Abstract
Dopamine is an essential neurotransmitter that plays an important role in a number of different physiological processes and disorders. There is substantial evidence that the neuropeptide neurotensin interacts with the mesolimbic dopamine system and can regulate dopamine neuron activity. In these studies we have used whole cell patch-clamp electrophysiology in brain slices from mice to examine how neurotensin regulates dopamine neuron activity by examining the effect of neurotensin on the inhibitory postsynaptic current generated by somatodendritic dopamine release (D2R IPSC) in ventral tegmental area (VTA) dopamine neurons. Neurotensin inhibited the D2R IPSC and activated an inward current in VTA dopamine neurons that appeared to be at least partially mediated by activation of a transient receptor potential C-type channel. Neither the inward current nor the inhibition of the D2R IPSC was affected by blocking PKC or calcium release from intracellular stores, and the inhibition of the D2R IPSC was greater with neurotensin compared with activation of other Gq-coupled receptors. Interestingly, the effects of neurotensin were not specific to D2R signaling as neurotensin also inhibited GABAB inhibitory postsynaptic currents in VTA dopamine neurons. Finally, the effects of neurotensin were significantly larger when intracellular Ca2+ was strongly buffered, suggesting that reduced intracellular calcium facilitates these effects. Overall these results suggest that neurotensin may inhibit the D2R and GABAB IPSCs downstream of receptor activation, potentially through regulation of G protein-coupled inwardly rectifying potassium channels. These studies provide an important advance in our understanding of dopamine neuron activity and how it is controlled by neurotensin.
Keywords: dopamine, neurotensin, D2R, GABAB, GIRK, calcium, TrpC
dopamine (DA) is an essential neurotransmitter involved in many different behaviors including motor behavior, incentive motivation, reward and reinforcement, learning, memory, drug intake, and habit formation (Wise 2004), and disruptions in DA signaling have been implicated in many disorders such as drug addiction, obesity, Parkinson's disease, and schizophrenia (Grace et al. 2007; Howes et al. 2015; Kenny 2011a,b; Lewis and Barker 2009). Most DA-producing neurons are found in the ventral tegmental area (VTA) and the substantia nigra pars compacta (SNc) of the midbrain (Wise 2004). At rest VTA/SNc DA neurons fire tonically (2–10 Hz) causing a baseline low level of DA at efferent target sites, but in response to a reward DA neurons fire in bursts causing phasic increases in DA release (Grace et al. 2007; Morikawa and Paladini 2011; Paladini and Roeper 2014). Phasic increases in DA release at efferent target sites are thought to be a salient signal and learning cue (Grace et al. 2007; Morikawa and Paladini 2011; Paladini and Roeper 2014). DA burst firing is primarily controlled by glutamatergic afferent inputs, but DA neuron activity can also be modulated by other neurotransmitters and neuropeptides acting either directly on DA neurons or indirectly through regulation of GABAergic or glutamatergic inputs to DA neurons (Grace et al. 2007; Morikawa and Paladini 2011; Paladini and Roeper 2014). Characterizing how DA neuron activity is regulated is important for understanding the function of DA under normal and pathological conditions.
In addition to releasing DA from their axon terminals, DA neurons release DA locally within the VTA/SNc from their soma and dendrites (Beckstead et al. 2004; Bjorklund and Lindvall 1975; Geffen et al. 1976; Kalivas and Duffy 1991; Rice et al. 1997). This somatodendritic DA release inhibits neighboring DA neurons through dopamine D2 receptor (D2R)-mediated activation of G-coupled inward rectifying potassium (GIRK) channels (Aghajanian and Bunney 1977; Beckstead et al. 2004; Lacey et al. 1987; Mercuri et al. 1997). D2Rs in VTA DA neurons regulate DA neuron activity and also regulate DA-mediated behaviors. For example, injection of quinpirole, a D2R agonist, directly into the VTA causes conditioned place aversion, blocks food-induced conditioned place preference, decreases food intake, and decreases cocaine-induced reinstatement (Liu et al. 2008; Xue et al. 2011). Furthermore selective knockout of autoreceptor D2Rs within midbrain DA neurons causes increased motor activity to a novel environment, increased food self-administration, and increased responses to cocaine such as increased locomotor activity and conditioned place preference compared with wild-type mice (Anzalone et al. 2012; Bello et al. 2011). In addition, DA neuron burst firing is followed by a pause, and it has been proposed that D2R-mediated inhibition terminates bursts of action potentials and is responsible for the pause following burst firing (Beckstead et al. 2004). Thus this autoinhibitory D2R signaling in the VTA plays an important role in the regulation of DA activity and DA-mediated behaviors.
Neurotensin is a tricapeptide that was first isolated and characterized from bovine hypothalamus (Carraway and Leeman 1973) and is widely expressed in both the central and peripheral nervous systems. The actions of neurotensin are mediated by three known neurotensin receptors: NTS1, NTS2, and NTS3 (for review, see Vincent et al. 1999). There is abundant evidence that neurotensin interacts with the DA system (for review, see Binder et al. 2001) and dysregulation of these interactions has been proposed to be involved in pathologies such as schizophrenia, drug abuse, and Parkinson's disease (Binder et al. 2001; Mustain et al. 2011; St-Gelais et al. 2006; Tanganelli et al. 2012). Fibers containing neurotensin heavily innervate midbrain DA neurons (Hokfelt et al. 1984; Woulfe and Beaudet 1989), and DA neurons of the VTA and SNc express neurotensin receptors, primarily the NTS1 receptor (Binder et al. 2001; Fassio et al. 2000; Lepee-Lorgeoux et al. 1999; Nicot et al. 1995; Palacios and Kuhar 1981; Szigethy and Beaudet 1989). Furthermore, D2Rs and NTS1 receptors have been shown to form heteromers in heterologous expression systems, which resulted in a decrease in D2R agonist binding and decreases in D2R signaling after treatment with neurotensin (Borroto-Escuela et al. 2013; Koschatzky et al. 2011). Previous research has shown that neurotensin modifies midbrain DA neuron activity through two NTS1 receptor-dependent mechanisms: increased DA neuron firing through activation of a nonselective cation channel (Farkas et al. 1996; Jiang et al. 1994; Jomphe et al. 2006; Shi and Bunney 1991; St-Gelais et al. 2004; Werkman et al. 2000) and a reduction in the inhibition of firing caused by D2R activation (Jomphe et al. 2006; Nimitvilai et al. 2012; Shi and Bunney 1990; Shi and Bunney 1991, 1992; Werkman et al. 2000). The majority of evidence suggests that the effects of neurotensin on DA neurons occur through activation of signaling pathways downstream of Gq-proteins, specifically through PKC, IP3, and calcium (Jomphe et al. 2006; Nimitvilai et al. 2013; St-Gelais et al. 2004; Thibault et al. 2011; Wu et al. 1995), although neurotensin has also been reported to affect DA neuron activity through a PKA dependent mechanism (Shi and Bunney 1992). In these studies we sought to examine the mechanisms by which neurotensin reduces the D2R-mediated inhibition of DA neuron activity by testing the hypothesis that neurotensin inhibits the D2R-mediated inhibitory postsynaptic current (D2R IPSC) (Beckstead et al. 2004) that occurs in response to the local, somatodendritic release of DA within the VTA.
MATERIALS AND METHODS
Animals.
Male C57BL/6J male mice (5–12 wk old) purchased from The Jackson Laboratories were used in all experiments. All protocols and procedures were approved by the Institutional Animal Care and Use Committee at Georgia State University and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Slice preparation and electrophysiology.
Acute brain slices were prepared as previously described (Roseberry et al. 2007). Briefly, adult male mice were anesthetized with isofluorane and decapitated. The brain was then removed and placed in carbogen (95% O2-5% CO2) saturated ice-cold artificial cerebral spinal fluid (aCSF), containing the following (in mM): 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.2 NaH2PO4, 1.2 MgCl2, 11.1 glucose, and 21.4 NaHCO3. A brain block containing the VTA was made and pseudohorizontal sections (220 μM) were cut with a vibrating blade microtome. Slices were then incubated in aCSF (∼35°C) containing 10 μM MK-801 {(+)-5-methyl-10, 11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate} for at least 30 min before recording. Slices were placed in a recording chamber and perfused with carbogen-saturated aCSF at a flow rate of ∼1–2 ml/min. Whole cell recordings were made using an Axon multiclamp 700B microelectrode amplifier and Axograph software. Putative DA neurons were identified by their location relative to the medial terminal nucleus of the accessory optic tract, the presence of hyperpolarization-activated cation currents (H current), the presence of spontaneous pacemaker firing, and the sensitivity to DA (Johnson and North 1992). Although recent studies have raised questions on the utility of using these measures to identify VTA DA neurons (Margolis et al. 2006), the characteristics described above have been widely used in electrophysiology studies to identify DA neurons within the VTA (Beckstead et al. 2004; Nimitvilai et al. 2012; Nimitvilai et al. 2013; Perra et al. 2011; Roseberry et al. 2007).
Electrodes (2.0–3.0 MΩ) were filled with a potassium gluconate (K-gluconate) based internal solution containing the following (in mM): 128 K gluconate, 10 NaCl, 1 MgCl2, 10 HEPES, 10 BAPTA, 2 ATP, 0.3 GTP, and 10 creatine phosphate. For the experiments testing the effects of neurotensin under reduced calcium buffering conditions, low-calcium buffering potassium methyl sulfate (KMeSO4) or K-gluconate-based internal solutions were used containing the followng: (in mM) 115 KMeSO4, 20 NaCl, 1 MgCl2, 10 HEPES, 0.1 EGTA, 2 ATP, 0.3 GTP, and 10 creatine phosphate; or the following (in mM) 128 K-gluconate, 10 NaCl, 1 MgCl2, 10 HEPES, 0.1 EGTA, 2 ATP, 0.3 GTP, and 10 creatine phosphate. No differences were observed between these two low calcium buffering internal solutions, so experiments using these two different internal solutions were pooled. Series resistance values were ∼3–15 MΩ. If the series resistance increased by >20% or if the IPSC or holding current was unstable in any of the experiments, the experiment was terminated and excluded from analysis. Neurons were voltage clamped at −60 mV for all experiments, and D2R IPSCs and GABAB IPSCs were evoked using a bipolar stimulating electrode placed 100–300 μM posterior to the recorded cell. D2R IPSCs were evoked with five stimuli (0.5 ms) at 40 Hz, and GABAB IPSCs were evoked with six stimuli (0.3 ms) at 50 Hz. D2R-mediated currents were also evoked using the iontophoretic application of DA. DA was applied iontophoretically through a ∼70–100 MΩ glass pipette filled with 1 M DA and ejected as a cation with a single pulse (10 nA, 25 ms). Leak of DA from the pipette was prevented with a constant negative back current (2 nA). To isolate D2R IPSCs, picrotoxin (100 μM), CGP 55845 (0.5 μM), and DNQX (10 μM) were included in the perfusion solution to block GABAA, GABAB, and AMPA receptors, respectively. GABAB IPSCs were isolated by including sulpiride (200 nM), picrotoxin (100 μM), and DNQX (10 μM) to block D2R, GABAA, and AMPA receptors, respectively. The peak amplitude of all IPSCs was measured from baseline and calculated as the mean current 30 ms before and after the peak IPSC amplitude. For all experiments cells were held for 10 min before drug application to allow for diffusion of the internal solution into the cell. For the experiments examining muscarinic acetylcholine receptor-induced currents, the nicotinic receptor antagonist mecamylamine (30 μM) was included both before and during application of acetylcholine. To determine the voltage current relationship and reversal potential of the neurotensin current, voltage ramps were applied (−120 to +40 mV at 160 mV/s or −120 to +20 mV at 140 mV/s) in the presence of tetrodotoxin (TTX).
Drugs.
The 8–13 active fragment of neurotensin (referred to as neurotensin) was used in all experiments. Neurotensin (8–13) was purchased from Bachem Americas (Torrance, CA). CGP 55845, SKF 96365, cyclopiazonic acid, and (R,S)-3,5-dihydroxyphenylglycine (DHPG) were purchased from Tocris Biosciences (Minneapolis, MN). Chelerythrine was purchased from Sigma Aldrich (St. Louis, MO). Mecamylamine and acetylcholine were generous gifts from the laboratory of Dr. Chun Jiang. All other reagents were from common commercial sources.
Data analysis and statistics.
Data are represented as the means ± SE unless otherwise noted. Data were analyzed using Axograph X (v1.3.5), LabChart (v7.3.6; ADInstruments), and Excel (v14.0; Microsoft) software. Statistics were calculated using Sigmastat (v11.0; Systat Software,). EC50 values were calculated using GraphPad Prism (v6.0f; GraphPad Software). Pearson's correlation coefficient was used to calculate correlation. All data were initially tested for normality using the Shapiro-Wilk test and were then analyzed with Student's t-tests, Mann-Whitney U-tests, ANOVAs, or a Kruskal-Wallis one-way ANOVA on ranks as appropriate with a significance level of P < 0.05 set a priori.
RESULTS
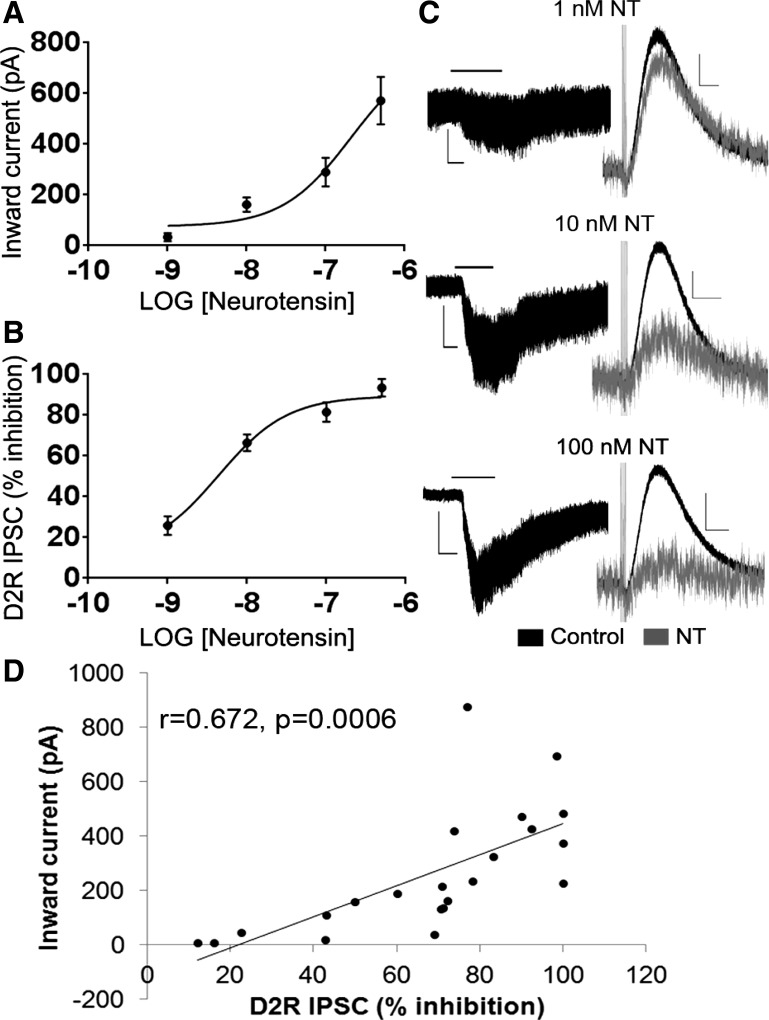
We initially examined how neurotensin affects DA neuron activity by assessing both the inward current activated by neurotensin and its ability to affect the inhibitory current generated by somatodendritic DA release in the VTA (D2R IPSC) (Beckstead et al. 2004). Neurotensin dose-dependently activated an inward current in DA neurons and inhibited the D2R IPSC (Fig. 1, A–C). Neurotensin also caused an increase in noise at all doses tested (Fig. 1C). The calculated maximal current and maximal inhibition of the D2R IPSC were 774.9 pA and 89.45% respectively, and the EC50 values of the effects of neurotensin on VTA DA neurons were 208.7 nM for the inward current and 4.38 nM for the inhibition of the D2R IPSC (Fig. 1, A and B). The inhibition of the D2R IPSC positively correlated with the magnitude of the inward current (r = 0.672, P = 0.0006; Fig. 1D), suggesting that neurotensin may activate the inward current and inhibit the D2R IPSC in VTA DA neurons through a common mechanism.
Fig. 1.
Neurotensin dose dependently activates an inward current and inhibits the inhibitory postsynaptic current generated by somatodendritic dopamine release (D2R IPSC) in ventral tegmental area (VTA) dopamine (DA) neurons. A: dose-response curve of the inward current induced by neurotensin. B: dose-response curve of the inhibitory effect of neurotensin on the D2R IPSC. C: sample traces of the neurotensin (NT)-induced current (left) and inhibition of the D2R IPSC (right). D: size of the inward current is positively correlated to the amount of inhibition of the D2R IPSC. Bars in C indicate time of neurotensin application; n = 5–7 cells from 4–7 mice for each dose. Scale bars = 50 pA/2 min (1-nM NT current); 100 pA/2 min (10-nM NT current); 200 pA/2 min (100-nM NT current); and 20 pA/500 ms (NT inhibition of D2R IPSC).
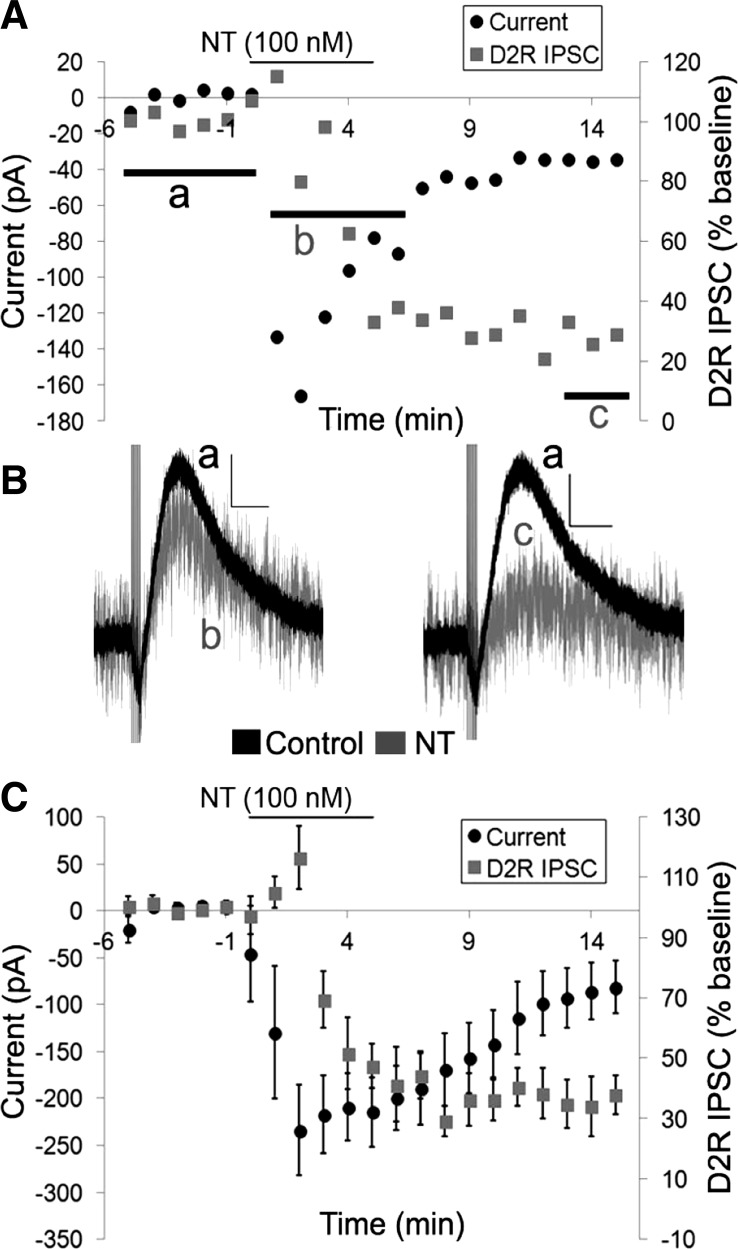
The timing of the activation of the inward current and the inhibition of the D2R IPSC differed, however. The inward current caused by 100 nM neurotensin reached its peak quickly, whereas the onset and peak of the inhibition of the D2R IPSC was delayed compared with the inward current (Fig. 2, A–C). The washout and recovery of the effects of neurotensin also differed as the neurotensin current and the increase in noise slowly reversed (in ∼10–15 min), while the inhibition of the D2R IPSC never recovered during the drug washout period (Fig. 2, A and C). Furthermore, the D2R IPSC remained inhibited 10 min after neurotensin application when the neurotensin current had almost completely recovered suggesting that the inward current is not simply occluding the D2R IPSC (Fig. 2, A–C). Thus, although the magnitude of the inward current correlated with the amount of inhibition of the D2R IPSC, there were differences in the timing of the two effects of neurotensin, suggesting that they may actually be mediated through different mechanisms.
Fig. 2.
The neurotensin-induced inward current precedes the inhibition of the D2R IPSC. A: sample cell of the effects of neurotensin (100 nM) on the inward current and the D2R IPSC. B: sample traces of the D2R IPSCs in A before neurotensin (a; black trace), during the peak of the neurotensin inward current (b; grey trace), and at 8–10 min after neurotensin washout (c; grey trace). C: mean responses of the effects of neurotensin (100 nM) on the inward current and the D2R IPSC. Bars in A and C indicate time of neurotensin application; n = 6 cells from 6 mice. Scale bars = 20 pA/500 ms.
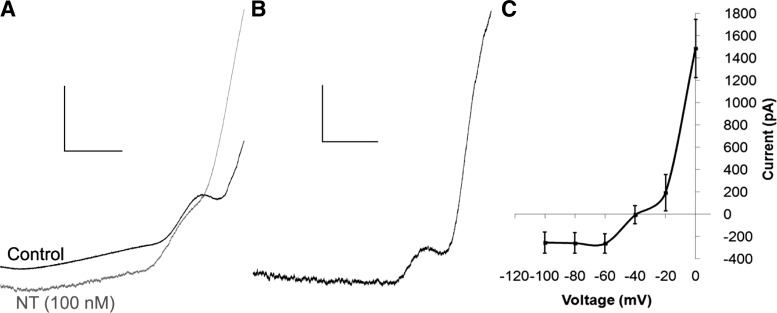
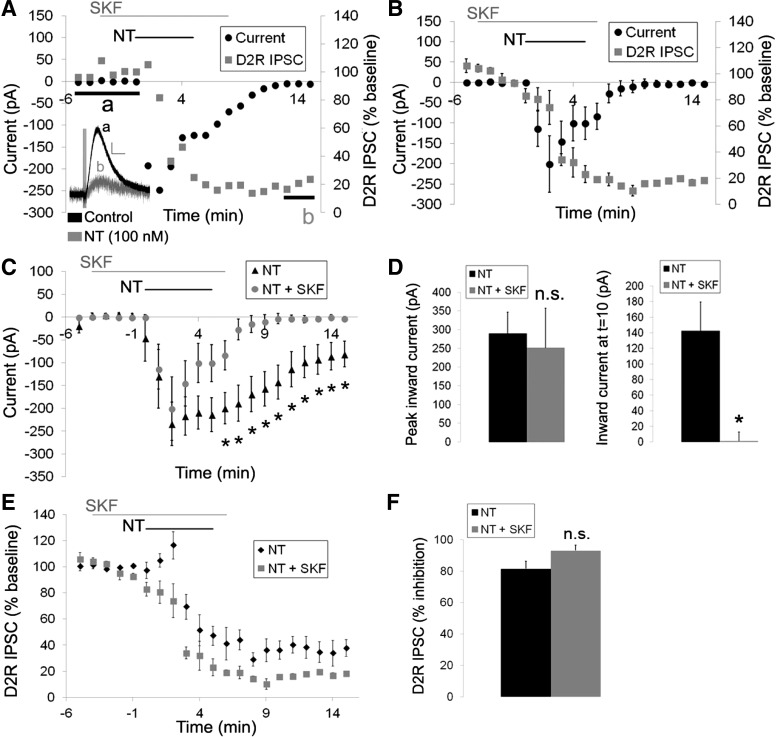
We next sought to confirm whether the neurotensin-caused inward current and inhibition of the D2R IPSC were independent by inhibiting the inward current and measuring the effect of neurotensin on the D2R ISPC. Thus we attempted to confirm previous experiments identifying the ion channels mediating the neurotensin-induced inward current in DA neurons. The neurotensin-induced current obtained from slow voltage ramps showed a unique current-voltage (I–V) relationship with outward rectification and a extended zero slope region around the reversal potential, which was calculated to be −36 ± 6.6 mV (Fig. 3). This unique I–V curve, combined with previous reports demonstrating that neurotensin activates a slow nonselective cation conductance permeable to Na+, K+, and Cs+ (Chien et al. 1996; Farkas et al. 1996; Jiang et al. 1994), suggests that neurotensin may be activating a member of the transient receptor potential C channel (TrpC) family (Kim et al. 2012; Zhang and Trebak 2014). Thus we tested whether SKF 96365, a TrpC channel blocker that was previously shown to block the neurotensin-caused increase in firing frequency in DA neurons (St-Gelais et al. 2004), could inhibit the neurotensin-induced inward current in VTA DA neurons. SKF 96365 (100 μM) partially inhibited the neurotensin-induced inward current (Fig. 4, C and D). Although the peak inward current caused by neurotensin (100 nM) was only slightly decreased by SKF 96365, the sustained neurotensin-induced current rapidly decreased, and the duration of the neurotensin-induced inward current was significantly shortened {Fig. 4, C and D; significant main effects of treatment [F(1,9) = 6.079, P < 0.05] and time [F(20,175) = 13.888, P < 0.001] and a significant treatment × time interaction [F(20,175) = 2.583, P < 0.001]}. In addition, the increase in noise caused by neurotensin also recovered more quickly during the washout and recovery of the neurotensin-induced current. We also tested whether SKF 96365 affected the ability of neurotensin to inhibit the D2R IPSC. SKF 96365 had no effect on the ability of neurotensin to inhibit the D2R IPSC, however, as the peak inhibition of the D2R IPSC caused by neurotensin was unaffected (Fig. 4, E and F). The D2R IPSC also remained inhibited throughout the experiment even when the neurotensin current recovered (Fig. 4, A and B), suggesting that the inward current and the inhibition of the D2R IPSC are likely independent and that the inward current caused by neurotensin does not simply occlude the D2R IPSC.
Fig. 3.
Current-voltage relationship of the neurotensin-induced current in VTA DA neurons. A: sample current traces resulting from slow voltage ramps (−120 to +20 mV at 140 mV/s) before (black trace) and after neurotensin (100 nM; grey trace). B: sample trace of the net neurotensin (100 nM)-induced current. C: mean current-voltage relationship of the neurotensin (100 nM)-induced current; n = 7 neurons from 4 mice. Scale bars = 1 nA/200 ms.
Fig. 4.
The transient receptor potential C channel (TrpC) channel inhibitor SKF 96365 partially blocks the neurotensin-induced inward current but does not affect the inhibition of the D2R IPSC. A: sample cell of the effects of neurotensin (100 nM) on the inward current and the D2R IPSC in the presence of SKF 96365 (100 μM). Inset: sample trace of the D2R IPSC before neurotensin (a; black trace) and 8–10 min after neurotensin washout (b; grey trace) in the presence of SKF 96365. B: mean responses of the effects of neurotensin (100 nM) on the inward current and the D2R IPSC in the presence of SKF 96365 (100 μM). C: mean inward current generated by neurotensin (100 nM) in the absence and presence of SKF 96365 (100 μM). D: mean neurotensin-induced current at the peak and 5 min after neurotensin washout in the presence and absence of SKF 96365. E and F: mean effect of neurotensin (100 nM) on the D2R IPSC in the absence and presence of SKF 96365 (100 μM). Bars in A–C and E indicate time of neurotensin and SKF 96365 application; n = 5–6 neurons from 4–6 mice for each group. Scale bar = 20 pA/500 ms. *P < 0.05 vs. neurotensin alone.
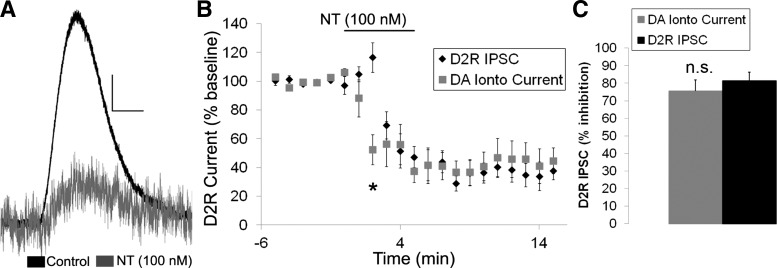
We next sought to confirm that the effects of neurotensin on the D2R IPSC were mediated postsynaptically and not through alterations in DA release by testing whether neurotensin also inhibited the D2R-mediated current generated by the iontophoretic application of DA. As predicted, neurotensin inhibited the iontophoresis evoked D2R current (Fig. 5A) to the same magnitude as the electrically evoked D2R IPSC (Fig. 5, B and C). Thus neurotensin appears to act at the postsynaptic membrane to inhibit the D2R IPSC and not through a presynaptic change in DA release.
Fig. 5.
Neurotensin inhibits the D2R-mediated current caused by DA iontophoresis. A: sample trace of the effect of neurotensin (100 nM) on the D2R-mediated current caused by DA iontophoresis. B and C: mean effect of neurotensin (100 nM) on the D2R-mediated current caused by DA iontophoresis and on the D2R IPSC evoked with electrical stimulation. Bar in B indicates time of neurotensin application; n = 6 neurons from 5–6 mice for each group. Scale bar = 20 pA/500 ms. *P < 0.05.
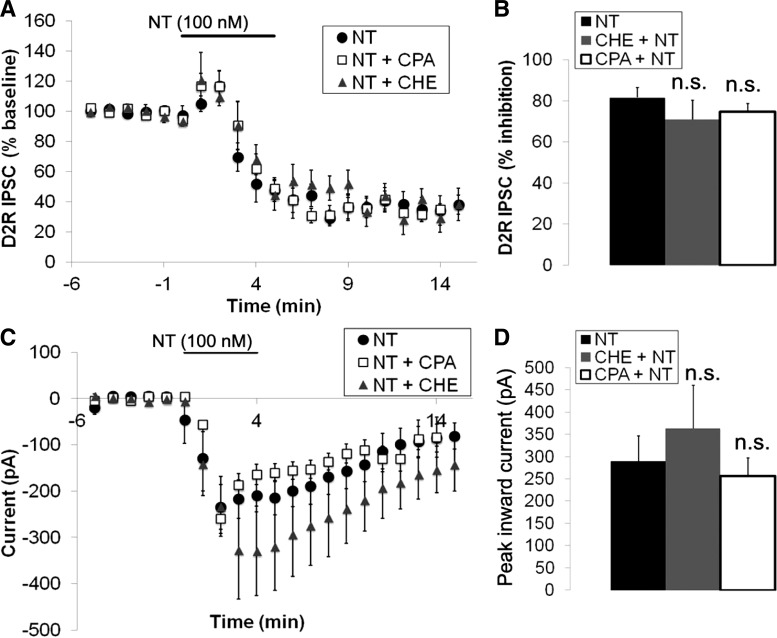
We next attempted to identify the mechanism by which neurotensin inhibits the D2R IPSC. Previous studies suggested that neurotensin inhibits D2R signaling through a Ca2+- and PKC-dependent mechanism (Jomphe et al. 2006; Nimitvilai et al. 2013; Thibault et al. 2011). Therefore, we tested the role of the release of Ca2+ from intracellular stores in the neurotensin-caused inhibition of the D2R IPSC through the use of the reversible sarcoplasmic reticulum Ca2+ ATPase inhibitor cycopiazonic acid (CPA). Pretreatment of brain slices with CPA (10 μM) for at least 15 min before neurotensin application had no effect on the neurotensin (100 nM) inhibition of the D2R IPSC (Fig. 6, A and B). In addition CPA had no effect on the peak inward current induced by neurotensin (Fig. 6, C and D). We also tested whether neurotensin inhibits the D2R IPSC through a PKC-dependent process through the use of the nonspecific PKC inhibitor chelerythrine. Chelerythrine (10 μM) also had no effect on the neurotensin-induced inhibition of the D2R IPSC (Fig. 6, A and B), and there was no significant difference between the peak inward current induced by neurotensin after chelerythrine treatment compared with neurotensin alone (Fig. 6, C and D). Thus, in contrast to previous reports (Jomphe et al. 2006; Nimitvilai et al. 2013; Thibault et al. 2011), the inhibition of the D2R IPSC in VTA DA neurons by neurotensin does not appear to depend on PKC activation, nor does it depend on release of Ca2+ from intracellular stores.
Fig. 6.
Neurotensin inhibition of the D2R IPSC and the neurotensin-induced current is not dependent on PKC activity or release of Ca2+ from intracellular stores. A and B: mean inhibition of the D2R IPSC caused by neurotensin (100 nM) in the presence and absence of cycopiazonic acid (CPA; 10 μM) or chelerythrine (CHE; 10 μM). C and D: mean neurotensin (100 nM)-induced current in the presence and absence of CPA (10 μM) or CHE (10 μM). Bars in A and C indicate time of neurotensin application; n = 5–6 cells from 4–6 mice for each group.
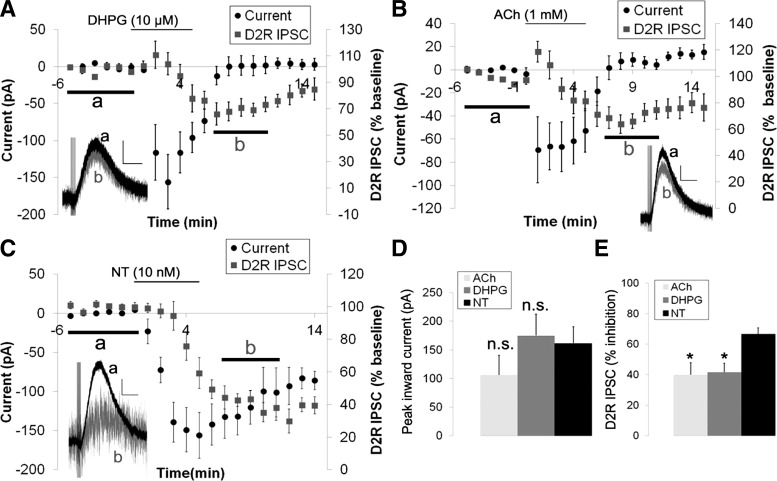
We next tested whether the inhibition of the D2R IPSC was specific to neurotensin or could be achieved by activation of other Gq-coupled receptors by examining whether activation of metabotropic glutamate receptors or muscarinic acetylcholine receptors also inhibited the D2R IPSC. To activate muscarinic receptors, acetylcholine (ACh) was added in the presence of mecamylamine (30 μM), a nicotinic acetylcholine receptor antagonist. For this experiment, we chose doses of the metabotropic glutamate receptor agonist DHPG (10 μM), ACh (1 mM), and neurotensin (10 nM) that caused approximately the same peak inward current to allow for direct comparison of their effects on the D2R IPSC (Fig. 7, A–D). There were no significant differences between the peak current activated by DHPG, ACh, and neurotensin [F(2,16) = 1.217, P = 0.322; Fig. 7D], but there were significant differences in the inhibition of the D2R IPSC, as neurotensin inhibited the D2R IPSC to a significantly greater extent than DHPG or ACh [F(2,16) = 5.181, P < 0.05; Fig. 7E]. In addition, the effects of DHPG and ACh on the D2R IPSC started to reverse upon removal of the agonist, whereas the effects of neurotensin on the D2R IPSC showed no reversal (Fig. 7, A–C). Thus it appears that activation of Gq-coupled receptors can inhibit the D2R IPSC, but activation of NTS1 appears to engage an additional mechanism that causes significantly greater inhibition of the D2R IPSC.
Fig. 7.
Neurotensin inhibits D2R IPSC significantly more than (R,S)-3,5-dihydroxyphenylglycine (DHPG) or acetylcholine. A: mean inward current and inhibition of the D2R IPSC caused by DHPG (10 μM). Inset: sample trace of the D2R IPSC before DHPG (a; black trace) and at 7–11 min after applying DHPG (b; grey trace). B: mean inward current and inhibition of the D2R IPSC caused by ACh (1 mM) in the presence of mecamylamine (30 μM). Inset: Sample trace of the D2R IPSC before ACh (a; black trace) and at 7–11 min after applying ACh (b; grey trace). C: mean inward current and inhibition of the D2R IPSC caused by neurotensin (10 nM). Inset: sample trace of the D2R IPSC before neurotensin (a; black trace) and at 7–11 min after applying neurotensin (b; grey trace). D: peak inward currents caused by DHPG (10 μM), ACh (1 mM), and neurotensin (10 nM) were not significantly different. E: neurotensin (10 nM) inhibited the D2R IPSC significantly more than DHPG and ACh. Bars in A–C indicate time of drug application. DHPG: n = 5 cells from 4 mice; ACh: n = 8 cells from 7 mice; neurotensin: n = 6 cells from 5 mice. Scale bars = 30 pA/500 ms. *P < 0.05 vs. neurotensin.
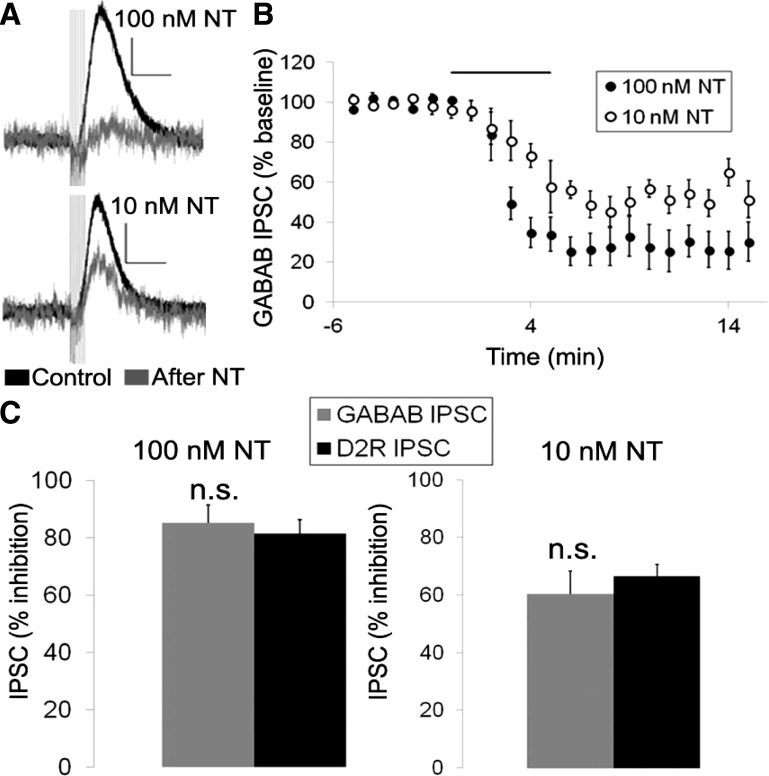
We next sought to test whether the ability of neurotensin to inhibit the D2R IPSC was specific to D2Rs or if it would also affect other inhibitory responses in VTA DA neurons. GABAB receptors and D2Rs both inhibit VTA DA neurons through the activation of G protein-coupled inward rectifying potassium channels (GIRK channels). Thus we next tested whether neurotensin also affected the IPSC generated by activation of GABAB receptors (GABAB IPSC) (Fig. 8, A and B). Interestingly, neurotensin inhibited the GABAB IPSC to a similar degree as the D2R IPSC (Fig. 8C). This was true for both the maximal dose (100 nM) of neurotensin that fully inhibited the D2R IPSC and an intermediate dose (10 nM) closer to the EC50 value for neurotensin inhibition of the D2R IPSC (Fig. 8C). Thus neurotensin does not appear to specifically inhibit the D2R IPSC in VTA DA neurons but can also inhibit the GABAB IPSC, possibly through a common mechanism.
Fig. 8.
Neurotensin inhibits the GABAB IPSC in VTA DA neurons. A and B: sample traces (A) and mean effect (B) of neurotensin (100 and 10 nM; grey trace) on the GABAB IPSC. C: neurotensin (100 and 10 nM) inhibited the GABAB and D2R IPSCs by the same magnitude. Bar in B indicates time of NT application; n = 5–8 cells from 4–6 mice for each group. Scale bars = 20 pA/300 ms.
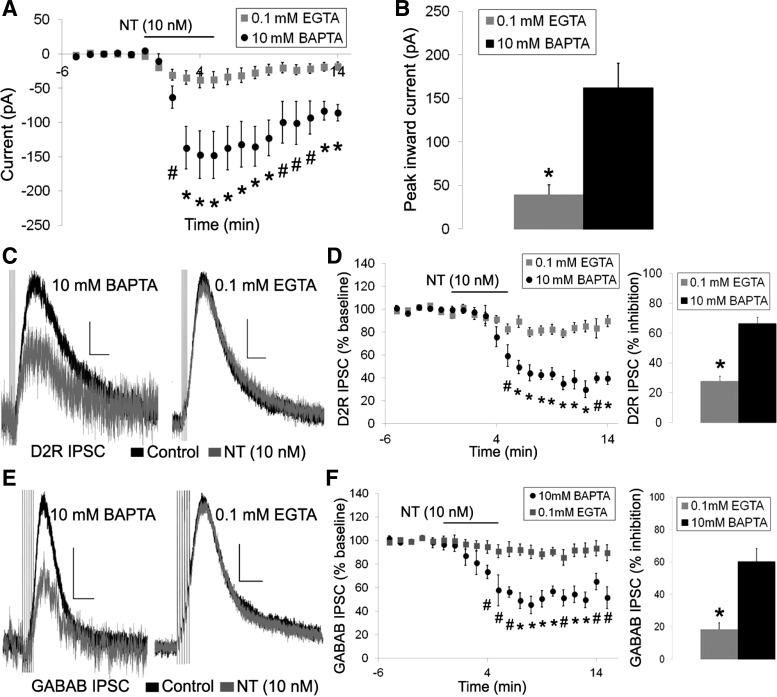
We next sought to examine the similarity of the inhibition of the D2R IPSC and the GABAB IPSC in more detail. The D2R and GABAB IPSCs differ in their sensitivity to intracellular Ca2+ levels as the D2R IPSC shows increased desensitization and long-term depression when intracellular free Ca2+ levels are weakly buffered, whereas the GABAB IPSC is insensitive to changes in intracellular Ca2+ (Beckstead and Williams 2007). Thus we next tested whether the effects of neurotensin on the D2R and GABAB IPSCs were affected by basal intracellular Ca2+ levels by switching from a high Ca2+ buffering internal solution (containing 10 mM BAPTA = low levels of basal free intracellular Ca2+) to a low Ca2+ buffering internal solution (containing 0.1 mM EGTA = higher levels of basal free intracellular Ca2+). A dose of neurotensin near the EC50 value for inhibition of the D2R IPSC was used in these experiments to allow for the identification of potential increases or decreases in the effect of neurotensin on the D2R and GABAB IPSCs with the reduced calcium buffering internal solution. Neurotensin (10 nM) still caused an inward current and slightly reduced both the D2R and GABAB IPSCs when measured with a 0.1 mM EGTA internal solution (Fig. 9). Interestingly, the inward current induced by neurotensin was significantly reduced compared with the inward current with the internal solution containing 10 mM BAPTA {Fig. 9, A and B; significant main effects of treatment [F(1, 15) = 23.357, P < 0.001] and time [F(19,250) = 33.674, P < 0.001] and significant treatment × time interaction [F(19,250) = 14.087, P < 0.001]}. The neurotensin-caused inhibition of both the D2R and GABAB IPSCs were also significantly reduced with the 0.1 mM EGTA internal solution compared with the 10 mM BAPTA internal solution with no differences in the magnitude of inhibition of the D2R IPSC vs. the GABAB ISPC {Fig. 9, C–F; D2R IPSC: significant main effects of treatment [F(1,17) = 32.102, P < 0.001] and time [F(19,288) = 33.157, P < 0.001] and a significant treatment × time interaction [F(19,288) = 11.708, P < 0.001]; GABAB IPSC: significant main effects of treatment [F(1,12) = 17.439, P = 0.001] and time [F(20,240) = 18.321, P < 0.001] and a significant treatment × time interaction [F(20,240) = 8.165, P < 0.001]}. Thus the effects of neurotensin on DA neurons were greater when intracellular Ca2+ was strongly buffered and resting levels of free intracellular Ca2+ were low and were attenuated when intracellular Ca2+ was weakly buffered and resting levels of free intracellular Ca2+ were high.
Fig. 9.
Reduced buffering of intracellular calcium attenuates the effects of neurotensin. A and B: mean neurotensin (10 nM)-induced inward current with an internal solution containing 10 mM BAPTA or 0.1 mM EGTA. C–F: sample traces (C and E) and mean effect (D and F) of neurotensin (10 nM; grey trace) on the D2R IPSC (C and D) and GABAB IPSC (E and F) using internal solutions containing 10 mM BAPTA or 0.1 mM EGTA. Bars in A, D, and F indicate time of neurotensin application; n = 5–12 cells from 4–11 mice for each group. Scale bars = 20 pA/400 ms (C) and 20 pA/200 ms (D). #P < 0.05, *P ≤ 0.001.
DISCUSSION
In these studies we have demonstrated that neurotensin increases DA neuron activity through multiple mechanisms. In addition to directly activating an inward current to increase DA neuron activity, neurotensin also significantly reduced the inhibition of DA neurons caused by activation of both D2R and GABAB receptors. These effects did not appear to depend on release of Ca2+ from intracellular stores or on PKC activation but were sensitive to basal levels of free intracellular Ca2+.
The effects of neurotensin on the inward current and the D2R and GABAB IPSCs appear to be independent and mediated by different mechanisms. The timing of neurotensin inhibition of the D2R IPSC was significantly delayed compared with the inward current caused by neurotensin, and the neurotensin-induced current recovered during the washout period after neurotensin application, whereas the inhibition of the D2R IPSC did not. The EC50 values for the two effects of neurotensin were also different further supporting the argument that the two effects of neurotensin occur through separate mechanisms. The EC50 value for inhibition of the D2R IPSC by neurotensin was ∼4 nM, much lower than the EC50 value for the neurotensin-induced current (∼200 nM), and lower doses of neurotensin (1–10 nM) have often been used previously to examine the effect of neurotensin on D2R signaling (Jomphe et al. 2006; Nimitvilai et al. 2012; Shi and Bunney 1990; Shi and Bunney 1991, 1992; Werkman et al. 2000) while higher doses of neurotensin (1 nM to 5 μM) have been used to examine the neurotensin-induced inward current in DA neurons (Farkas et al. 1996; Jiang et al. 1994; Jomphe et al. 2006; Shi and Bunney 1991; St-Gelais et al. 2004; Werkman et al. 2000). In addition, partially inhibiting the neurotensin current with SKF 96365 did not have any effect on the neurotensin-caused inhibition of the D2R IPSC. Finally, ACh-, DHPG-, and neurotensin-caused inward currents that were similar in magnitude, but neurotensin had a much larger effect on the D2R IPSC. If the inward current caused the inhibition of the D2R IPSC, then it would be expected that neurotensin, ACh, and DHPG would inhibit the D2R IPSC to the same magnitude when activating inward currents of the same size. In agreement with our findings, it was previously reported that neurotensin reduces quinpirole (D2R agonist)-induced inhibition of DA activity even when the excitatory effect of neurotensin is blocked with heparin, an IP3 receptor antagonist (Jomphe et al. 2006). Therefore, it appears that the neurotensin inward current does not block the D2R IPSC because of occlusion or net excitation and that these are independent effects downstream of NTS1 activation.
The neurotensin-induced inward current has been characterized as a slow nonselective cation current that is equally permeable to both Na+ and K+, a characteristic of Trp channels (Farkas et al. 1996). The neurotensin-induced current in DA neurons was similar to that generated by activation of specific TrpC channels expressed in HEK-293 cells (Kim et al. 2012; Zhang and Trebak 2014), suggesting that neurotensin may be activating a member of the TrpC family, potentially through the activation of phospholipase C and release of DAG (Harteneck and Gollasch 2011; Pena and Ordaz 2008). We found that the TrpC channel blocker SKF 96365 significantly shortened the duration of the neurotensin-induced current, which is in agreement with a previous report showing that SKF 96365 blocks the neurotensin-caused increase in DA neuron firing frequency (St-Gelais et al. 2004). Thus it appears that the neurotensin-activated inward current is at least partially mediated by activation of TrpC channels in VTA DA neurons.
The majority of evidence suggests that the effects of neurotensin on DA neurons are mediated by signals that are downstream of PLC activation. Previously, it was reported that the neurotensin-induced inward current and increase in firing frequency is dependent on Ca2+ and the IP3 receptor (Jomphe et al. 2006; St-Gelais et al. 2004; Wu et al. 1995). Another study found that the neurotensin-induced inward current was not dependent on Ca2+, however, as the neurotensin current was not affected by buffering intracellular Ca2+ with 20 mM BAPTA (Farkas et al. 1996). In addition, it has also been reported that neurotensin inhibits D2R signaling through a PKC- and Ca2+-dependent mechanism (Jomphe et al. 2006; Nimitvilai et al. 2013; Thibault et al. 2011). In contrast to these previous reports, we found that the neurotensin inhibition of the D2R IPSC and the neurotensin-induced current was not dependent on PKC or Ca2+ and the effects of neurotensin were actually potentiated when intracellular Ca2+ was buffered with 10 mM BAPTA. In addition, the neurotensin inhibition of the D2R IPSC and the inward current was not dependent on Ca2+ release from intracellular stores. Interestingly, it has also been reported that neurotensin reduces the DA-caused inhibition of DA neuronal firing through the cAMP pathway and not through a PKC dependent mechanism (Shi and Bunney 1992). Additional experiments are needed to resolve these differences, however, and to determine if the effects of neurotensin occur through dual pathways.
Although neurotensin likely inhibits the D2R IPSC via activation of Gq-coupled signaling through a mechanism similar to metabotropic glutamate receptors and muscarinic acetylcholine receptors, here we have shown that there appears to be an additional mechanism activated by neurotensin to inhibit the D2R IPSC to a larger extent than other Gq-coupled receptors. Interestingly, we also found that the effects of neurotensin were not specific to the D2R IPSC as neurotensin also inhibited the GABAB IPSC to the same magnitude. This suggests that the effects of neurotensin are likely not due to modulation of D2R activity by direct heterodimer interactions with NTS1 as has been observed in HEK-293 cells (Borroto-Escuela et al. 2013; Koschatzky et al. 2011). Previously, it was reported that neurotensin does not block GABA-caused inhibition of DA neuronal firing (Shi and Bunney 1991). GABA inhibits DA neurons through the activation of both GABAA and GABAB receptors, however, so neurotensin may only block the inhibition produced by GABAB receptors and not GABAA receptors, which could explain the differences in these results. Thus the results presented here suggest that neurotensin inhibits both GABAB and D2R signaling in VTA DA neurons.
In these studies, we have shown a novel mechanism for modulation of VTA DA neuron activity by neurotensin: inhibition of GABAB IPSCs. GABAB receptors and D2Rs both activate GIRK channels to inhibit DA neurons, so it is possible that neurotensin modulates GIRK channel activity downstream of both D2R and GABAB activation to reduce D2R- and GABAB-caused inhibition of DA neurons (Fig. 10). In support of this notion, it was previously shown that neurotensin blocks D2R signaling downstream of the D2R (Farkas et al. 1997). In addition, it was recently reported that inducing high-frequency bursting or depolarization in VTA DA neurons causes potentiation of GIRK currents and this potentiation was due to modulation to the GIRK channels themselves rather than regulation of the GABAB or D2 receptors (Lalive et al. 2014). Thus neurotensin may reduce both D2R- and GABAB-mediated GIRK currents by causing direct modulation of GIRK channels, although further experiments will be required to test this hypothesis.
Fig. 10.
Diagram of the proposed model of neurotensin inhibition of G-coupled inward rectifying potassium (GIRK) currents activated by D2 and GABAB receptors. Activation of NTS1 with neurotensin in VTA DA neurons causes robust inhibition of D2R and GABAB GIRK currents when the relative levels of free intracellular Ca2+ are low due to strong Ca2+ buffering, while neurotensin-induced inhibition of D2R and GABAB GIRK currents is significantly attenuated when relative levels of free intracellular Ca2+ are higher due to weak Ca buffering.
D2R IPSC desensitization and long-term depression have been reported to increase when intracellular Ca2+ is weakly buffered and free intracellular Ca2+ levels are high, whereas GABAB IPSCs do not show the same sensitivity to intracellular Ca2+ levels (Beckstead and Williams 2007). Combined with the ability of neurotensin to increase Ca2+ in DA neurons (Jomphe et al. 2006; St-Gelais et al. 2004), these results suggest that reducing the Ca2+ buffering capacity of the internal solution would result in a larger effect of neurotensin on the D2R IPSC but not the GABAB IPSC. Surprisingly, the neurotensin-caused inhibition of both the GABAB and D2R IPSCs was significantly attenuated with low intracellular Ca2+ buffering (i.e., 0.1 mM EGTA solution). Thus neurotensin appears to inhibit GIRK current activation downstream of D2R and GABAB receptors through a Ca2+-sensitive mechanism, whereby low levels of free intracellular calcium are required for the full inhibition of GIRK channel activation (Fig. 10). Alternatively, it is also possible that the NTS1 receptor is Ca2+ sensitive. We found that the neurotensin-induced inward current was also significantly reduced with low intracellular Ca2+ buffering. Thus all effects of neurotensin in DA neurons were reduced with low intracellular Ca2+ buffering and high intracellular levels of free Ca2+. Therefore, it is possible that the NTS1 receptor may be sensitive to free intracellular Ca2+ levels and may desensitize or internalize with high levels of free intracellular Ca2+ resulting in a reduced effect of neurotensin, although future studies will be required to test this hypothesis.
In summary, we have demonstrated that neurotensin affects DA neuron activity through two seemingly independent effects: direct activation of an inward current mediated in part by TrpC channels, and inhibition of both the D2R and GABAB IPSCs. Overall, these studies advance our understanding of how neurotensin regulates DA neuron activity, and further research characterizing how neurotensin affects DA neuron activity may lead to a better understanding and treatments of disorders caused by a disruption in the function of the mesolimbic DA system.
GRANTS
Funding for these studies was provided by the Department of Biology and the Brains and Behavior Program at Georgia State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.S. and A.G.R. conception and design of research; K.S. performed experiments; K.S. analyzed data; K.S. and A.G.R. interpreted results of experiments; K.S. prepared figures; K.S. drafted manuscript; K.S. and A.G.R. edited and revised manuscript; K.S. and A.G.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Anna I. Dunigan for helpful comments and suggestions during the preparation of this manuscript.
REFERENCES
- Aghajanian GK, Bunney BS. Dopamine “autoreceptors”: pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedebergs Arch Pharmacol 297: 1–7, 1977. [DOI] [PubMed] [Google Scholar]
- Anzalone A, Lizardi-Ortiz JE, Ramos M, De Mei C, Hopf FW, Iaccarino C, Halbout B, Jacobsen J, Kinoshita C, Welter M, Caron MG, Bonci A, Sulzer D, Borrelli E. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci 32: 9023–9034, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron 42: 939–946, 2004. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Williams JT. Long-term depression of a dopamine IPSC. J Neurosci 27: 2074–2080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci 14: 1033–1038, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacol Rev 53: 453–486, 2001. [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res 83: 531–537, 1975. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Ravani A, Tarakanov AO, Brito I, Narvaez M, Romero-Fernandez W, Corrales F, Agnati LF, Tanganelli S, Ferraro L, Fuxe K. Dopamine D2 receptor signaling dynamics of dopamine D2-neurotensin 1 receptor heteromers. Biochem Biophys Res Commun 435: 140–146, 2013. [DOI] [PubMed] [Google Scholar]
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem 248: 6854–6861, 1973. [PubMed] [Google Scholar]
- Chien PY, Farkas RH, Nakajima S, Nakajima Y. Single-channel properties of the nonselective cation conductance induced by neurotensin in dopaminergic neurons. Proc Natl Acad Sci USA 93: 14917–14921, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas RH, Chien PY, Nakajima S, Nakajima Y. Neurotensin and dopamine D2 activation oppositely regulate the same K+ conductance in rat midbrain dopaminergic neurons. Neurosci Lett 231: 21–24, 1997. [DOI] [PubMed] [Google Scholar]
- Farkas RH, Chien PY, Nakajima S, Nakajima Y. Properties of a slow nonselective cation conductance modulated by neurotensin and other neurotransmitters in midbrain dopaminergic neurons. J Neurophysiol 76: 1968–1981, 1996. [DOI] [PubMed] [Google Scholar]
- Fassio A, Evans G, Grisshammer R, Bolam JP, Mimmack M, Emson PC. Distribution of the neurotensin receptor NTS1 in the rat CNS studied using an amino-terminal directed antibody. Neuropharmacology 39: 1430–1442, 2000. [DOI] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature 260: 258–260, 1976. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30: 220–227, 2007. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Gollasch M. Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels. Curr Pharm Biotechnol 12: 35–41, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Everitt BJ, Theodorsson-Norheim E, Goldstein M. Occurrence of neurotensinlike immunoreactivity in subpopulations of hypothalamic, mesencephalic, and medullary catecholamine neurons. J Comp Neurol 222: 543–559, 1984. [DOI] [PubMed] [Google Scholar]
- Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol 29: 97–115, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Pessia M, North RA. Neurotensin excitation of rat ventral tegmental neurones. J Physiol 474: 119–129, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol 450: 455–468, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomphe C, Lemelin PL, Okano H, Kobayashi K, Trudeau LE. Bidirectional regulation of dopamine D2 and neurotensin NTS1 receptors in dopamine neurons. Eur J Neurosci 24: 2789–2800, 2006. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. A comparison of axonal and somatodendritic dopamine release using in vivo dialysis. J Neurochem 56: 961–967, 1991. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci 12: 638–651, 2011a. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Reward Mechanisms in Obesity: New Insights and Future Directions. Neuron 69: 664–679, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim J, Jeon JP, Myeong J, Wie J, Hong C, Kim HJ, Jeon JH, So I. The roles of G proteins in the activation of TRPC4 and TRPC5 transient receptor potential channels. Channels (Austin) 6: 333–343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschatzky S, Tschammer N, Gmeiner P. Cross-receptor interactions between dopamine D2L and neurotensin NTS1 receptors modulate binding affinities of dopaminergics. ACS Chem Neurosci 2: 308–316, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol 392: 397–416, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalive AL, Munoz MB, Bellone C, Slesinger PA, Luscher C, Tan KR. Firing modes of dopamine neurons drive bidirectional GIRK channel plasticity. J Neurosci 34: 5107–5114, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepee-Lorgeoux I, Betancur C, Rostene W, Pelaprat D. Differential ontogenetic patterns of levocabastine-sensitive neurotensin NT2 receptors and of NT1 receptors in the rat brain revealed by in situ hybridization. Brain Res Dev Brain Res 113: 115–131, 1999. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Barker RA. Understanding the dopaminergic deficits in Parkinson's disease: insights into disease heterogeneity. J Clin Neurosci 16: 620–625, 2009. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Shin R, Ikemoto S. Dual role of medial A10 dopamine neurons in affective encoding. Neuropsychopharmacology 33: 3010–3020, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol 577: 907–924, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, Borrelli E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience 79: 323–327, 1997. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Paladini CA. Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms. Neuroscience 198: 95–111, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustain WC, Rychahou PG, Evers BM. The role of neurotensin in physiologic and pathologic processes. Curr Opin Endocrinol Diabetes Obes 18: 75–82, 2011. [DOI] [PubMed] [Google Scholar]
- Nicot A, Rostene W, Berod A. Differential expression of neurotensin receptor mRNA in the dopaminergic cell groups of the rat diencephalon and mesencephalon. J Neurosci Res 40: 667–674, 1995. [DOI] [PubMed] [Google Scholar]
- Nimitvilai S, McElvain MA, Arora DS, Brodie MS. Reversal of quinpirole inhibition of ventral tegmental area neurons is linked to the phosphatidylinositol system and is induced by agonists linked to G(q). J Neurophysiol 108: 263–274, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, McElvain MA, Brodie MS. Reversal of dopamine D2 agonist-induced inhibition of ventral tegmental area neurons by Gq-linked neurotransmitters is dependent on protein kinase C, G protein-coupled receptor kinase, and dynamin. J Pharmacol Exp Ther 344: 253–263, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios JM, Kuhar MJ. Neurotensin receptors are located on dopamine-containing neurones in rat midbrain. Nature 294: 587–589, 1981. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Roeper J. Generating bursts (and pauses) in the dopamine midbrain neurons. Neuroscience 282C: 109–121, 2014. [DOI] [PubMed] [Google Scholar]
- Pena F, Ordaz B. Non-selective cation channel blockers: potential use in nervous system basic research and therapeutics. Mini Rev Med Chem 8: 812–819, 2008. [DOI] [PubMed] [Google Scholar]
- Perra S, Clements MA, Bernier BE, Morikawa H. In vivo ethanol experience increases D(2) autoinhibition in the ventral tegmental area. Neuropsychopharmacology 36: 993–1002, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J Neurophysiol 77: 853–862, 1997. [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Painter T, Mark GP, Williams JT. Decreased vesicular somatodendritic dopamine stores in leptin-deficient mice. J Neurosci 27: 7021–7027, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WS, Bunney BS. Neurotensin attenuates dopamine D2 agonist quinpirole-induced inhibition of midbrain dopamine neurons. Neuropharmacology 29: 1095–1097, 1990. [DOI] [PubMed] [Google Scholar]
- Shi WX, Bunney BS. Neurotensin modulates autoreceptor mediated dopamine effects on midbrain dopamine cell activity. Brain Res 543: 315–321, 1991. [DOI] [PubMed] [Google Scholar]
- Shi WX, Bunney BS. Roles of intracellular cAMP and protein kinase A in the actions of dopamine and neurotensin on midbrain dopamine neurons. J Neurosci 12: 2433–2438, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Gelais F, Jomphe C, Trudeau LE. The role of neurotensin in central nervous system pathophysiology: what is the evidence? J Psychiatry Neurosci 31: 229–245, 2006. [PMC free article] [PubMed] [Google Scholar]
- St-Gelais F, Legault M, Bourque MJ, Rompre PP, Trudeau LE. Role of calcium in neurotensin-evoked enhancement in firing in mesencephalic dopamine neurons. J Neurosci 24: 2566–2574, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szigethy E, Beaudet A. Correspondence between high affinity 125I-neurotensin binding sites and dopaminergic neurons in the rat substantia nigra and ventral tegmental area: a combined radioautographic and immunohistochemical light microscopic study. J Comp Neurol 279: 128–137, 1989. [DOI] [PubMed] [Google Scholar]
- Tanganelli S, Antonelli T, Tomasini MC, Beggiato S, Fuxe K, Ferraro L. Relevance of dopamine D(2)/neurotensin NTS1 and NMDA/neurotensin NTS1 receptor interaction in psychiatric and neurodegenerative disorders. Curr Med Chem 19: 304–316, 2012. [DOI] [PubMed] [Google Scholar]
- Thibault D, Albert PR, Pineyro G, Trudeau LE. Neurotensin triggers dopamine D2 receptor desensitization through a protein kinase C and beta-arrestin1-dependent mechanism. J Biol Chem 286: 9174–9184, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JP, Mazella J, Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol Sci 20: 302–309, 1999. [DOI] [PubMed] [Google Scholar]
- Werkman TR, Kruse CG, Nievelstein H, Long SK, Wadman WJ. Neurotensin attenuates the quinpirole-induced inhibition of the firing rate of dopamine neurons in the rat substantia nigra pars compacta and the ventral tegmental area. Neuroscience 95: 417–423, 2000. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 5: 483–494, 2004. [DOI] [PubMed] [Google Scholar]
- Woulfe J, Beaudet A. Immunocytochemical evidence for direct connections between neurotensin-containing axons and dopaminergic neurons in the rat ventral midbrain tegmentum. Brain Res 479: 402–406, 1989. [DOI] [PubMed] [Google Scholar]
- Wu T, Li A, Wang HL. Neurotensin increases the cationic conductance of rat substantia nigra dopaminergic neurons through the inositol 1,4,5-trisphosphate-calcium pathway. Brain Res 683: 242–250, 1995. [DOI] [PubMed] [Google Scholar]
- Xue Y, Steketee JD, Rebec GV, Sun W. Activation of D(2)-like receptors in rat ventral tegmental area inhibits cocaine-reinstated drug-seeking behavior. Eur J Neurosci 33: 1291–1298, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Trebak M. Transient receptor potential canonical 7: a diacylglycerol-activated non-selective cation channel. Hand Exp Pharmacol 222: 189–204, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]