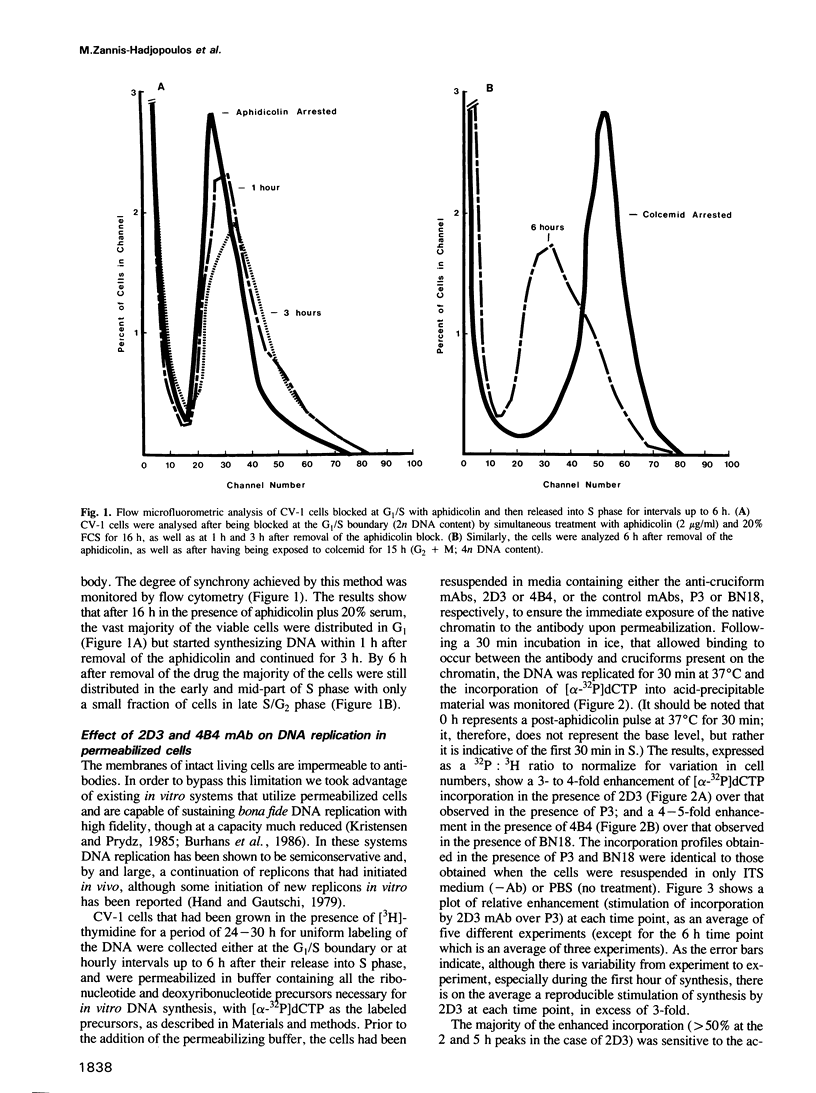
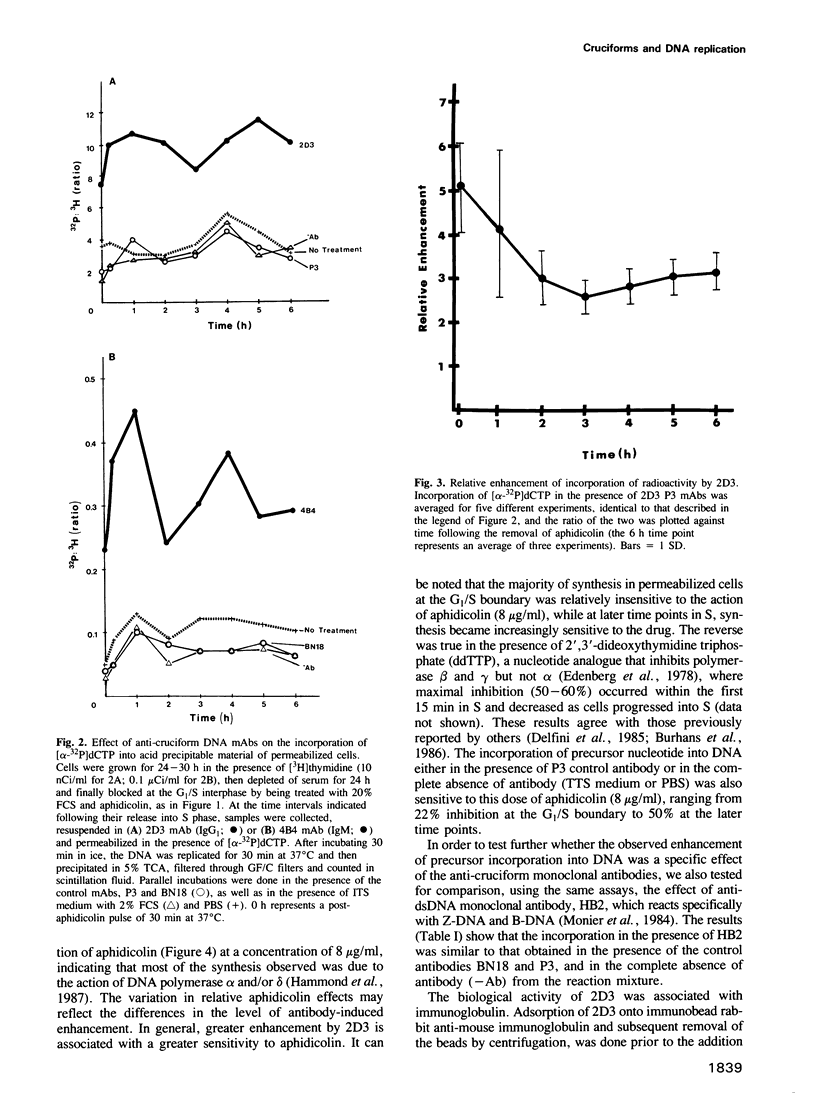
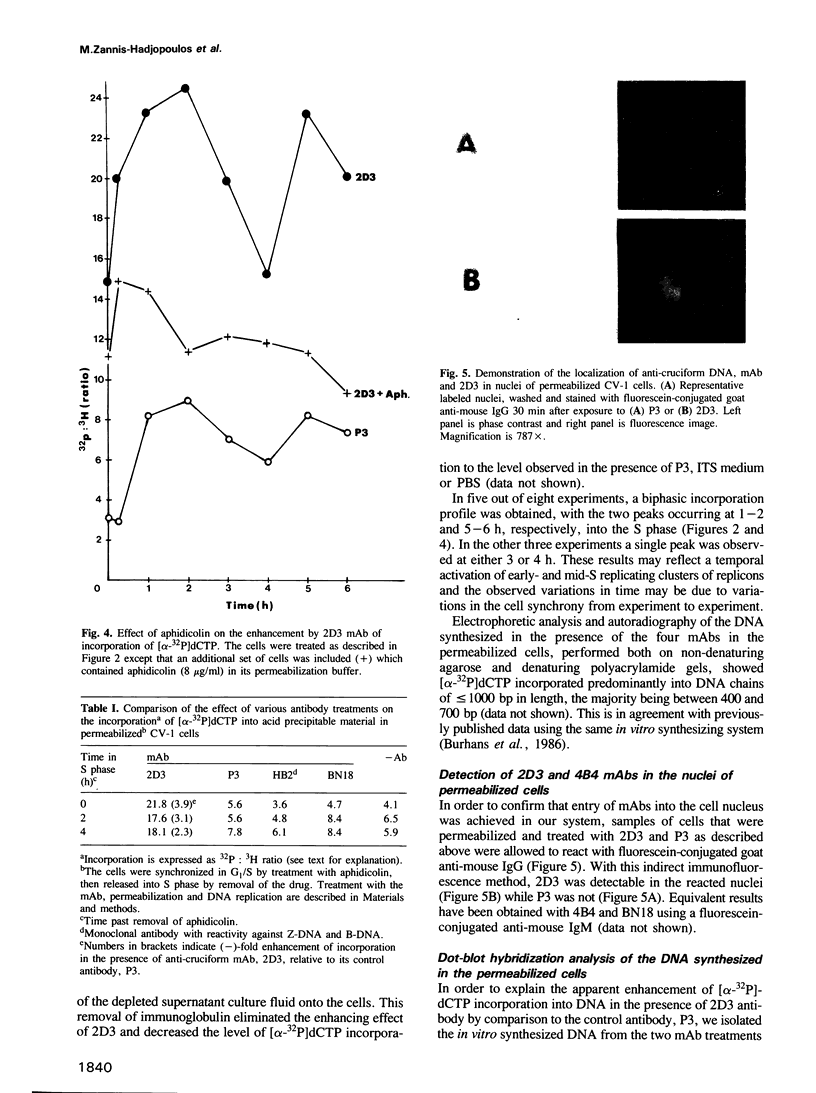
Abstract
To study the possible involvement of DNA cruciforms in the initiation of DNA replication, we used two monoclonal antibodies, 2D3 and 4B4, with anti-cruciform DNA specificity. Synchronized CV-1 cells were released into S phase for hourly intervals up to 6 h and permeabilized in the presence of monoclonal antibodies, under conditions that allow limited DNA replication. Exposure of the permeabilized cells to 2D3 or 4B4 resulted in a 2- to 6-fold enhancement of incorporation of labeled precursor nucleotide over the 6 h period. Approximately 50% of the enhanced synthesis was sensitive to aphidicolin, and the enhancing effect of 2D3 was abolished by absorption with immunobead anti-mouse immunoglobulin. Dot-blot hybridization analyses of DNA isolated from anti-cruciform antibody treatment groups showed a similar 2- to 11-fold increase in the relative copy number of low copy probes. In contrast, exposure of the permeabilized cells to a monoclonal antibody directed against Z-DNA and B-DNA had no significant effect on DNA synthesis. The results suggest that cruciforms are present in replicating DNA and that they are recognized and stabilized by the monoclonal antibodies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollum F. J. Mammalian DNA polymerases. Prog Nucleic Acid Res Mol Biol. 1975;15(0):109–144. doi: 10.1016/s0079-6603(08)60118-x. [DOI] [PubMed] [Google Scholar]
- Burhans W. C., Selegue J. E., Heintz N. H. Isolation of the origin of replication associated with the amplified Chinese hamster dihydrofolate reductase domain. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7790–7794. doi: 10.1073/pnas.83.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Collins J. M., Glock M. S., Chu A. K. Nuclease S1 sensitive sites in parental deoxyribonucleic acid of cold-and temperature-sensitive mammalian cells. Biochemistry. 1982 Jul 6;21(14):3414–3419. doi: 10.1021/bi00257a026. [DOI] [PubMed] [Google Scholar]
- Decker R. S., Yamaguchi M., Possenti R., DePamphilis M. L. Initiation of simian virus 40 DNA replication in vitro: aphidicolin causes accumulation of early-replicating intermediates and allows determination of the initial direction of DNA synthesis. Mol Cell Biol. 1986 Nov;6(11):3815–3825. doi: 10.1128/mcb.6.11.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfini C., Alfani E., De Venezia V., Oberholtzer G., Tomasello C., Eremenko T., Volpe P. Cell-cycle dependence and properties of the HeLa cell DNA polymerase system. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2220–2224. doi: 10.1073/pnas.82.8.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinter-Gottlieb G., Kaufmann G. Uncoupling of SV40 tsA replicon activation from DNA chain elongation by temperature shifts and aphidicolin arrest. Nucleic Acids Res. 1982 Jan 22;10(2):763–773. doi: 10.1093/nar/10.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Anderson S., DePamphilis M. L. Involvement of DNA polymerase alpha in simian virus 40 DNA replication. J Biol Chem. 1978 May 10;253(9):3273–3280. [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Hice R. H., Chlebowicz-Sledziewska E. ARS replication during the yeast S phase. Cell. 1983 Mar;32(3):831–838. doi: 10.1016/0092-8674(83)90069-7. [DOI] [PubMed] [Google Scholar]
- Ford M., Fried M. Large inverted duplications are associated with gene amplification. Cell. 1986 May 9;45(3):425–430. doi: 10.1016/0092-8674(86)90328-4. [DOI] [PubMed] [Google Scholar]
- Frappier L., Price G. B., Martin R. G., Zannis-Hadjopoulos M. Monoclonal antibodies to cruciform DNA structures. J Mol Biol. 1987 Feb 20;193(4):751–758. doi: 10.1016/0022-2836(87)90356-1. [DOI] [PubMed] [Google Scholar]
- Frappier L., Zannis-Hadjopoulos M. Autonomous replication of plasmids bearing monkey DNA origin-enriched sequences. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6668–6672. doi: 10.1073/pnas.84.19.6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J. Nucleotide sequence of the region encompassing the JC virus origin of DNA replication. J Virol. 1983 Apr;46(1):170–176. doi: 10.1128/jvi.46.1.170-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L. Effect of damage to early, middle, and late-replicating DNA on progress through the S period in Chinese hamster ovary cells. Exp Cell Res. 1978 Mar 15;112(2):225–232. doi: 10.1016/0014-4827(78)90204-5. [DOI] [PubMed] [Google Scholar]
- Hammond R. A., Byrnes J. J., Miller M. R. Identification of DNA polymerase delta in CV-1 cells: studies implicating both DNA polymerase delta and DNA polymerase alpha in DNA replication. Biochemistry. 1987 Oct 20;26(21):6817–6824. doi: 10.1021/bi00395a035. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Hand R., Gautschi J. R. Replication of mammalian DNA in vitro. Evidence for initiation from fiber autoradiography. J Cell Biol. 1979 Aug;82(2):485–493. doi: 10.1083/jcb.82.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. H., Hamlin J. L. An amplified chromosomal sequence that includes the gene for dihydrofolate reductase initiates replication within specific restriction fragments. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4083–4087. doi: 10.1073/pnas.79.13.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hixson J. E., Wong T. W., Clayton D. A. Both the conserved stem-loop and divergent 5'-flanking sequences are required for initiation at the human mitochondrial origin of light-strand DNA replication. J Biol Chem. 1986 Feb 15;261(5):2384–2390. [PubMed] [Google Scholar]
- Hobom G., Grosschedl R., Lusky M., Scherer G., Schwarz E., Kössel H. Functional analysis of the replicator structure of lambdoid bacteriophage DNAs. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):165–178. doi: 10.1101/sqb.1979.043.01.023. [DOI] [PubMed] [Google Scholar]
- Hsieh T. S., Wang J. C. Thermodynamic properties of superhelical DNAs. Biochemistry. 1975 Feb 11;14(3):527–535. doi: 10.1021/bi00674a011. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer K. M., Gall J. G. The macronuclear ribosomal DNA of Tetrahymena pyriformis is a palindrome. J Mol Biol. 1976 Jun 25;104(2):421–453. doi: 10.1016/0022-2836(76)90280-1. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Zannis-Hadjopoulos M., Martin R. G. Cloning of nascent monkey DNA synthesized early in the cell cycle. Mol Cell Biol. 1985 Apr;5(4):721–727. doi: 10.1128/mcb.5.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen T., Prydz H. Isolated HeLa cell nuclei synthesize meaningful DNA. Nucleic Acids Res. 1985 May 24;13(10):3551–3560. doi: 10.1093/nar/13.10.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacatena R. M., Cesareni G. Base pairing of RNA I with its complementary sequence in the primer precursor inhibits ColE1 replication. Nature. 1981 Dec 17;294(5842):623–626. doi: 10.1038/294623a0. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. The inverted repeat as a recognizable structural feature in supercoiled DNA molecules. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6468–6472. doi: 10.1073/pnas.77.11.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockshon D., Galloway D. A. Cloning and characterization of oriL2, a large palindromic DNA replication origin of herpes simplex virus type 2. J Virol. 1986 May;58(2):513–521. doi: 10.1128/jvi.58.2.513-521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Backman K., Kield D., Flashman S., Jeffrey A., Maurer R. Recognition sequences of repressor and polymerase in the operators of bacteriophage lambda. Cell. 1975 Jun;5(2):109–113. doi: 10.1016/0092-8674(75)90018-5. [DOI] [PubMed] [Google Scholar]
- Masukata H., Tomizawa J. Control of primer formation for ColE1 plasmid replication: conformational change of the primer transcript. Cell. 1986 Jan 17;44(1):125–136. doi: 10.1016/0092-8674(86)90491-5. [DOI] [PubMed] [Google Scholar]
- Masukata H., Tomizawa J. Effects of point mutations on formation and structure of the RNA primer for ColE1 DNA replication. Cell. 1984 Feb;36(2):513–522. doi: 10.1016/0092-8674(84)90244-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCutchan T., Hsu H., Thayer R. E., Singer M. F. Organization of African green monkey DNA at junctions between alpha-satellite and other DNA sequences. J Mol Biol. 1982 May 15;157(2):195–211. doi: 10.1016/0022-2836(82)90230-3. [DOI] [PubMed] [Google Scholar]
- Meijer M., Beck E., Hansen F. G., Bergmans H. E., Messer W., von Meyenburg K., Schaller H. Nucleotide sequence of the origin of replication of the Escherichia coli K-12 chromosome. Proc Natl Acad Sci U S A. 1979 Feb;76(2):580–584. doi: 10.1073/pnas.76.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Mizuuchi M., Gellert M. Cruciform structures in palindromic DNA are favored by DNA supercoiling. J Mol Biol. 1982 Apr 5;156(2):229–243. doi: 10.1016/0022-2836(82)90325-4. [DOI] [PubMed] [Google Scholar]
- Monier J. C., Brochier J., Moreira A., Sault C., Roux B. Generation of hybridoma antibodies to double-stranded DNA from non-autoimmune BALB/c strain: studies on anti-idiotype. Immunol Lett. 1984;8(2):61–68. doi: 10.1016/0165-2478(84)90051-8. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Fontaine A. A native cruciform DNA structure probed in bacteria by recombinant T7 endonuclease. J Biol Chem. 1987 Aug 15;262(23):11364–11368. [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Cruciform structures in supercoiled DNA. Nature. 1981 Feb 5;289(5797):466–470. doi: 10.1038/289466a0. [DOI] [PubMed] [Google Scholar]
- Pepe G., Holtrop M., Gadaleta G., Kroon A. M., Cantatore P., Gallerani R., De Benedetto C., Quagliariello C., Sbisà E., Saccone C. Non-random patterns of nucleotide substitutions and codon strategy in the mammalian mitochondrial genes coding for identified and unidentified reading frames. Biochem Int. 1983 Apr;6(4):553–563. [PubMed] [Google Scholar]
- Platt J. R. POSSIBLE SEPARATION OF INTERTWINED NUCLEIC ACID CHAINS BY TRANSFER-TWIST. Proc Natl Acad Sci U S A. 1955 Mar 15;41(3):181–183. doi: 10.1073/pnas.41.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rivin C. J., Fangman W. L. Replication fork rate and origin activation during the S phase of Saccharomyces cerevisiae. J Cell Biol. 1980 Apr;85(1):108–115. doi: 10.1083/jcb.85.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Houck C. M., Deininger P. L., Friedmann T., Schmid C. W. Partial nucleotide sequence of the 300-nucleotide interspersed repeated human DNA sequences. Nature. 1980 Mar 27;284(5754):372–374. doi: 10.1038/284372a0. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Manning J. E., Davidson N. Inverted repeat sequences in the Drosophila genome. Cell. 1975 Jun;5(2):159–172. doi: 10.1016/0092-8674(75)90024-0. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Broyles S. S., Pettijohn D. E. Perfect palindromic lac operator DNA sequence exists as a stable cruciform structure in supercoiled DNA in vitro but not in vivo. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1797–1801. doi: 10.1073/pnas.80.7.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., McMonagle E. C. Characterization of the TRS/IRS origin of DNA replication of herpes simplex virus type 1. Virology. 1983 Oct 30;130(2):427–438. doi: 10.1016/0042-6822(83)90097-1. [DOI] [PubMed] [Google Scholar]
- Studzinski G. P., Brelvi Z. S., Feldman S. C., Watt R. A. Participation of c-myc protein in DNA synthesis of human cells. Science. 1986 Oct 24;234(4775):467–470. doi: 10.1126/science.3532322. [DOI] [PubMed] [Google Scholar]
- Sullivan K. M., Lilley D. M. A dominant influence of flanking sequences on a local structural transition in DNA. Cell. 1986 Dec 5;47(5):817–827. doi: 10.1016/0092-8674(86)90524-6. [DOI] [PubMed] [Google Scholar]
- Taylor J. H. Origins of replication and gene regulation. Mol Cell Biochem. 1984;61(2):99–109. doi: 10.1007/BF00222489. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T., Selzer G., Som T. Inhibition of ColE1 RNA primer formation by a plasmid-specified small RNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1421–1425. doi: 10.1073/pnas.78.3.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Spadaro A., Schaffer J. E., Murray A. W., Maxam A. M., Schaffer P. A. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol Cell Biol. 1985 May;5(5):930–942. doi: 10.1128/mcb.5.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Hydroxyapatite chromatography of short double-helical DNA. Biochim Biophys Acta. 1973 Dec 21;331(3):333–340. doi: 10.1016/0005-2787(73)90019-1. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Palindromes in chromosomes. J Mol Biol. 1974 Mar 25;84(1):115–138. doi: 10.1016/0022-2836(74)90216-2. [DOI] [PubMed] [Google Scholar]
- Wong J. F., Ma D. P., Wilson R. K., Roe B. A. DNA sequence of the Xenopus laevis mitochondrial heavy and light strand replication origins and flanking tRNA genes. Nucleic Acids Res. 1983 Jul 25;11(14):4977–4995. doi: 10.1093/nar/11.14.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Kaufmann G., Martin R. G. Mammalian DNA enriched for replication origins is enriched for snap-back sequences. J Mol Biol. 1984 Nov 15;179(4):577–586. doi: 10.1016/0022-2836(84)90156-6. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Kaufmann G., Wang S. S., Lechner R. L., Karawya E., Hesse J., Martin R. G. Properties of some monkey DNA sequences obtained by a procedure that enriches for DNA replication origins. Mol Cell Biol. 1985 Jul;5(7):1621–1629. doi: 10.1128/mcb.5.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Cleary J. M., Brusilow W. S., Harding N. E., Smith D. W. Chromosomal replication origin from the marine bacterium Vibrio harveyi functions in Escherichia coli: oriC consensus sequence. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1164–1168. doi: 10.1073/pnas.80.5.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]