those in the non-elderly population.18 However, the effects of TLPD on perioperative morbidity and mortality, specifically in elderly patients, in comparison with OPD have not been evaluated. Thus, the objectives of the present study were: (i) to compare postoperative outcomes in elderly (≥70 years) and non-elderly patients undergoing PD, and (ii) to determine if TLPD will mitigate increased postoperative morbidity in elderly patients compared with OPD. The study hypothesis assumed that TLPD might mitigate risks associated with OPD in elderly patients.
Materials and methods
Data sources
A single-institution, retrospective review of a prospectively maintained pancreatic surgery database was conducted. Details regarding patient demographics, comorbidities, clinical presentation, operative details, pathology reports, biochemical data, postoperative outcomes and surgical follow-up were extracted by chart review of medical records. The study protocol was approved by the Institutional Review Board of the Mayo Clinic (Rochester, MN, USA).
Study subjects
Consecutive patients submitted to PD between June 2007 (when TLPD was introduced at this institution) and June 2014 were evaluated. The study institution states a preference for pylorus preservation, two-layer duct-to-mucosa pancreaticojejunostomy, single-layer end-to-side hepaticojejunostomy, and double-layer duodenojejunostomy in both TLPD and OPD. The techniques of TLPD with and without vein resection have been previously described.14,19 Decisions on the placement of operative drains and feeding jejunostomy tubes are made at the time of operation and are based on the surgeon's judgement and preferences. The study protocol permitted the inclusion of patients undergoing elective PD for any indication with or without concomitant procedures such as diagnostic laparoscopy, biopsy, lysis of adhesions, resection of an adjacent organ and/or resection of other intra-abdominal organ(s). Exclusion criteria denied the inclusion of patients in whom PD was performed emergently and patients without explicit institutional research authorization. Elderly patients were defined as patients aged ≥70 years. This age cut-off was consistent with a prior review of outcomes conducted at the study institution7 and other comparative studies evaluating outcomes in elderly patients undergoing minimally invasive PD,18 and provided a distribution of patients that allowed for meaningful statistical analyses. In the first analysis, the entire cohort was evaluated and perioperative outcomes were compared among elderly and non-elderly patients. In the second analysis, outcomes of OPD were compared with those of TLPD in the elderly patient subgroup. Evaluation was based on the two study objectives.
Outcomes
Outcomes of interest were postoperative mortality, postoperative morbidity and hospital resource utilization. Mortality was defined as any in-hospital death or death within 30 days of the time of surgery. Surgical morbidity was captured for 30 days from the date of operation based upon in-hospital occurrences, readmissions and routine follow-up information. Cardiac events were captured and defined as any myocardial infarction, cardiac arrest, unstable arrhythmias requiring intervention and transfer to a monitored unit, or congestive heart failure. Respiratory events were defined as any respiratory failure, prolonged ventilator support for >48 h or pneumonia. Surgical site infections were defined as any superficial, deep or organ space infection with or without associated wound and/or fascial dehiscence. Complications were graded on severity according to the Clavien–Dindo classification. Major morbidity was defined as a complication of Clavien–Dindo Grade IIIb or higher.20 Pancreas-specific outcomes such as postoperative pancreatic fistula (POPF), post-pancreatectomy haemorrhage (PPH) and delayed gastric emptying (DGE) were classified based on International Study Group of Pancreatic Surgery definitions.21–23
Outcomes related to hospital resource utilization were defined by admission to ICUs, reoperations, readmissions, hospital LoS, receipt of blood transfusion(s), and discharge disposition other than home, such as another hospital, rehabilitation centre or skilled nursing facility. Reoperations and readmissions were captured for up to 30 days. Reoperations and readmissions outside the study institution were confirmed through outside institutional charts that were also reviewed to capture additional morbidity related to these occurrences. Any unplanned admissions to the ICU on either the index hospital admission or readmission were also captured. Postoperative blood transfusion was defined as any receipt of packed red blood cells (PRBC) during the course of hospitalization. Length of stay was the duration of hospitalization from the date of surgery until the time of index discharge.
Statistical analysis
Univariate tests of association were conducted to identify statistically significant differences between elderly and non-elderly patients in the cohort analysis, as well as between patients undergoing OPD and TLPD, respectively, in the elderly patient subgroup. Data were analysed using t-tests for continuous variables and chi-squared or Fisher's exact tests for categorical variables. Statistical significance was defined at the 0.05 level. Intention-to-treat and as-treated analyses for univariate outcomes were conducted for the subgroup analysis comparing OPD with TLPD. No differences in the results were noted and hence the as-treated analysis is presented. Based upon these univariate analyses, multivariable logistic regression models were constructed to risk-adjust outcomes and evaluate for goodness of fit. All analyses were performed using sas Version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
Descriptive statistics
The entire cohort consisted of 860 patients who met the study inclusion and exclusion criteria for evaluation. These included 522 (60.7%) non-elderly patients and 338 (39.3%) elderly patients. Among the elderly subgroup, 113 (33.4%) patients underwent TLPD and 225 (66.6%) underwent OPD. The age range of the entire cohort was 26.7–91.2 years. The mean ± standard deviation postoperative follow-up time to data acquisition in the entire cohort was 48.6 ± 43.8 days from the day of surgery; the median length of follow-up was 39 days. Basic demographics for the entire cohort and the elderly subgroup are summarized in Tables1 and 2, respectively.
Table 1.
Baseline characteristics in the entire patient cohort
| Variable | Entire cohort (n = 860) | Age <70 years (non-elderly) (n = 522) | Age ≥70 years (elderly) (n = 338) | P-value |
|---|---|---|---|---|
| Basic demographics | ||||
| Age, years, mean ± SD | 65.2 ± 11.7 | 57.9 ± 8.8 | 76.4 ± 4.4 | <0.001 |
| <70 years, n (%) | 522 (60.7%) | |||
| ≥70 years, n (%) | 338 (39.3%) | |||
| Gender, n (%) | 0.656 | |||
| Male | 494 (57.4%) | 303 (58.0%) | 191 (56.5%) | |
| Female | 366 (42.6%) | 219 (42.0%) | 147 (43.5%) | |
| BMI, kg/m2, mean ± SD | 27.4 ± 5.3 | 27.8 ± 5.8 | 26.8 ± 4.4 | 0.006 |
| Comorbidities, n (%) | ||||
| Obesity (BMI ≥30 kg/m2) | 235 (27.3%) | 162 (31.0%) | 73 (21.6%) | 0.002 |
| Any alcohol use | 453 (59.5%) | 282 (63.4%) | 171 (54.1%) | 0.010 |
| Current smoker | 139 (16.2%) | 110 (21.1%) | 29 (8.6%) | <0.001 |
| Steroid use within 6 monthsa | 38 (4.4%) | 21 (4.0%) | 17 (5.0%) | 0.483 |
| CADa | 159 (18.5%) | 64 (12.3%) | 95 (28.1%) | <0.001 |
| COPDa | 65 (7.6%) | 30 (5.8%) | 35 (10.4%) | 0.012 |
| Hypertensiona | 461 (53.6%) | 229 (43.9%) | 232 (68.6%) | <0.001 |
| Diabetesa | 234 (27.2%) | 132 (25.3%) | 102 (30.2%) | 0.116 |
| Renal diseasea | 78 (9.1%) | 41 (7.9%) | 37 (11.0%) | 0.123 |
| Liver diseasea | 56 (6.5%) | 41 (7.9%) | 15 (4.4%) | 0.047 |
| History of VTEa | 51 (5.9%) | 32 (6.1%) | 19 (5.6%) | 0.758 |
| Vascular diseasea | 109 (12.7%) | 43 (8.2%) | 66 (19.5%) | <0.001 |
| Clinical symptoms, n (%) | ||||
| History of cholangitis | 40 (4.7%) | 24 (4.6%) | 16 (4.7%) | 0.926 |
| History of jaundice | 413 (48.0%) | 246 (47.1%) | 167 (49.4%) | 0.513 |
| History of pancreatitis | ||||
| Any | 140 (16.3%) | 104 (19.9%) | 36 (10.7%) | 0.003 |
| Acute | 101 (11.7%) | 73 (14.0%) | 28 (8.3%) | 0.001 |
| Chronic | 39 (4.5%) | 31 (5.9%) | 8 (2.4%) | |
| Anaemiab | ||||
| Haemoglobin, g/dl, mean ± SD | 12.9 ± 1.7 | 13.0 ± 1.7 | 12.6 ± 1.6 | 0.001 |
| Haemoglobin ≤10.5 g/dl, n (%) | 154 (17.9%) | 83 (15.9%) | 71 (21.0%) | 0.057 |
| Operative characteristics | ||||
| ASA class, n (%) | ||||
| Class III/IV versus Class I/II | 516 (60.6%) | 275 (53.2%) | 241 (71.9%) | <0.001 |
| Laparoscopic Whipple, n (%) | 281 (32.7%) | 168 (32.2%) | 113 (33.4%) | 0.703 |
| Converted, n (%) | 20 (2.3%) | 15 (2.9%) | 5 (1.5%) | 0.185 |
| PD versus PPPD, n (%) | 103 (12.0%) | 60 (11.5%) | 43 (12.7%) | 0.588 |
| Vein resection, n (%) | 132 (15.4%) | 75 (14.4%) | 57 (16.9%) | 0.321 |
| Operative drain, n (%) | 692 (80.5%) | 420 (80.5%) | 272 (80.5%) | 0.996 |
| Total operation time, min, mean ± SD | 371.9 ± 96.5 | 378.7 ± 95.6 | 361.4 ± 97.2 | 0.010 |
| EBL, ml, mean ± SD | 713.7 ± 820.1 | 728.9 ± 711.3 | 690.3 ± 965.0 | 0.501 |
| Diagnostic characteristics | ||||
| Malignant diagnosis, n (%) | 648 (75.5%) | 381 (73.3%) | 267 (79.0%) | 0.057 |
| Diagnosisc, n (%) | ||||
| Pancreatic cancer | 416 (48.4%) | 242 (46.4%) | 174 (51.5%) | <0.001 |
| Cholangiocarcinoma | 35 (4.1%) | 16 (3.1%) | 19 (5.6%) | |
| Duodenal cancer | 28 (3.3%) | 15 (2.9%) | 13 (3.9%) | |
| Ampullary cancer | 97 (11.3%) | 55 (10.5%) | 42 (12.4%) | |
| Renal cell carcinoma | 9 (1.1%) | 3 (0.6%) | 6 (1.8%) | |
| Neuroendocrine tumour | 69 (8.0%) | 55 (10.4%) | 14 (4.1%) | |
| Pancreatitis | 34 (4.0%) | 28 (5.4%) | 6 (1.8%) | |
| Cystic neoplasm | 13 (1.5%) | 11 (2.1%) | 2 (0.6%) | |
| IPMN | 97 (11.3%) | 46 (8.8%) | 51 (15.1%) | |
| Other | 62 (7.2%) | 51 (9.8%) | 11 (3.3%) | |
| Neoadjuvant therapyd, n (%) | 71 (8.3%) | 55 (10.5%) | 16 (4.7%) | 0.003 |
Percentage values for categorical variables reflect proportions according to the total available data for that variable.
Any steroid use: systemic steroid administration within 6 months of operation. CAD: history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass graft. COPD: documented medical history supported by radiological and/or functional evaluation. Hypertension: elevated blood pressure requiring medications for adequate control. Diabetes: impaired glycaemic regulation requiring insulin, oral hypoglycaemic agents, and/or dietary modification. Renal disease: impaired renal function based on biochemical evaluation and/or solitary kidney. Liver disease: history of viral hepatitis, steatohepatitis, or cirrhosis. History of VTE: any prior history of deep vein thrombosis and/or pulmonary embolus. Vascular disease: peripheral arterial disease and/or arterial aneurysm with or without repair.
Clinical symptoms documented were based upon initial presentation. Anaemia was defined as a haemoglobin level ≤10.5 g/dl, which represented the 25th percentile of the cohort, for the purposes of risk-adjusted multivariate analyses.
Diagnosis: cystic neoplasm does not include IPMN. Other: sarcoma, lymphoma, gastrointestinal stromal tumour, adenoma, benign stricture, solid pseudopapillary epithelial neoplasm, etc.
Neoadjuvant therapy: any preoperative chemotherapy, radiation therapy or both.
ASA, American Society of Anesthesiologists; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EBL, estimated blood loss; IPMN, intraductal papillary mucinous neoplasm; PD, pancreatoduodenectomy without pylorus preservation; PPPD, pylorus-preserving pancreatoduodenectomy; SD, standard deviation; VTE, venous thromboembolism.
Table 2.
Baseline characteristics in the elderly patient subgroup
| Variable | Elderly only (n = 338) | Laparoscopic PD (n = 113) | Open PD (n = 225) | P-value |
|---|---|---|---|---|
| Basic demographics | ||||
| Age, years, mean ± SD | 76.4 ± 4.4 | 76.5 ± 4.3 | 76.4 ± 4.5 | 0.816 |
| Gender, n (%) | 0.003 | |||
| Male | 191 (56.5%) | 51 (45.1%) | 140 (62.2%) | |
| Female | 147 (43.5%) | 62 (54.9%) | 85 (37.8%) | |
| BMI, kg/m2, mean ± SD | 26.8 ± 4.4 | 26.9 ± 4.7 | 26.8 ± 4.3 | 0.919 |
| Comorbidities, n (%) | ||||
| Obesity (BMI ≥30 kg/m2) | 73 (21.6%) | 28 (24.8%) | 45 (20.0%) | 0.314 |
| Any alcohol use | 171 (54.1%) | 47 (42.3%) | 124 (60.5%) | 0.002 |
| Current smoker | 29 (8.6%) | 9 (8.0%) | 20 (8.9%) | 0.775 |
| Steroid use within 6 monthsa | 17 (5.0%) | 9 (8.0%) | 8 (3.6%) | 0.080 |
| CADa | 95 (28.1%) | 34 (30.1%) | 61 (27.1%) | 0.566 |
| COPDa | 35 (10.4%) | 15 (13.3%) | 20 (8.9%) | 0.212 |
| Hypertensiona | 232 (68.6%) | 78 (69.0%) | 154 (68.4%) | 0.913 |
| Diabetesa | 102 (30.2%) | 29 (25.7%) | 73 (32.4%) | 0.200 |
| Renal diseasea | 37 (11.0%) | 12 (10.6%) | 25 (11.1%) | 0.891 |
| Liver diseasea | 15 (4.4%) | 6 (5.3%) | 9 (4.0%) | 0.581 |
| History of VTEa | 19 (5.6%) | 6 (5.3%) | 13 (5.8%) | 0.860 |
| Vascular diseasea | 66 (19.5%) | 26 (23.0%) | 40 (17.8%) | 0.2524 |
| Clinical symptoms, n (%) | ||||
| History of cholangitis | 16 (4.7%) | 6 (5.3%) | 10 (4.4%) | 0.724 |
| History of jaundice | 167 (49.4%) | 47 (41.6%) | 120 (53.3%) | 0.042 |
| History of pancreatitis | ||||
| Any | 36 (10.7%) | 15 (13.3%) | 21 (9.3%) | 0.268 |
| Acute | 28 (8.3%) | 15 (13.3%) | 13 (5.8%) | 0.010 |
| Chronic | 8 (2.4%) | 0 (0%) | 8 (3.6%) | |
| Anaemiab | ||||
| Haemoglobin, g/dl, mean ± SD | 12.6 ± 1.6 | 12.8 ± 1.5 | 12.5 ± 1.6 | 0.099 |
| Haemoglobin ≤10.5 g/dl, n (%) | 71 (21.0%) | 21 (18.6%) | 50 (22.2%) | 0.439 |
| Operative characteristics | ||||
| ASA class | ||||
| Class III/IV versus class I/II, n (%) | 241 (71.9%) | 83 (74.1%) | 158 (70.9%) | 0.532 |
| PD versus PPPD, n (%) | 43 (12.7%) | 13 (11.5%) | 30 (13.3%) | 0.634 |
| Vein resection, n (%) | 57 (16.9%) | 18 (15.9%) | 39 (17.3%) | 0.745 |
| Operative drain, n (%) | 272 (80.5%) | 64 (56.6%) | 208 (92.4%) | <0.001 |
| Total operation time, min, mean ± SD | 361.4 ± 97.2 | 364.5 ± 110.6 | 359.8 ± 90.0 | 0.681 |
| EBL, ml, mean ± SD | 690.3 ± 965.0 | 344.7 ± 346.5 | 868.8 ± 1118.2 | <0.001 |
| Diagnostic characteristics | ||||
| Malignant diagnosis, n (%) | 267 (79.0%) | 75 (66.4%) | 192 (85.3%) | <0.001 |
| Diagnosisc, n (%) | <0.001 | |||
| Pancreatic cancer | 174 (51.5%) | 53 (46.9%) | 121 (53.8%) | |
| Cholangiocarcinoma | 19 (5.6%) | 4 (3.5%) | 15 (6.7%) | |
| Duodenal cancer | 13 (3.9%) | 2 (1.8%) | 11 (4.9%) | |
| Ampullary cancer | 42 (12.4%) | 9 (8.0%) | 33 (14.7%) | |
| Renal cell carcinoma | 6 (1.8%) | 0 | 6 (2.7%) | |
| Neuroendocrine tumour | 14 (4.1%) | 6 (5.3%) | 8 (3.6%) | |
| Pancreatitis | 6 (1.8%) | 3 (2.7%) | 3 (1.3%) | |
| Cystic neoplasm | 2 (0.6%) | 0 (0%) | 2 (0.9%) | |
| IPMN | 51 (15.1%) | 29 (25.7%) | 22 (9.8%) | |
| Other | 11 (3.3%) | 7 (6.2%) | 4 (1.8%) | |
| Neoadjuvant therapyd, n (%) | 16 (4.7%) | 5 (4.4%) | 11 (4.9%) | 0.850 |
Percentage values for categorical variables reflect proportions according to the total available data for that variable.
Any steroid use: systemic steroid administration within 6 months of operation. CAD: history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass graft. COPD: documented medical history supported by radiological and/or functional evaluation. Hypertension: elevated blood pressure requiring medications for adequate control. Diabetes: impaired glycaemic regulation requiring insulin, oral hypoglycaemic agents, and/or dietary modification. Renal disease: impaired renal function based on biochemical evaluation and/or solitary kidney. Liver disease: history of viral hepatitis, steatohepatitis, or cirrhosis. History of VTE: any prior history of deep vein thrombosis and/or pulmonary embolus. Vascular disease: peripheral arterial disease and/or arterial aneurysm with or without repair.
Clinical symptoms documented were based upon initial presentation. Anaemia was defined as a haemoglobin level ≤10.5 g/dl, which represented the 25th percentile of the cohort, for the purposes of risk-adjusted multivariate analyses.
Other: sarcoma, lymphoma, gastrointestinal stromal tumour, adenoma, benign stricture, solid pseudopapillary epithelial neoplasm, etc.
Neoadjuvant therapy: any preoperative chemotherapy, radiation therapy or both.
ASA, American Society of Anesthesiologists; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EBL, estimated blood loss; IPMN, intraductal papillary mucinous neoplasm; PD, pancreatoduodenectomy without pylorus preservation; PPPD, pylorus-preserving pancreatoduodenectomy; SD, standard deviation; VTE, venous thromboembolism.
A comparison of baseline characteristics in the elderly and non-elderly patient subgroups showed differences in age (by definition) and body mass index (BMI). Elderly patients had a lower BMI than non-elderly patients. Differences in comorbidities were noted: elderly patients showed decreased rates of obesity, alcohol use and tobacco use, and increased rates of coronary artery disease, chronic obstructive pulmonary disease, hypertension, liver disease and vascular disease. Elderly patients also had higher American Society of Anesthesiologists (ASA) class scores, shorter operative times, and a decreased likelihood of receiving neoadjuvant therapy. Clinical presentations also differed. Elderly patients were more likely to have a history of pancreatitis or preoperative anaemia. A cut-off haemoglobin level of 10.5 g/dl represented the 25th percentile for this cohort and was used as an indicator variable for preoperative anaemia for the purposes of multivariate analysis.
Evaluations of the subgroups of elderly patients submitted to OPD and TLPD, respectively, showed minimal differences in baseline comorbidities. There were differences in gender in that female patients represented a greater proportion of the TLPD subgroup but not the OPD subgroup. There were also differences in current alcohol use, which was lower in the TLPD group than in the OPD group. In the multivariate analysis, attempts to risk-adjust for alcohol use were compromised by the number of unavailable data for this variable. Notably, there were differences in indications for PD, whereby malignant diagnoses were more common in the OPD group. Differences in indications for PD were also reflected in differences in clinical presentation in that the likelihood of a history of pancreatitis was greater in TLPD patients and the likelihood of a history of jaundice was lower in TLPD patients than in OPD patients. Fewer operative drains were placed in patients in the TLPD subgroup compared with those in the OPD subgroup, based on surgeon practice preference. Estimated blood loss was also lower in the TLPD subgroup than in the OPD subgroup. There were no differences in venous resection, pylorus preservation or operative time between the TLPD and OPD subgroups.
Entire cohort: elderly versus non-elderly patients
Univariate and multivariate analyses for the entire cohort comparing outcomes in elderly and non-elderly patients, respectively, are summarized in Table3. There were no differences between elderly and non-elderly patients in terms of postoperative 30-day or in-hospital mortality, unplanned ICU admission, reoperation requiring a general anaesthetic, readmission, surgical site infection (superficial or deep with or without wound or fascial dehiscence), POPF (Grades B and C) or PPH (Grades B and C). On univariate analysis, elderly patients had a 59% increased odds of major morbidity (Clavien–Dindo Grade IIIb complication and higher; P = 0.036), 84% increased odds of DGE (Grades B and C; P < 0.001), 71% increased odds of a respiratory complication (failure, prolonged ventilator support or pneumonia; P = 0.014), 55% increased odds of blood transfusion (receipt of any PRBC during the course of hospitalization; P = 0.003), more than three-fold increased odds of cardiac complications (myocardial infarction, arrest, failure or unstable arrhythmia; P < 0.001), and almost nine-fold increased odds of a discharge disposition other than home (skilled nursing facility, other hospital or rehabilitation centre; P < 0.001) than non-elderly patients. The association between elderly age and these outcomes was confirmed on multivariate analyses controlling for gender, obesity, current smoker status, coronary artery disease, chronic obstructive pulmonary disease, hypertension, liver disease, vascular disease, history of pancreatitis, operative time, preoperative anaemia, ASA class and neoadjuvant therapy. After risk adjustment, elderly age was no longer predictive of postoperative major morbidity (P = 0.677). With respect to hospital resource utilization, the median number of units of PRBC transfused was greater in elderly than in non-elderly patients (1 unit versus 0 units; P < 0.001). Median LoS was also greater in elderly than in non-elderly patients (9 days versus 8 days; P < 0.001).
Table 3.
Outcomes for the entire cohort (elderly versus non-elderly patients)
| Outcome | Non-elderly (n = 522) n (%) | Elderly (n = 338) n (%) | UnivariateaOR | Univariate P-value | MultivariatebOR (95% CI) | Multivariate P-value |
|---|---|---|---|---|---|---|
| Mortalityc | 7 (1.3%) | 8 (2.4%) | 1.78 | 0.252 | 1.49 (0.44–5.08) | 0.523 |
| Major morbidityd | 46 (8.8%) | 45 (13.3%) | 1.59 | 0.036 | 1.53 (0.93–2.53) | 0.677 |
| ICU admissione | 62 (11.9%) | 55 (16.3%) | 1.44 | 0.066 | 1.40 (0.89–2.22) | 0.145 |
| Reoperationf | 24 (4.6%) | 18 (5.3%) | 1.17 | 0.629 | 0.98 (0.49–1.95) | 0.943 |
| Readmissiong | 87 (16.7%) | 56 (16.7%) | 1.00 | 0.985 | 1.44 (0.94–2.19) | 0.092 |
| POPFh(Grade B/C) | 119 (22.8%) | 83 (24.6%) | 1.10 | 0.552 | 1.05 (0.79–1.52) | 0.783 |
| PPHh(Grade B/C) | 46 (8.8%) | 28 (8.3%) | 0.94 | 0.787 | 0.881 (0.50–1.54) | 0.657 |
| DGEh(Grade B / C) | 104 (19.9%) | 106 (31.4%) | 1.84 | <0.001 | 1.73 (1.21–2.47) | 0.003 |
| Cardiac eventi | 41 (7.8%) | 81 (24.0%) | 3.70 | <0.001 | 3.21 (2.03–5.06) | <0.001 |
| Respiratory eventj | 45 (8.6%) | 47 (13.9%) | 1.71 | 0.014 | 1.68 (1.02–2.78) | 0.041 |
| SSIk | 134 (25.7%) | 100 (29.6%) | 1.22 | 0.208 | 1.29 (0.91–1.82) | 0.154 |
| Transfusionl | 161 (31.2%) | 136 (41.3%) | 1.55 | 0.003 | 1.41 (1.00–1.98) | 0.048 |
| Discharge dispositionm | 8 (1.6%) | 41 (12.4%) | 9.00 | <0.001 | 8.14 (3.41–19.5) | <0.001 |
Univariate OR: calculated based on frequency tables with P-values obtained from chi-squared tests or Fisher's exact test if cell counts were ≤5 for any given event.
Multivariate logistic regression computed ORs that controlled for the following variables: elderly, sex, obesity, current smoker status, coronary artery disease, chronic obstructive pulmonary disease, hypertension, liver disease, vascular disease, history of pancreatitis, operative time, preoperative anaemia, ASA class, and neoadjuvant therapy.
Mortality: in-hospital or 30-day death from date of surgery.
Major morbidity: Grade IIIb or higher complication based on Clavien–Dindo classification of surgical complications.
Unplanned/unanticipated ICU admission, excluding monitored stepdown units.
Reoperation: any major reoperation within 30 days of surgery or during course of hospitalization requiring a general anaesthetic.
Readmission: any hospital readmission within 30 days of date of surgery.
Classifications of POPF, PPH and DGE are based upon International Study of Pancreatic Surgery Group definitions.
Cardiac event: any myocardial infarction, cardiac arrest, unstable arrhythmias requiring intervention and transfer to monitored unit, or congestive heart failure.
Respiratory event: any respiratory failure necessitating positive pressure ventilation, prolonged ventilatory support >48 h, or pneumonia.
SSI: any superficial, deep/organ space infection or wound/fascial dehiscence.
Transfusion: receipt of any packed red blood cells during the course of hospitalization.
Discharge disposition: discharge to skilled nursing facility, rehabilitation centre or other hospital compared with home, with or without home health services. Odds ratios calculated excluded expired patients.
95% CI, 95% confidence interval; ASA, American Society of Anesthesiologists; DGE, delayed gastric emptying; ICU, intensive care unit; OR, odds ratio; POPF, postoperative pancreatic fistula; PPH, post-pancreatectomy haemorrhage; SSI, surgical site infection.
Elderly subgroup: OPD versus TLPD
Univariate and multivariate analyses for the elderly subgroup comparing outcomes between OPD and TLPD are summarized in Table4. There were no differences between OPD and TLPD in terms of postoperative 30-day or in-hospital mortality, major morbidity, unplanned ICU admission, reoperation requiring a general anaesthetic, readmission, surgical site infection, POPF (Grades B and C) or PPH (Grades B and C). On univariate analysis, OPD was associated with 72% increased odds for DGE (Grades B and C) and more than three-fold increased odds for any blood transfusion compared with TLPD. The association between OPD and these outcomes was confirmed on multivariate analyses controlling for gender, history of pancreatitis, history of jaundice, malignant diagnosis and preoperative anaemia. Notably, there were no differences in the odds for cardiorespiratory complications or discharge disposition other than home between OPD and TLPD. With respect to hospital resource utilization, the median number of PRBC units transfused was greater in the OPD subgroup than in the TLPD subgroup (2 units versus 0 units; P < 0.001) (Fig.1a). Median LoS was also greater in the OPD subgroup than in the TLPD subgroup (9 days versus 8 days; P < 0.001) (Fig.1b).
Table 4.
Outcomes for the elderly subgroup (open versus laparoscopic pancreatoduodenectomy)
| Outcome | Laparoscopy (n = 113) n (%) | Open (n = 225) n (%) | UnivariateaOR | Univariate P-value | MultivariatebOR (95% CI) | Multivariate P-value |
|---|---|---|---|---|---|---|
| Mortalityc | 5 (4.4%) | 3 (1.3%) | 0.29 | 0.123 | 0.35 (0.07–1.67) | 0.186 |
| Major morbidityd | 11 (9.7%) | 34 (15.1%) | 1.65 | 0.170 | 1.83 (0.86–3.90) | 0.116 |
| ICU admissione | 16 (14.2%) | 39 (17.3%) | 1.27 | 0.456 | 1.28 (0.66–2.47) | 0.462 |
| Reoperationf | 3 (2.7%) | 15 (6.7%) | 2.62 | 0.197 | 3.25 (0.87–12.1) | 0.079 |
| Readmissiong | 19 (17.1%) | 37 (16.5%) | 0.96 | 0.890 | 1.03 (0.53–2.00) | 0.927 |
| POPFh(Grade B/C) | 26 (23.0%) | 57 (25.3%) | 1.14 | 0.640 | 1.02 (0.59–1.79) | 0.934 |
| PPHh(Grade B/C) | 9 (8.0%) | 19 (8.4%) | 1.07 | 0.880 | 1.11 (0.47–2.63) | 0.809 |
| DGEh(Grade B/C) | 27 (23.9%) | 79 (35.1%) | 1.72 | 0.036 | 1.80 (1.05–3.07) | 0.032 |
| Cardiac eventi | 25 (22.1%) | 56 (24.9%) | 1.17 | 0.574 | 1.11 (0.63–1.95) | 0.712 |
| Respiratory eventj | 14 (12.4%) | 33 (14.7%) | 1.22 | 0.568 | 1.30 (0.65–2.63) | 0.458 |
| SSIk | 32 (28.3%) | 68 (30.2%) | 1.10 | 0.718 | 0.92 (0.54–1.57) | 0.771 |
| Transfusionl | 26 (23.4%) | 110 (50.5%) | 3.33 | <0.001 | 2.89 (1.70–4.91) | <0.001 |
| Discharge dispositionm | 10 (9.3%) | 31 (14.0%) | 1.59 | 0.224 | 1.72 (0.80–3.81) | 0.161 |
Univariate OR: calculated based on frequency tables with P-values obtained from chi-squared tests or Fisher's exact test if cell counts were ≤5 for any given event.
Multivariate logistic regression computed ORs that controlled for the following variables: elderly, sex, obesity, current smoker status, coronary artery disease, chronic obstructive pulmonary disease, hypertension, liver disease, vascular disease, history of pancreatitis, operative time, preoperative anaemia, ASA class and neoadjuvant therapy.
Mortality: in-hospital or 30-day death from date of surgery.
Major morbidity: Grade IIIb or higher complication based on Clavien–Dindo classification of surgical complications.
Unplanned/unanticipated ICU admission, excluding monitored stepdown units.
Reoperation: any major reoperation within 30 days of surgery or during course of hospitalization requiring a general anaesthetic.
Readmission: any hospital readmission within 30 days of date of surgery.
Classifications of POPF, PPH and DGE are based upon International Study of Pancreatic Surgery Group definitions.
Cardiac event: any myocardial infarction, cardiac arrest, unstable arrhythmias requiring intervention and transfer to monitored unit, or congestive heart failure.
Respiratory event: any respiratory failure necessitating positive pressure ventilation, prolonged ventilatory support >48 h, or pneumonia.
SSI: any superficial, deep/organ space infection or wound/fascial dehiscence.
Transfusion: receipt of any packed red blood cell during the course of hospitalization.
Discharge disposition: discharge to skilled nursing facility, rehabilitation centre, or other hospital compared to home, with or without home health services. Odds ratios calculated excluded expired patients.
95% CI, 95% confidence interval; ASA, American Society of Anesthesiologists; DGE, delayed gastric emptying; ICU, intensive care unit; OR, odds ratio; POPF, postoperative pancreatic fistula; PPH, post-pancreatectomy haemorrhage; SSI, surgical site infection.
Figure 1.
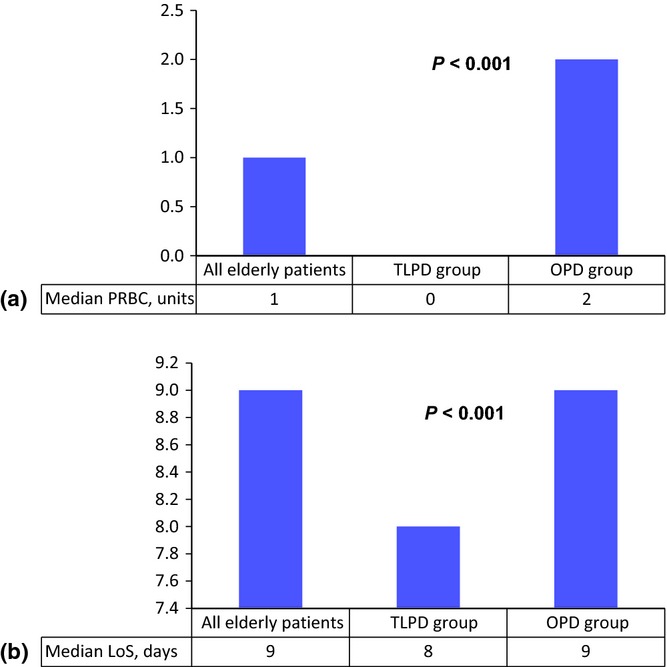
Hospital resource utilization is represented by the median number of units of packed red blood cells (PRBC) transfused during the course of hospitalization and hospital length of stay (LoS). (a) The median number of units of PRBC transfused was 2 in the open pancreatoduodenectomy (OPD) subgroup compared with 0 in the totally laparoscopic pancreatoduodenectomy (TLPD) subgroup (P < 0.001). (b) The median LoS was 9 days in the OPD subgroup and 8 days in the TLPD subgroup (P < 0.001). Median values are represented as data were skewed and not normally distributed. Non-parametric tests of association (Kruskal–Wallis test) were performed to determine P-values in comparisons between the experiment and control groups
Discussion
Key findings in this study show, firstly, that elderly patients are at increased risk for cardiac complications (adjusted OR 3.21, P < 0.001), respiratory complications (adjusted OR 1.68, P = 0.041), DGE (adjusted OR 1.73, P = 0.003), receipt of blood transfusion (adjusted OR 1.41, P = 0.048), discharge disposition other than home (adjusted OR 8.14, P < 0.001), and increased hospital LoS (9 days compared with 8 days; P < 0.001). Secondly, the present findings show that TLPD does not mitigate the increased risk for cardiorespiratory complications. The advantages of TLPD over OPD in elderly patients include a decreased likelihood of blood transfusion (adjusted OR 2.89, P < 0.001, OPD versus TLPD), DGE (adjusted OR 1.80, P = 0.032, OPD versus TLPD), and shorter LoS (9 days versus 8 days; P < 0.001, OPD versus TLPD).
These results are supported by those of other studies in relation to postoperative morbidity but are discordant in relation to postoperative mortality.8,9,18 A recent meta-analysis pooled seven studies comprising over 5000 patients and demonstrated that elderly patients (defined as those aged 76–80 years) had increased postoperative mortality compared with non-elderly patients undergoing PD.9 When elderly patients were defined as those aged >80 years, they were found to have increased risk for postoperative complications compared with non-elderly patients.9 When elderly patients were defined as those aged >75 years, they were found to have increased risk for pulmonary complications compared with non-elderly patients.9 In another study conducted in patients undergoing pancreatectomy, those aged >75 years had increased rates of mortality, ICU admission, major cardiac events, and discharge to skilled nursing facilities compared with patients aged 16–74 years.8
The present results confirm prior findings that elderly patients are more likely to have postoperative cardiorespiratory complications.8,9 However, they do not demonstrate an increased risk for postoperative mortality in elderly patients. This finding may reflect differences between this and other studies in definitions of ‘elderly patients’ or may indicate that the present study is underpowered to detect statistically significant differences for such a rare event, given that the overall mortality rate in the study cohort was 1.7%. The current study also did not demonstrate increased risk for ICU admission or major morbidity after risk adjustment. The present authors acknowledge that the inclusion criteria for ICU admission were stringent in that they focused on those patients with unplanned admissions for treatment or invasive monitoring. Admissions to the ICU that had been scheduled preoperatively for postoperative cardiorespiratory monitoring and equivalent monitoring outside the ICU were not categorized as ICU admissions. Major morbidity in this study was defined using the Clavien–Dindo system of classification rather than other modifications or unique classification systems.8,9,13,24
Only one prior study has evaluated minimally invasive approaches to PD in elderly patients.18 This single-institution, retrospective review of 41 consecutive patients evaluated only patients undergoing robotic PD. In a comparison of elderly patients (defined as those aged >70 years) and non-elderly patients, Buchs et al.18 identified no differences in operative time, blood loss, conversion rate, postoperative mortality or overall morbidity between the two groups. The authors concluded that robotic PD could be safely offered to elderly patients and that age should not be a contraindication to this procedure.18 The present study is distinct from that prior report18 in that it focused specifically on elderly patients in its comparison of operative approaches in order to determine if TLPD would offer advantages to this higher-risk group.
Several limitations of this study warrant discussion. The retrospective nature of the study makes it prone to selection bias. As the elderly subgroup analysis shows, the TLPD subgroup showed greater proportions of female patients, non-alcoholic patients and patients with non-malignant indications for resection than the OPD subgroup. Although there is no intended bias in the selection of patients for TLPD, these variables are known to affect outcomes and therefore risk adjustment was performed to control for these differences.25 The present authors speculate that these differences may be explained by referral patterns as the choice of operative approach is based on surgeon preference because only one surgeon at the study institution performs TLPD. Additional comparisons based on intention-to-treat analyses (in which patients in whom TLPD was converted to OPD were included in the TLPD group) found no differences in the results. Moreover, there were no significant differences in operative variables such as prevalence of venous resection, operative time and pylorus preservation between the TLPD and OPD subgroups.
In conclusion, the present study suggests that morbidity but not mortality is increased in elderly patients submitted to PD compared with non-elderly patients. The laparoscopic approach may improve selected perioperative outcomes in elderly patients, such as by decreasing requirements for blood transfusion and the incidence of DGE, and may reduce hospital LoS, but it does not lower the risk for cardiorespiratory complications. The indications for PD should not be expanded based on ability to perform the procedure with minimally invasive approaches in the elderly patient population.
Conflicts of interest
None declared.
References
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408–410. doi: 10.1007/BF00642443. [DOI] [PubMed] [Google Scholar]
- Gagner M, Pomp A. Laparoscopic pancreatic resection: is it worthwhile? J Gastrointest Surg. 1997;1:20–25. doi: 10.1007/s11605-006-0005-y. ; discussion 25–26. [DOI] [PubMed] [Google Scholar]
- Whipple AO. Pancreaticoduodenectomy for islet carcinoma: a five-year follow-up. Ann Surg. 1945;121:847–852. doi: 10.1097/00000658-194506000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg. 1935;102:763–779. doi: 10.1097/00000658-193510000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg. 1987;206:358–365. doi: 10.1097/00000658-198709000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. ; discussion 1210–1211. [DOI] [PubMed] [Google Scholar]
- Spencer MP, Sarr MG, Nagorney DM. Radical pancreatectomy for pancreatic cancer in the elderly. Is it safe and justified? Ann Surg. 1990;212:140–143. doi: 10.1097/00000658-199008000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner AM, Glasgow RE, Jordan TH, Krassner AD, Way LW, Mulvihill SJ, et al. Pancreatic resection in the elderly. J Am Coll Surg. 2004;198:697–706. doi: 10.1016/j.jamcollsurg.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Sukharamwala P, Thoens J, Szuchmacher M, Smith J, DeVito P. Advanced age is a risk factor for post-operative complications and mortality after a pancreaticoduodenectomy: a meta-analysis and systematic review. HPB. 2012;14:649–657. doi: 10.1111/j.1477-2574.2012.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzaras I, Schmidt C, Klemanski D, Muscarella P, Melvin WS, Ellison EC, et al. Pancreatic resection in the octogenarian: a safe option for pancreatic malignancy. J Am Coll Surg. 2011;212:373–377. doi: 10.1016/j.jamcollsurg.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakeeb A. Laparoscopic pancreatic resections. Adv Surg. 2009;43:91–102. doi: 10.1016/j.yasu.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Sharpe SM, Talamonti MS, Wang E, Bentrem DJ, Roggin KK, Prinz RA, et al. The laparoscopic approach to distal pancreatectomy for ductal adenocarcinoma results in shorter lengths of stay without compromising oncologic outcomes. Am J Surg. 2015;209:557–563. doi: 10.1016/j.amjsurg.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810–819. doi: 10.1016/j.jamcollsurg.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg. 2010;145:19–23. doi: 10.1001/archsurg.2009.243. [DOI] [PubMed] [Google Scholar]
- Palanivelu C, Jani K, Senthilnathan P, Parthasarathi R, Rajapandian S, Madhankumar MV. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg. 2007;205:222–230. doi: 10.1016/j.jamcollsurg.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, et al. Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg. 2015;19:189–194. doi: 10.1007/s11605-014-2644-8. ; discussion 194. [DOI] [PubMed] [Google Scholar]
- Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg. 2014;260:633–638. doi: 10.1097/SLA.0000000000000937. ; discussion 638–640. [DOI] [PubMed] [Google Scholar]
- Buchs NC, Addeo P, Bianco FM, Gangemi A, Ayloo SM, Giulianotti PC. Outcomes of robot-assisted pancreaticoduodenectomy in patients older than 70 years: a comparative study. World J Surg. 2010;34:2109–2114. doi: 10.1007/s00268-010-0650-x. [DOI] [PubMed] [Google Scholar]
- Kendrick ML, Sclabas GM. Major venous resection during total laparoscopic pancreaticoduodenectomy. HPB. 2011;13:454–458. doi: 10.1111/j.1477-2574.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Baker MS, Sherman KL, Stocker SJ, Hayman AV, Bentrem DJ, Prinz RA, et al. Using a modification of the Clavien–Dindo system accounting for readmissions and multiple interventions: defining quality for pancreaticoduodenectomy. J Surg Oncol. 2014;110:400–406. doi: 10.1002/jso.23663. [DOI] [PubMed] [Google Scholar]
- Shubert CR, Kendrick ML, Thomsen KM, Farnell MB, Habermann EB. Identification of risk categories for in pancreaticoduodenectomy based on diagnosis. HPB. 2015;17:428–437. doi: 10.1111/hpb.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]


