Abstract
Phrenic long-term facilitation (pLTF) is a persistent increase in phrenic nerve activity after acute intermittent hypoxia (AIH). Distinct cell-signaling cascades give rise to pLTF depending on the severity of hypoxemia within hypoxic episodes. Moderate AIH (mAIH; three 5-min episodes, PaO2 ∼35–55 mmHG) elicits pLTF by a serotonin (5-HT)-dependent mechanism that requires new synthesis of brain-derived neurotrophic factor (BDNF), activation of its high-affinity receptor (TrkB), and ERK MAPK signaling. In contrast, severe AIH (sAIH; three 5-min episodes, PaO2 ∼25–30 mmHG) elicits pLTF by an adenosine-dependent mechanism that requires new TrkB synthesis and Akt signaling. Although both mechanisms require spinal protein synthesis, the newly synthesized proteins are distinct, as are the neurochemicals inducing plasticity (serotonin vs. adenosine). In many forms of neuroplasticity, new protein synthesis requires translational regulation via mammalian target of rapamycin (mTOR) signaling. Since Akt regulates mTOR activity, we hypothesized that mTOR activity is necessary for sAIH- but not mAIH-induced pLTF. Phrenic nerve activity in anesthetized, paralyzed, and ventilated rats was recorded before, during, and 60 min after mAIH or sAIH. Rats were pretreated with intrathecal injections of 20% DMSO (vehicle controls) or rapamycin (0.1 mM, 12 μl), a selective mTOR complex 1 inhibitor. Consistent with our hypothesis, rapamycin blocked sAIH- but not mAIH-induced pLTF. Thus spinal mTOR activity is required for adenosine-dependent (sAIH) but not serotonin-dependent (mAIH) pLTF, suggesting that distinct mechanisms regulate new protein synthesis in these forms of spinal neuroplasticity.
Keywords: phrenic, long-term facilitation, hypoxia, plasticity, motor neuron, spinal, mTOR, rapamycin, translational regulation
phrenic long-term facilitation (pLTF) is an important model of spinal, respiratory motor plasticity (Mitchell and Johnson 2003) elicited by acute intermittent hypoxia [AIH (Bach and Mitchell 1996; Baker and Mitchell 2000)]. The magnitude (Nichols et al. 2012) and cellular mechanisms of pLTF vary depending on the characteristics of AIH (Dale-Nagle et al. 2010a; Devinney et al. 2013; Nichols et al. 2012). For example, the severity of hypoxemia within individual hypoxic episodes of the same AIH protocol (three 5-min episodes; 5-min intervals) determines which cellular mechanism gives rise to pLTF. Moderate AIH (mAIH; PaO2 = 35–55 mmHg) elicits pLTF by a mechanism that requires spinal serotonin (5-HT) type 2 receptor activation (Baker-Herman and Mitchell 2002; Fuller et al. 2001; Kinkead and Mitchell 1999), new synthesis of brain-derived neurotrophic factor [BDNF (Baker-Herman et al. 2004)], activation of the high-affinity BDNF receptor [TrkB (Baker-Herman et al. 2004)], and ERK MAPK signaling (Hoffman et al. 2012). In contrast, severe AIH (sAIH; PaO2 = 25–30 mmHg) produces pLTF (Nichols et al. 2012) that requires spinal adenosine 2A (A2A) receptor activation (Nichols et al. 2012), synthesis of an immature TrkB isoform (Golder et al. 2008), and phosphatidylinositol 3 (PI3)-kinase/Akt signaling (Golder et al. 2008). These signaling cascades interact via mutual, cross-talk inhibition (Hoffman et al. 2010). Although serotonin-dependent mechanisms predominate with mAIH, adenosine-dependent mechanisms predominant with sAIH. The biological significance of diverse pLTF mechanisms remains unclear, but we have suggested that they confer flexible respiratory responses to diverse challenges that differ in quality [e.g., pattern (Devinney et al. 2013), severity (Nichols et al. 2012), and/or duration (Dale et al. 2012; Dale-Nagle et al. 2011)]. mAIH- and sAIH-induced pLTF both require new protein synthesis, although the specific proteins synthesized differ (i.e., BDNF vs. immature TrkB). Neuroplasticity often requires new protein synthesis via translational regulation initiated by a mammalian target of rapamycin (mTOR)-dependent mechanism (Hoeffer and Klann 2010; Jaworski and Sheng 2006). mTOR is a serine/threonine protein kinase that activates p70S6K and 4E-BP1 (Klann and Dever 2004), enhancing cellular translational activity (Jaworski and Sheng 2006). Since both mAIH and sAIH increase translation, mTOR may be a point of convergence between their respective signaling pathways. Although canonical mTOR activation requires PI3-kinase/Akt signaling (Garami et al. 2003; Hoeffer and Klann 2010; Jaworski and Sheng 2006; Manning et al. 2002), ERK MAPKs activate mTOR in some cell types/conditions, including cancer pathogenesis (Ma et al. 2005; Tee et al. 2003). Because of common associations between Akt and mTOR activation, and less frequent reports of ERK-dependent mTOR activation, we tested the hypothesis that spinal mTOR is necessary for sAIH- but not mAIH-induced pLTF.
MATERIALS AND METHODS
Animals.
Three- to four-month-old male Sprague-Dawley rats were studied in all experiments (n = 40; colony 211A; Harlan, Indianapolis, IN). All procedures were approved by the Institutional Animal Care and Use Committee, School of Veterinary Medicine, University of Wisconsin-Madison.
Experimental preparation.
Rats were anesthetized with isoflurane in a closed chamber, followed by tracheotomy and ventilation with a rodent ventilator (model 683; Harvard Apparatus, South Natick, MA; tidal volume ∼2.5 ml, frequency ∼75 per min). Tracheal pressure was monitored via pressure transducer (model P23ID Gould Stratham) attached to the inspired line of the ventilator. The lungs were briefly hyperinflated by occluding the ventilator expiratory line every hour to minimize atelectasis. Anesthesia was maintained with isoflurane (3–4% in 50% O2, balance N2) throughout the surgical procedures; adequacy of anesthesia was confirmed by the lack of toe-pinch or eye-blink reflexes. After surgery, rats were slowly converted to urethane anesthesia (1.8 mg/kg) via a femoral venous catheter. While surgery was performed on a temperature-controlled stainless steel surgical table, rectal temperature was monitored with a temperature sensor (Physitemp Instruments, Clifton, NJ) and maintained by adjusting the surgical table temperature. The inspired O2 concentration was monitored with a fuel cell sensor (TED 60T; Teledyne Analytical Instruments, City of Industry, CA). Rats were vagotomized, and a catheter was inserted into the right femoral artery to monitor blood pressure with a pressure transducer (APT300; Harvard Apparatus, Holliston, MA). Arterial blood samples were analyzed for O2 (PaO2) and CO2 (PaCO2) partial pressures, pH, and standard base excess (SBE) with a blood gas analyzer (ABL 800 Flex; Radiometer, Copenhagen, Denmark). A slow, continuous intravenous infusion of an 8:1:1 solution of veterinary lactated Ringer's solution, hetastarch, and sodium bicarbonate was maintained after conversion to urethane anesthesia to maintain blood pressure and acid-base balance throughout the experiment.
The left phrenic nerve was dissected and exposed via a dorsal approach, cut distally, and desheathed. The nerve was submerged in mineral oil and placed on a bipolar silver recording electrode to record spontaneous neural activity. The adequacy of anesthesia was tested before protocols commenced and immediately after the protocol was complete. Adequacy of anesthetic depth was assessed as the lack of pressor or phrenic neural responses to toe pinch with a hemostat; we did not observe increased blood pressure or phrenic nerve activity in any rat in response to toe pinch, consistent with previous studies. Urethane anesthesia is known to remain stable and effective in rats for hours longer than our typical experimental protocol (Maggi and Meli 1986). After adequate anesthesia was confirmed, neuromuscular block with pancuronium bromide (∼1.2 ml iv, 1 mg/ml) was initiated to minimize movement artifacts associated with respiratory muscle activity and to prevent asynchrony between ventilator and spontaneous breaths. End-tidal CO2 was monitored using a flow-through capnograph (model 1265; Philips Respironics, Murrysville, PA) with sufficient response time to measure end-tidal CO2 levels in rats and was maintained at ∼40 mmHg throughout the surgical preparation. Nerve activity was amplified (gain, 10,000×; A-M Systems, Everett, WA), bandpass filtered (300 Hz to 10 kHz), rectified, and integrated (CWE 821 filter; Paynter, Ardmore, PA; time constant, 50 ms). The signal was then digitized and recorded using a WINDAQ data acquisition system (DATAQ Instruments, Akron, OH). Data analysis was performed using custom software on a LabVIEW platform (National Instruments, Austin, TX).
An intrathecal catheter was placed to enable localized rapamycin injections near the phrenic motor nucleus. In brief, after dorsal laminectomy at C2, an incision was made in the dura and a small silicone catheter (2-Fr; Access Technologies, Skokie, IL) was inserted and advanced ∼3 mm caudally, bringing the catheter tip to the rostral edge of cervical segments containing the phrenic motor nucleus (∼C3). The catheter was primed with either rapamycin (0.1 mM; MP Biomedicals, Santa Ana, CA) or vehicle (20% DMSO in saline) before catheter placement. Rapamycin dose was selected based on preliminary dose-response testing and previously published reports (Geranton et al. 2009; Jimenez-Diaz et al. 2008; Liang et al. 2013; Xu et al. 2011, 2014). Intrathecal solutions were prepared fresh for each experiment.
Experimental protocol.
Stable nerve activity was established while the rat was ventilated with a hyperoxic inspired gas mixture (FiO2, 0.5–0.6; PaO2 > 150 mmHg); inspired CO2 was elevated to maintain PaCO2 at a constant baseline level just sufficient to prevent the rat from becoming apneic (typically between 40 and 45 mmHg). After the preparation stabilized, the apneic threshold for rhythmic phrenic activity was determined by progressively lowering the inspired (and arterial) CO2 until rhythmic phrenic activity ceased. The end-tidal CO2 was then progressively increased in 1-mmHg increments every 1–2 min until nerve activity resumed, documenting the recruitment threshold. Baseline nerve activity was established with PaCO2 set 2–3 mmHg above the CO2 recruitment threshold. This procedure allows a standardized level of respiratory drive during baseline conditions in different rats. Fifteen minutes after baseline conditions were set, rats received an intrathecal injection of rapamycin or vehicle (12 μl over ∼3 min). Approximately 15 min postinjection, a baseline blood sample was taken and the rats were exposed to AIH, consisting of three 5-min hypoxic episodes with 5-min intervals of 50% O2. Since cellular signaling cascades responsible for LTF differ depending on severity of hypoxemia during AIH (Nichols et al. 2012), rats were divided into the following experimental groups: 1) rats receiving mAIH (∼11% inspired O2) with intrathecal vehicle, 2) rats with mAIH and intrathecal rapamycin, 3) rats receiving sAIH (∼9% inspired O2) and intrathecal vehicle, and 4) rats with sAIH and intrathecal rapamycin. Additional rats were used as time controls (i.e., without hypoxia) to test for time-dependent effects of intrathecal vehicle or rapamycin alone. Nerve activity was monitored for 60 min after the last hypoxic episode while baseline blood gas levels were maintained. Blood samples (0.1–0.2 ml in a capillary tube) were drawn and analyzed before AIH (baseline), during the first hypoxic episode, and at 15, 30, and 60 min post-AIH. An experiment was considered successful only if blood gas regulation conformed to the de novo criteria outlined below. At the conclusion of experiments, rats were humanely euthanized via intravenous urethane overdose followed by ceased pump ventilation.
Statistical analysis.
Peak phrenic amplitude and burst frequency were averaged in 1-min bins at each recorded data point (baseline, during hypoxia, and 15, 30, and 60 min post-AIH). Changes in nerve burst amplitude were normalized as a percent change from baseline (BL) values; burst frequency is reported as a change from baseline in bursts/min. We compared phrenic nerve responses of rats receiving vehicle vs. rapamycin injections (time control and AIH for each). Because rapamycin time control and vehicle time control groups were similar across all studies and could not be distinguished statistically (Table 1), they were combined into a single time control group (“combined time controls”). Statistical comparisons were made for time and drug treatment effects within AIH groups using two-way, repeated-measures ANOVA. Bonferroni's post hoc test was used to identify statistically significant individual comparisons. A three-way ANOVA and Bonferroni post hoc test were used to assess interactions between mAIH and sAIH when animals were treated with rapamycin. Differences were considered significant if P < 0.05. All values are means ± SE.
Table 1.
Body temperature, pH, blood gases, SBE, and MAP in each treatment group
| Experimental Groups | Tb, °C | pH | PaCO2, mmHg | Pao2, mmHg | SBE | MAP, mmHg |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| mAIH + vehicle | 37.37 ± 0.25 | 7.349 ± 0.006 | 46.60 ± 1.18 | 312.6 ± 14.9 | −0.14 ± 0.45 | 104.0 ± 8.7 |
| mAIH + rapamycin | 37.66 ± 0.24 | 7.342 ± 0.006 | 48.38 ± 0.77 | 293.4 ± 20.3 | 0.26 ± 0.55 | 98.7 ± 7.4 |
| sAIH + vehicle | 37.33 ± 0.11 | 7.376 ± 0.009 | 45.60 ± 1.97 | 309.9 ± 8.2 | 0.99 ± 0.48 | 89.5 ± 2.1 |
| sAIH + rapamycin | 37.17 ± 0.15 | 7.368 ± 0.012 | 45.37 ± 1.11 | 336.7 ± 13.1 | 0.62 ± 0.41 | 86.2 ± 3.9 |
| TC vehicle | 37.73 ± 0.23 | 7.364 ± 0.005 | 47.75 ± 1.65 | 304.2 ± 19.6 | 1.23 ± 0.30 | 88.8 ± 7.8 |
| TC rapamycin | 37.58 ± 0.13 | 7.356 ± 0.005 | 47.38 ± 0.36 | 304.0 ± 7.5 | 0.63 ± 0.21 | 101.6 ± 4.1 |
| 15 min | ||||||
| mAIH + vehicle | 37.49 ± 0.23 | 7.336 ± 0.005 | 46.70 ± 1.39 | 245.6 ± 19.2* | −1.09 ± 0.50 | 104.8 ± 7.8 |
| mAIH + rapamycin | 37.72 ± 0.20 | 7.328 ± 0.007 | 48.90 ± 0.80 | 217.8 ± 22.0*†‡ | −0.34 ± 0.67 | 106.6 ± 7.2 |
| sAIH + vehicle | 37.38 ± 0.16 | 7.348 ± 0.007* | 45.56 ± 1.90 | 226.8 ± 13.1*†‡ | −0.85 ± 0.74* | 97.3 ± 2.2* |
| sAIH + rapamycin | 37.12 ± 0.09 | 7.360 ± 0.011 | 45.02 ± 1.60 | 215.3 ± 22.2*†‡ | −0.17 ± 0.51 | 98.4 ± 4.3* |
| TC vehicle | 37.78 ± 0.15 | 7.353 ± 0.009 | 47.73 ± 1.41 | 301.5 ± 14.0 | 0.65 ± 0.33 | 90.4 ± 6.1 |
| TC rapamycin | 37.60 ± 0.04 | 7.358 ± 0.002 | 47.00 ± 0.20 | 308.5 ± 13.1 | 0.58 ± 0.14 | 95.0 ± 2.7 |
| 30 min | ||||||
| mAIH + vehicle | 37.47 ± 0.21 | 7.345 ± 0.006 | 47.39 ± 1.14 | 308.3 ± 10.6 | −0.10 ± 0.31 | 98.7 ± 7.7 |
| mAIH + rapamycin | 37.86 ± 0.19 | 7.343 ± 0.003 | 47.90 ± 0.75 | 278.8 ± 11.2 | 0.22 ± 0.46 | 93.7 ± 6.5 |
| sAIH + vehicle | 37.45 ± 0.16 | 7.357 ± 0.008* | 46.56 ± 2.16 | 275.6 ± 11.5* | 0.14 ± 0.77 | 87.7 ± 3.8 |
| sAIH + rapamycin | 37.15 ± 0.10 | 7.367 ± 0.010 | 46.27 ± 1.43 | 292.3 ± 7.9* | 0.92 ± 0.39 | 91.8 ± 4.9 |
| TC vehicle | 37.75 ± 0.22 | 7.351 ± 0.010 | 47.88 ± 1.86 | 305.0 ± 12.2 | 0.50 ± 0.17 | 86.7 ± 3.4 |
| TC rapamycin | 37.55 ± 0.10 | 7.354 ± 0.006 | 47.50 ± 0.59 | 309.3 ± 11.9 | 0.55 ± 0.22 | 98.2 ± 5.6 |
| 60 min | ||||||
| mAIH + vehicle | 37.53 ± 0.24 | 7.349 ± 0.010 | 46.99 ± 1.22 | 312.6 ± 7.5 | −0.03 ± 0.45 | 90.0 ± 8.9* |
| mAIH + rapamycin | 37.72 ± 0.17 | 7.354 ± 0.005 | 48.08 ± 1.02 | 285.4 ± 12.4 | 1.08 ± 0.67 | 85.7 ± 7.0* |
| sAIH + vehicle | 37.41 ± 0.18 | 7.364 ± 0.010 | 46.45 ± 1.99 | 289.8 ± 9.8 | 0.56 ± 0.43 | 80.6 ± 3.4* |
| sAIH + rapamycin | 37.30 ± 0.14 | 7.373 ± 0.007 | 45.57 ± 1.38 | 302.8 ± 9.8* | 1.03 ± 0.35 | 84.1 ± 2.9 |
| TC vehicle | 37.45 ± 0.26 | 7.356 ± 0.009 | 47.23 ± 1.89 | 315.3 ± 9.3 | 0.55 ± 0.22 | 87.8 ± 6.4 |
| TC rapamycin | 37.45 ± 0.17 | 7.352 ± 0.007 | 47.20 ± 0.29 | 311.8 ± 14.8 | 0.38 ± 0.44 | 107.8 ± 6.2 |
Values are means ± SE for body temperature (Tb), pH, blood gases, standard base excess (SBE), and mean arterial pressure (MAP) in each treatment group: moderate acute intermittent hypoxia (mAIH) + vehicle (n= 7), mAIH + rapamycin (n= 5), severe AIH (sAIH) + vehicle (n= 8), sAIH + rapamycin (n= 6), time control (TC) vehicle (n= 4), and TC rapamycin (n= 4) at baseline and 15, 30, and 60 min after mAIH or sAIH.
P< 0.05, significantly different from baseline.
P< 0.05, significantly different from vehicle time controls.
P< 0.05, significantly different from rapamycin time controls.
RESULTS
mAIH-induced pLTF does not require mTOR complex 1 activity.
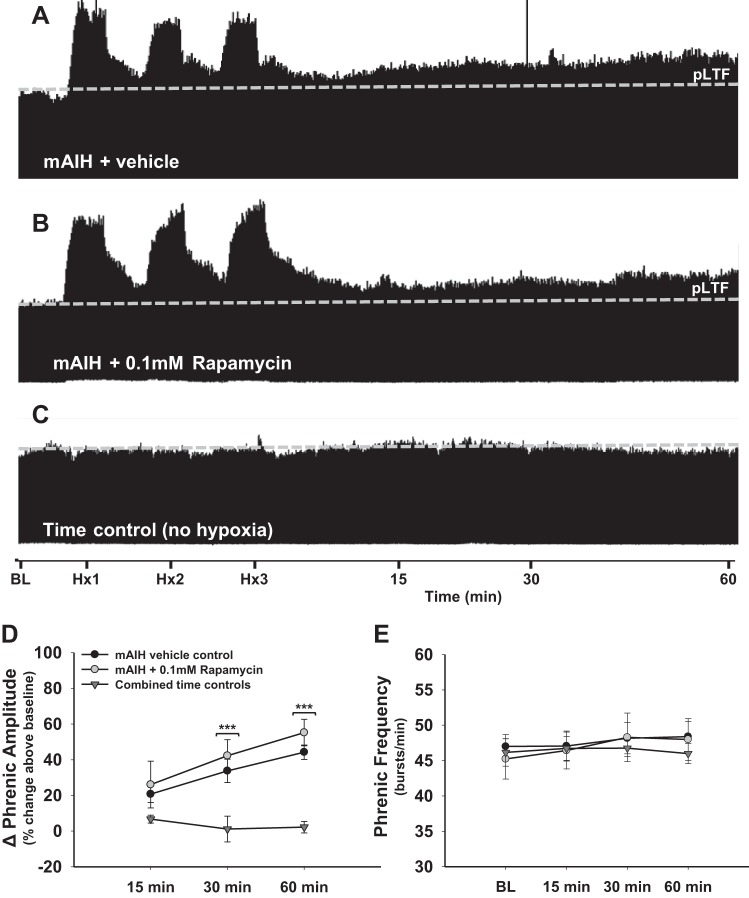
To determine if mAIH-induced pLTF requires mTOR complex 1 (mTORC1) activity, rats were pretreated with rapamycin (0.1 mM) or an equal volume of vehicle (20% DMSO in saline). Vehicle control rats exhibited progressive increases in phrenic burst amplitude following mAIH, consistent with previous reports (Baker-Herman and Mitchell 2002, 2008; Hoffman et al. 2010; Nichols et al. 2012; Wilkerson et al. 2008). Indeed, phrenic amplitude was significantly higher than time control rats at 30 (34 ± 7% above BL,; P < 0.001; Fig. 1, A, C, and D) and 60 min post-mAIH (44 ± 4% above BL; P > 0.001; Fig. 1, A, C, and D). Intrathecal rapamycin had no impact on mAIH-induced pLTF; in rats pretreated with rapamycin, phrenic burst amplitudes were significantly higher than in time controls at 30 (42 ± 9% above BL; P < 0.001; Fig. 1, B–D) and 60 min post-mAIH (55 ± 7% above BL; Fig. 1, B–D). No differences in mAIH-induced pLTF were observed between rapamycin- and vehicle-treated rats (P > 0.05; Fig. 1D).
Fig. 1.
Moderate acute intermittent hypoxia (mAIH)-induced phrenic long-term facilitation (pLTF) does not require mammalian target of rapamycin complex 1 (mTORC1) activity. A–C: representative traces of integrated phrenic neurograms during and after mAIH in vehicle control (20% DMSO in saline; A), rapamycin-treated (0.1 mM, 12 μl; B), and time control rats (no AIH; C). The dashed line indicates baseline (BL) phrenic amplitude in each trace. In A and B, typical pLTF in response to mAIH is shown, demonstrating that mTORC1 signaling is not necessary for mAIH-induced pLTF. In C, no time-dependent change in phrenic burst amplitude is shown, demonstrating experimental preparation stability. D: phrenic burst amplitude (percent change above BL) in vehicle controls (n = 7, filled circles), rapamycin-treated (n = 5, open circles), and combined time control rats (n = 8, triangles). pLTF is significant in vehicle control and rapamycin-treated rats at 30 and 60 min post-mAIH compared with time controls (both P < 0.001). E: frequency of phrenic bursting was unaffected by mAIH or rapamycin. Burst frequency remained consistent across groups at all time points (P > 0.05). ***P < 0.001 vs. time controls.
Rapamycin had no discernible effect on phrenic nerve burst frequency LTF; frequency was consistent across groups at all times post-mAIH (P > 0.05; Fig. 1E). Thus mTORC1 activity is not necessary for mAIH-induced pLTF.
sAIH-induced pLTF requires mTORC1 activity.
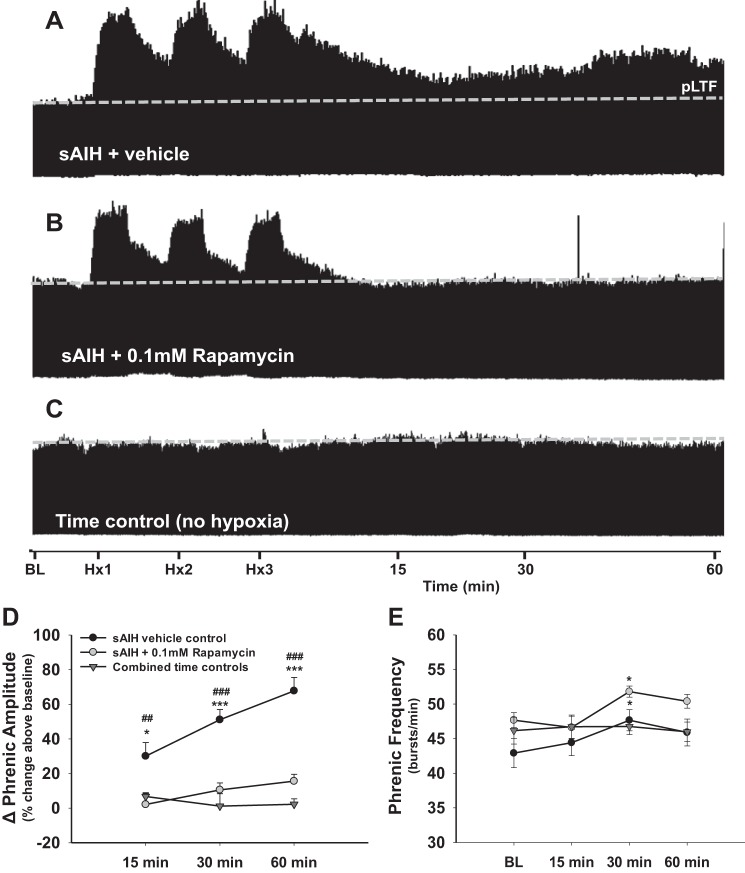
The role of mTORC1 activity in the expression of sAIH-induced pLTF was tested by pretreating rats with intrathecal rapamycin (0.1 mM) or vehicle (20% DMSO in saline) before inducing sAIH (∼9% O2). Vehicle control rats exhibited a robust and progressive post-sAIH increase in phrenic burst amplitude, consistent with previous reports (Nichols et al. 2012). By 15 min post-sAIH, phrenic burst amplitude was significantly enhanced compared with time controls (30 ± 8%; P < 0.05; Fig. 2, A, C, and D), a difference persisting at 30 (51 ± 6%; P < 0.001; Fig. 2, A, C, and D) and 60 min post-sAIH (68 ± 8%; P < 0.001; Fig. 2, A, C, and D). However, in contrast to mAIH-induced pLTF, rapamycin abolished sAIH-induced pLTF (Fig. 2B); phrenic burst amplitude was similar to that in time controls in rapamycin-pretreated rats at all times post-sAIH (P > 0.05; Fig. 2, B–D), and vehicle-treated rats exhibited significantly greater pLTF than rapamycin-pretreated rats at all times (Fig. 2, A, B, and D; all P < 0.01; Fig. 2D). To confirm that rapamycin effects were specific to the sAIH vs. mAIH difference, a three-way ANOVA with factors of time, hypoxia type (i.e., mAIH or sAIH), and drug treatment (rapamycin or vehicle) was performed. Consistent with our hypothesis, rapamycin effects on pLTF were dependent on the AIH protocol. There was a highly significant interaction between AIH type and rapamycin (P = <0.001), confirming that sAIH-induced (not mAIH induced) pLTF requires mTORC1 signaling.
Fig. 2.
Severe AIH (sAIH)-induced pLTF requires mTOR activity. A–C: representative traces of integrated phrenic neurograms during and after sAIH in vehicle control (20% DMSO in saline; A), rapamycin-treated (mTORC1 inhibitor, 0.1 mM, 12 μl; B), and time control rats (no AIH; C). The dashed line indicates BL phrenic amplitude. In A, robust pLTF is shown following sAIH in control rats. As shown in B, rapamycin pretreatment abolishes sAIH-induced pLTF, demonstrating that mTORC1 signaling is necessary. In C, no time-dependent change in phrenic burst amplitude is shown without AIH (i.e., time controls). D: phrenic burst amplitude (percent change above BL) in vehicle control (n = 8, filled circles), rapamycin-pretreated (n = 6, open circles), and combined time control rats (n = 8, triangles). pLTF is significantly elevated in vehicle control rats compared with rapamycin-pretreated and time control rats at 15, 30, and 60 min post-sAIH (P < 0.05 for each). E: phrenic burst frequency was elevated in vehicle- and rapamycin-treated rats at 30 min post sAIH (P < 0.05), although frequency had returned to BL by 60 min post-sAIH. No differences in burst frequency were observed between treatment groups (P > 0.05), indicating the effect on burst frequency was a result of sAIH vs. rapamycin. *P < 0.05; ***P < 0.001 vs. time controls. #P < 0.01; ###P < 0.001 vs. rapamycin-treated rats.
sAIH had a transient effect on phrenic burst frequency, although this effect was again rapamycin insensitive (P < 0.05; Fig. 2E). The transient nature of sAIH-induced frequency LTF was illustrated by the fact that burst frequency had normalized by 60 min post-sAIH.
Blood gases, mean arterial pressures, and acid-base status.
Measured physiological variables are shown in Table 1. The following blood gas criteria were maintained in all rats: 1) for mAIH, PaO2 was between 35 and 45 mmHg during hypoxic episodes; 2) for sAIH, PaO2 was between 25 and 30 mmHg; 3) PaO2 during the hyperoxic baseline and recovery periods was >150 mmHg; and 4) PaCO2 was successfully regulated within 1.5 mmHg of baseline throughout the post-AIH recovery period. In rats analyzed in this study, there were no differences between groups in body temperature or PaCO2 at any time point. Rats receiving sAIH with vehicle displayed reduced (P < 0.05) arterial pH and SBE following AIH (vs. BL), but these values had returned to baseline by 60 min post-sAIH. All groups showed a transient reduction in PaO2 at 15 min post-AIH compared with baseline (P < 0.05) and time controls (P < 0.05; mAIH with vehicle showed a marginally significant trend, P = 0.056). This PaO2 reduction continued in rats receiving sAIH and rapamycin until 60 min post-AIH. Regardless, PaO2 values were well above 150 mmHg at all times in all groups post-AIH; thus the transient decrease in PaO2 was unlikely to have had any discernable physiological impact.
Rats receiving sAIH (with or without rapamycin) showed a transient increase in mean arterial blood pressure (MAP) compared with baseline at 15 min post-AIH (P < 0.05). However, consistent with previous studies using this experimental preparation (Baker-Herman and Mitchell 2008), a progressive reduction in MAP was observed in all groups during the posthypoxia recording period. Rats were excluded from analysis if MAP decreased more than 30 mmHg from baseline at 60 min post-AIH. Six rats were removed from the study due to excessive reductions in MAP. However, MAP decreases were similar across all groups and were significantly lower at 60 min compared with baseline (P < 0.05) in all but the sAIH plus rapamycin group.
DISCUSSION
Distinct signaling cascades associated with new protein synthesis underlie pLTF induced by mAIH vs. sAIH (Dale-Nagle et al. 2010a; Devinney et al. 2013; Nichols et al. 2012). Specifically, mTOR activity is necessary for sAIH- but not mAIH-induced pLTF, providing additional evidence that mAIH and sAIH elicit pLTF via distinct mechanisms (Nichols et al. 2012). This knowledge expands our fundamental understanding concerning the diversity of mechanisms giving rise to AIH-induced neuroplasticity.
Cellular mechanisms of pLTF.
The serotonin-dependent (mAIH) and adenosine-dependent (sAIH) forms of pLTF are mediated by the Q and S pathways to phrenic motor facilitation (pMF), respectively. These pathways are named for the G protein-coupled receptors most frequently associated with the metabotropic receptors initiating the respective pathways. For example, spinal Gs protein-coupled adenosine 2A (A2A) receptors (i.e., S pathway) are necessary (Nichols et al. 2012) and sufficient (Golder et al. 2008) for sAIH-induced pLTF. A2A receptor activation initiates new synthesis of an immature TrkB protein isoform and requires PI3-kinase/Akt signaling (Golder et al. 2008). PI3-kinase/Akt is in the canonical signaling cascade regulating mTOR activation (Garami et al. 2003; Hoeffer and Klann 2010; Jaworski and Sheng 2006; Manning et al. 2002), consistent with our finding that mTOR signaling is required for sAIH-induced pLTF. Akt activation releases endogenous constraints on mTORC1, enabling its effects on protein synthesis via translational regulation (Tee et al. 2003). Since rapamycin does not affect signaling via the mTOR/Rictor complex [i.e., mTORC2; (Sarbassov et al. 2004)], our results are consistent with a role for mTORC1 (vs. mTORC2) signaling in sAIH-induced pLTF. Although we did not attempt to demonstrate a direct link between mTORC1 activity and translational regulation of immature TrkB protein, we suggest that mTORC1 regulates TrkB synthesis, thereby giving rise to sAIH-induced pLTF.
In contrast, the widely studied mAIH-induced pLTF is initiated by Gq-coupled 5-HT type 2 metabotropic receptors, giving rise to pLTF via new BDNF (vs. TrkB) synthesis and ERK MAPK (vs. Akt) signaling (Hoffman et al. 2012). Because of its initiating metabotropic receptor and the fact that multiple ligands acting via Gq proteins elicit the same basic response, this mechanism is referred to as the Q pathway to pMF (Dale-Nagle et al. 2010a). Since Q pathway-induced pLTF also requires translational regulation of new protein synthesis, in this case, BDNF (Baker-Herman et al. 2004), and ERK/MAPK signaling activates mTOR under specific conditions (Ma et al. 2005; Tee et al. 2003), we considered the possibility that mTOR signaling also plays a role in mAIH-induced pLTF. However, since rapamycin pretreatment had no effect on mAIH-induced pLTF, the Q pathway to pLTF is mTORC1 independent, once again illustrating distinctions between serotonin-dependent and adenosine-dependent pMF pathways.
Previous studies support a complex interaction between the S and Q pathways, characterized as cross-talk inhibition (Devinney et al. 2013). For example, subthreshold A2A receptor activation during mAIH constrains pLTF via protein kinase A (PKA) activity (Hoffman et al. 2010; Hoffman and Mitchell 2013). Since rapamycin had no effect on mAIH-induced pLTF, we conclude that PKA mediates cross-talk inhibition upstream from mTOR signaling, reflecting divergent cAMP signaling pathways (Fig. 3; Fields et al. 2015). Although mTOR signaling does not modulate the Q pathway to pMF, we cannot rule out the possibility that the Q and S pathways converge downstream from mTOR signaling. For example, ERK directly activates p70S6K (an intermediate kinase between mTOR and 4E-BP1) in some model systems (Dufner and Thomas 1999; Klann and Dever 2004). Thus ERK could regulate translational activity via a p70S6K, Mnk-1, 4E-BP1, and eIF4E signaling pathway (Banko et al. 2004; Hoeffer and Klann 2010; Klann and Dever 2004). On the other hand, if the S and Q pathways do converge, it is difficult to explain specificity of the proteins synthesized (BDNF vs. TrkB).
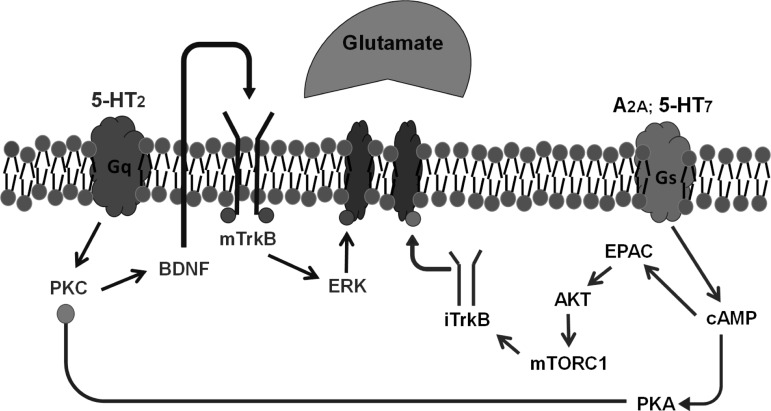
Fig. 3.
Working model of convergent pLTF pathways. The Q pathway to phrenic motor facilitation (pMF) is initiated by episodic activation of Gq protein-coupled 5-HT2 receptors (MacFarlane and Mitchell 2009; MacFarlane et al. 2011) followed by PKCθ activation (Devinney et al. 2015), new brain-derived neurotrophic factor (BDNF) synthesis (Baker-Herman et al. 2004), TrkB (Baker-Herman et al. 2004; Dale EA and Mitchell GS, unpublished observations), and ERK/MAP kinase activation (Hoffman et al. 2012). The mechanism whereby ERK elicits pLTF remains unknown. We propose a newly organized “S” pathway (right) to pLTF initiated by adenosine 2A (A2A) receptors (Golder et al. 2008), followed by cAMP production, EPAC, Akt, and mTOR activation (Fields et al. 2015) and new synthesis of immature TrkB isoforms (Golder et al. 2008). Mechanisms downstream of iTrkB are unknown. S-to-Q pathway inhibition via PKA (Hoffman and Mitchell 2013) diverges at cAMP based on recent observations (Fields et al. 2015).
Therapeutic implications of AIH.
Repetitive AIH is an exciting new strategy to promote functional recovery of lost breathing capacity (and nonrespiratory motor functions) with clinical disorders such as chronic spinal injury, motor neuron disease, and other neuromuscular disorders (Dale et al. 2014; Mitchell 2007; Navarrete-Opazo and Mitchell 2014). Repetitive AIH protocols investigated to date include mAIH exposures for 7 consecutive days (Lovett-Barr et al. 2012; Wilkerson and Mitchell 2009) or 3 times per week for 4 or 10 wk (Dale-Nagle et al. 2010b; Satriotomo et al. 2012). Since repetitive AIH enhances respiratory (Lovett-Barr et al. 2012) and nonrespiratory somatic motor function (Lovett-Barr et al. 2012) in rat models of spinal cord injury (SCI) and humans with chronic, incomplete SCI (Hayes et al. 2014; Trumbower et al. 2012), a detailed understanding of cellular mechanisms giving rise to AIH-induced spinal respiratory plasticity is vital to optimize the therapeutic potential of repetitive AIH.
The relative contributions of the Q vs. S pathways to functional recovery of breathing capacity following cervical spinal injury in rats are complex. For example, recent studies suggest that the mechanisms of moderate repetitive AIH-induced functional recovery of breathing capacity are adenosine vs. serotonin dependent shortly after spinal injury (Dale-Nagle et al. 2010b; Navarrete-Opazo et al. 2015), consistent with mTOR-dependent S pathway-mediated functional recovery. Indeed, A2A receptor inhibition with istradefylline (KW-6002) prevents daily AIH-induced recovery of respiratory motor function 2 wk following cervical spinal hemisection (Navarrete-Opazo et al. 2015). Furthermore, repetitive AIH up-regulates mTOR and its downstream targets in the cervical spinal cord shortly after injury (Gutierrez et al. 2013). Thus mTOR may be a useful molecular target to enhance the therapeutic benefits of repetitive AIH with acute spinal injury. Conversely, pharmacological treatments designed to suppress mTOR activity may undermine the ability of repetitive AIH to restore motor function. For example, specific mTOR inhibitors are used clinically to treat some cancers, including renal cell carcinoma (Sankin et al. 2015) and breast cancer (Chia et al. 2015). The most commonly prescribed drug to treat type II diabetes, metformin, inhibits mTOR signaling by activating AMP-activated protein kinase [AMPK (Potter et al. 2010; Zhou et al. 2001)]. Since metformin crosses the blood-brain barrier (Labuzek et al. 2010), it may decrease mTOR signaling in motor neurons and undermine repetitive AIH-induced functional recovery.
In conclusion, we demonstrate that sAIH- but not mAIH-induced pLTF requires spinal mTOR activity, providing additional evidence that distinct cellular mechanisms give rise to pLTF elicited by AIH protocols that differ in a single detail (35–45 vs. 25–30 mmHg PaO2). These data add to our mechanistic understanding of AIH-induced plasticity and further differentiate the S and Q pathways to pMF. mTORC1 is a unique contributor to S pathway-induced pMF, including sAIH-induced pLTF.
Because cellular mechanisms associated with sAIH-induced pLTF have been implicated in repetitive AIH-induced recovery of respiratory motor function shortly after experimental cervical spinal injury (Navarrete-Opazo et al. 2015), mTOR is an interesting target for pharmacological interventions to optimize repetitive AIH-induced functional recovery. Our working model suggests that spinal A2A receptors activate Akt and mTORC1, increasing protein synthesis via translational regulation. mTORC1 manipulation with the well-tolerated drug rapamycin may enable qualitative assessment of contributions made by the S vs. Q pathways to enhanced pLTF following repetitive AIH preconditioning.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL111598 and HL69064. B. J. Dougherty was supported by a fellowship from the Craig H. Neilsen Foundatio. D. P. Fields was supported by an Institutional Advanced Opportunity Award from the University of Wisconsin, Madison.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.J.D., D.P.F., and G.S.M. conception and design of research; B.J.D. and D.P.F. performed experiments; B.J.D. analyzed data; B.J.D., D.P.F., and G.S.M. interpreted results of experiments; B.J.D. and D.P.F. prepared figures; B.J.D. drafted manuscript; B.J.D., D.P.F., and G.S.M. edited and revised manuscript; B.J.D., D.P.F., and G.S.M. approved final version of manuscript.
REFERENCES
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162: 8–17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Hou L, Klann E. NMDA receptor activation results in PKA- and ERK-dependent Mnk1 activation and increased eIF4E phosphorylation in hippocampal area CA1. J Neurochem 91: 462–470, 2004. [DOI] [PubMed] [Google Scholar]
- Chia S, Gandhi S, Joy AA, Edwards S, Gorr M, Hopkins S, Kondejewski J, Ayoub JP, Califaretti N, Rayson D, Dent SF. Novel agents and associated toxicities of inhibitors of the pi3k/Akt/mtor pathway for the treatment of breast cancer. Curr Oncol 22: 33–48, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology 29: 39–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Satriotomo I, Mitchell GS. Cervical spinal erythropoietin induces phrenic motor facilitation via extracellular signal-regulated protein kinase and Akt signaling. J Neurosci 32: 5973–5983, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann NY Acad Sci 1198: 252–259, 2010b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Satriotomo I, Mitchell GS. Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci 31: 7682–7690, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS. Phrenic long-term facilitation requires PKCtheta activity within phrenic motor neurons. J Neurosci 35: 8107–8117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann NY Acad Sci 1279: 143–153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 253: 100–109, 1999. [DOI] [PubMed] [Google Scholar]
- Fields DP, Springborn S, Mitchell GS. Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophysiol (April 12, 2015). doi: 10.1152/jn.00374.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol 90: 2001–2006; discussion 2000, 2001. [DOI] [PubMed] [Google Scholar]
- Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 11: 1457–1466, 2003. [DOI] [PubMed] [Google Scholar]
- Geranton SM, Jimenez-Diaz L, Torsney C, Tochiki KK, Stuart SA, Leith JL, Lumb BM, Hunt SP. A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci 29: 15017–15027, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 28: 2033–2042, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez DV, Clark M, Nwanna O, Alilain WJ. Intermittent hypoxia training after C2 hemisection modifies the expression of PTEN and mTOR. Exp Neurol 248: 45–52, 2013. [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82: 104–113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33: 67–75, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A(2A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol 588: 255–266, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neuroscience 250: 632–643, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol 113: 1184–1193, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol 34: 205–219, 2006. [DOI] [PubMed] [Google Scholar]
- Jimenez-Diaz L, Geranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP. Local translation in primary afferent fibers regulates nociception. PLoS One 3: e1961, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. Am J Physiol Regul Integr Comp Physiol 277: R658–R666, 1999. [DOI] [PubMed] [Google Scholar]
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci 5: 931–942, 2004. [DOI] [PubMed] [Google Scholar]
- Labuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopien B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep 62: 956–965, 2010. [DOI] [PubMed] [Google Scholar]
- Liang L, Tao B, Fan L, Yaster M, Zhang Y, Tao YX. mTOR and its downstream pathway are activated in the dorsal root ganglion and spinal cord after peripheral inflammation, but not after nerve injury. Brain Res 1513: 17–25, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32: 3591–3600, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol 587: 5469–5481, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178: 45–55, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: Other systems and conclusions. Experientia 42: 531–537, 1986. [DOI] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10: 151–162, 2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Genetic Basis for Respiratory Control Disorders, edited by Gaultier C. New York: Springer, 2007. [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003. [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 307: R1181–R1197, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Vinit S, Dougherty BJ, Mitchell GS. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp Neurol 266C: 1–10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol 112: 1678–1688, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter WB, O'Riordan KJ, Barnett D, Osting SM, Wagoner M, Burger C, Roopra A. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS One 5: e8996, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankin A, Hakimi AA, Hsieh JJ, Molina AM. Metastatic non-clear cell renal cell carcinoma: an evidence based review of current treatment strategies. Front Oncol 5: 67, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004. [DOI] [PubMed] [Google Scholar]
- Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol 237: 103–115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin. J Biol Chem 278: 37288–37296, 2003. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 26: 163–172, 2012. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol 217: 116–123, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JE, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci 28: 2949–2958, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JT, Zhao JY, Zhao X, Ligons D, Tiwari V, Atianjoh FE, Lee CY, Liang L, Zang W, Njoku D, Raja SN, Yaster M, Tao YX. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest 124: 592–603, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Fitzsimmons B, Steinauer J, O'Neill A, Newton AC, Hua XY, Yaksh TL. Spinal phosphinositide 3-kinase-Akt-mammalian target of rapamycin signaling cascades in inflammation-induced hyperalgesia. J Neurosci 31: 2113–2124, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]