Abstract
The cerebellum is a critical sensorimotor structure that exhibits protracted postnatal development in mammals. Many aspects of cerebellar circuit development are activity dependent, but little is known about the nature and sources of the activity. Based on previous findings in 6-day-old rats, we proposed that myoclonic twitches, the spontaneous movements that occur exclusively during active sleep (AS), provide generalized as well as topographically precise activity to the developing cerebellum. Taking advantage of known stages of cerebellar cortical development, we examined the relationship between Purkinje cell activity (including complex and simple spikes), nuchal and hindlimb EMG activity, and behavioral state in unanesthetized 4-, 8-, and 12-day-old rats. AS-dependent increases in complex and simple spike activity peaked at 8 days of age, with 60% of units exhibiting significantly more activity during AS than wakefulness. Also, at all three ages, approximately one-third of complex and simple spikes significantly increased their activity within 100 ms of twitches in one of the two muscles from which we recorded. Finally, we observed rhythmicity of complex and simple spikes that was especially prominent at 8 days of age and was greatly diminished by 12 days of age, likely due to developmental changes in climbing fiber and mossy fiber innervation patterns. All together, these results indicate that the neurophysiological activity of the developing cerebellum can be used to make inferences about changes in its microcircuitry. They also support the hypothesis that sleep-related twitches are a prominent source of discrete climbing and mossy fiber activity that could contribute to the activity-dependent development of this critical sensorimotor structure.
Keywords: sensitive period, active sleep, REM sleep, activity-dependent development, myoclonic twitching, rhythmicity
across the early postnatal period in rats, active sleep [AS, or rapid eye movement (REM) sleep] is the predominant behavioral state (Gramsbergen et al. 1970; Jouvet-Mounier et al. 1969). AS is characterized by muscle atonia punctuated by myoclonic twitches of the muscles controlling the limbs, eyes, and whiskers (Blumberg et al. 2013). Twitches are discrete jerky movements that occur many thousands of times each day, and twitch-related sensory feedback shapes spinal circuitry (Petersson et al. 2003) and triggers substantial neural activity in many brain areas, including the thalamus, hippocampus, and cerebral cortex (Khazipov et al. 2004; Mohns and Blumberg 2008, 2010; Tiriac et al. 2012; Tiriac et al. 2014). We recently reported in 6-day-old rats that cerebellar Purkinje cells also exhibit increased activity in response to twitching (Sokoloff et al. 2014). This discovery introduced twitching as a potentially important source of the activity that is known to be important for many aspects of cerebellar circuit development (Hashimoto and Kano 2013; Kakizawa et al. 2000; Sherrard et al. 2013).
The cerebellum is a central contributor to sensorimotor processing (Apps and Garwicz 2005), receiving substantial inputs from the cerebral cortex, brain stem, and spinal cord (Apps and Garwicz 2005; Huang et al. 2013; Odeh et al. 2005). Despite a plethora of convergent afferent signals, cerebellar cortical anatomy is relatively simple, consisting of a constrained circuit constructed of comparatively few cell types (Eccles et al. 1967). Importantly, in rats, humans, and other mammals, the cerebellum undergoes substantial postnatal development (rats: Altman 1972a, 1972b, 1972c; McKay and Turner 2005; Shimono et al. 1976; humans: Dobbing and Sands 1973; Ellis 1920; Zecevic and Rakic 1976). At birth in rats, the Purkinje cell layer (PCL) contains immature Purkinje cells with short dendritic processes and disordered laminar organization (Altman 1972b; McKay and Turner 2005). Early in the first postnatal week, climbing fiber innervation to Purkinje cells increases (Crepel 1971; Shimono et al. 1976). Initially, Purkinje cells receive input from multiple climbing fibers, and this polyinnervation reaches its peak by the end of the first postnatal week (Crepel et al. 1976; Hashimoto et al. 2009; McKay and Turner 2005; Shimono et al. 1976; Watanabe and Kano 2011). At the same time, as granule cells are beginning to migrate to the internal granular layer (IGL), mossy fibers form transient direct connections with Purkinje cells (Kalinovsky et al. 2011; Manzini et al. 2006; Mason and Gregory 1984). During the second postnatal week, the “winners” of the competition among multiple climbing fibers begin to translocate along the Purkinje cells' apical dendrites (Hashimoto and Kano 2003), direct mossy fiber connections on Purkinje cells diminish as granule cells migrate to their final locations, and the parallel fiber system linking granule cells to Purkinje cells is established (Altman 1972a; Kalinovsky et al. 2011; Shimono et al. 1976). Also at this time, the interneurons in the molecular layer become functional (Altman 1972b; Carillo et al. 2013; Hashimoto et al. 2009; Shimono et al. 1976; Watanabe and Kano 2011).
Although activity-dependent processes are often invoked as critical for various aspects of cerebellar development (Andjus et al. 2003; Hashimoto et al. 2012; Kakizawa et al. 2000; Kalinovsky et al. 2011; Mikuni et al. 2013; Nakayama et al. 2012; Sherrard et al. 2013), the nature and sources of the activity have not been identified. Here we take advantage of established milestones in cerebellar cortical development to investigate circuit-related features of state-dependent activity in unanesthetized infant rats. Specifically, we recorded Purkinje cell activity across sleep-wake cycles at three ages: postnatal day (P)4, when both mossy fibers and climbing fibers form direct connections with Purkinje cells (Shimono et al. 1976); P8, when climbing fiber polyinnervation of Purkinje cells reaches its peak (Hashimoto and Kano, 2005; Kalinovsky et al. 2011; Watanabe and Kano 2011); and P12, when the direct mossy fiber connections on Purkinje cells begin to disappear, the parallel fiber system has emerged, and the translocation of climbing fibers to Purkinje cell dendrites has begun (Shimono et al. 1976; Watanabe and Kano 2011). Our results indicate distinct periods of state-dependent cerebellar activity, as well as rhythmicity, that parallel associated changes in cerebellar microcircuitry. Finally, if sleep-related processes are indeed important for the development of this system, then any of a variety of stressors, including sleep restriction or deprivation, could influence cerebellar circuit development, with possible implications for the emergence of neurodevelopmental disorders (e.g., Wang et al. 2014).
MATERIALS AND METHODS
Subjects
Male and female Sprague-Dawley Norway rats (Rattus norvegicus) at P3-4 (n = 13; 9–11.2 g body wt; designated as P4), P7-8 (n = 13; 14.6–21.2 g body wt; designated as P8), and P12 (n = 9; 29.6–36.4 g body wt) were used. Littermates were always assigned to different age groups. Litters were culled to eight pups by 3 days of age. Mothers with litters were housed in standard laboratory cages (48 × 20 × 26 cm). Water and food were available ad libitum and animals were maintained on a 12:12-h light-dark schedule with lights on at 0700. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Iowa and performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication No. 80-23).
Surgery
On the day of testing, a pup with a visible milk band was selected, removed from the litter, and anesthetized with 2–5% isoflurane. First, 2–4 bipolar stainless steel hook electrodes (50 μm diameter, California Fine Wire, Grover Beach, CA) were implanted into nuchal and hindlimb muscles and a ground wire was placed transdermally on the back. Electrodes and ground wires were secured with flexible collodion. Next, the skull was exposed and dried, and a custom-built head-fix apparatus was attached using cyanoacrylate adhesive (Blumberg et al. 2015). After this 10-min surgery, the pup's torso was wrapped lightly in gauze and allowed to recover in a humidified incubator maintained at thermoneutrality (35°C). After 1 h, the pup was removed from the incubator, anesthetized again, and secured in an infant stereotaxic apparatus. Three holes were drilled in the skull for insertion of the electrode for recording cerebellar activity, a thermocouple for measuring brain temperature, and a ground wire. After this second 10-min surgery, the pup was moved to a thermally controlled testing chamber that includes a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) located within a Faraday cage. Testing did not begin until after 1–2 h of acclimation when the experimenter noted regular, organized sleep-wake cycles. It should be stressed that under these conditions, pups cycle regularly between sleep and wakefulness and exhibit all the basic features of sleep-wake organization, including spatiotemporally organized limb twitching produced against a background of muscle atonia (Blumberg et al. 2015).
Cerebellar Neurophysiology
Unit activity from a total of 67 Purkinje cells was recorded. In 7 of the 9 pups at P12, we recorded from 11 cells that were not Purkinje cells; those data will not be presented. Extracellular recordings were performed using platinum/iridium single-ended electrodes (Thomas Recording, Germany; impedance: 1–2 MΩ; P4 and P8 rats only) or 16-channel silicon depth electrodes (NeuroNexus, Ann Arbor, MI; Models A1 × 16 × 100 × 177, A1 × 16 × 50 × 177Poly2) connected to a data acquisition system (Tucker-Davis Technologies, Alachua, FL) that amplified (10,000×) and filtered (500-5,000 Hz band pass) the neural signal. For histological verification of electrode placement, the electrode surface was coated with fluorescent DiI (Life Technologies, Grand Island, NY) before insertion. A ground electrode (Ag/AgCl, 0.25 mm diameter; Medwire, Mt. Vernon, NY) was inserted just beneath the surface of the cerebral cortex. Brain temperature, measured using a fine-wire thermocouple (Omega Engineering, Stamford, CT) that was also inserted into the cerebral cortex, was maintained at 36–37°C. Bipolar EMG hook electrodes were connected to a differential amplifier (A-M systems, Carlsborg, WA) that amplified (10,000×) and filtered (300–5,000 Hz bandpass) the EMG signal; a 60-Hz notch filter was also used. A digital interface and Spike2 software (Cambridge Electronic Design, Cambridge, UK) were used to acquire neural and EMG signals at 12.5 kHz and 1 kHz, respectively.
With the pup in the stereotaxic apparatus, the recording electrode was lowered into the cerebellar cortex using age-specific coordinates referenced to lambda (P4: AP = −1.0 to −2.4 mm, ML = 1.3–2.0 mm, 8–10° angle; P8: AP = −1.1 to −2.4 mm, ML = 1.7–2.0 mm, 8–10° angle; P12: AP = −2.0 to −3.3 mm, ML = 1.7–2.1 mm, 10–12° angle). The electrode was lowered until action potentials were detected on one or more channels (DV range: −1.4 to −3.5 mm). Large-amplitude action potentials (signal-to-noise ≥ 2:1), a relatively high spontaneous firing rate (i.e., ≥1 Hz), and the presence of complex spike waveforms similar to those described by others at similar ages (e.g., Crepel 1971; Puro and Woodward 1977a) served as criteria for identifying Purkinje cell activity. Data acquisition began once an identified Purkinje cell was held for at least 10 min. Recording sessions lasted 15–30 min, during which continuous neural and EMG data were collected as pups cycled spontaneously between sleep and wake. During acquisition, an experimenter digitally scored the subject's sleep and wake movements. Experimenters were blind to the physiological data while scoring behavior. Additional recordings from the same pup, when possible, were obtained by lowering the electrode sufficiently to ensure that the same Purkinje cells were not recorded more than once.
Histology
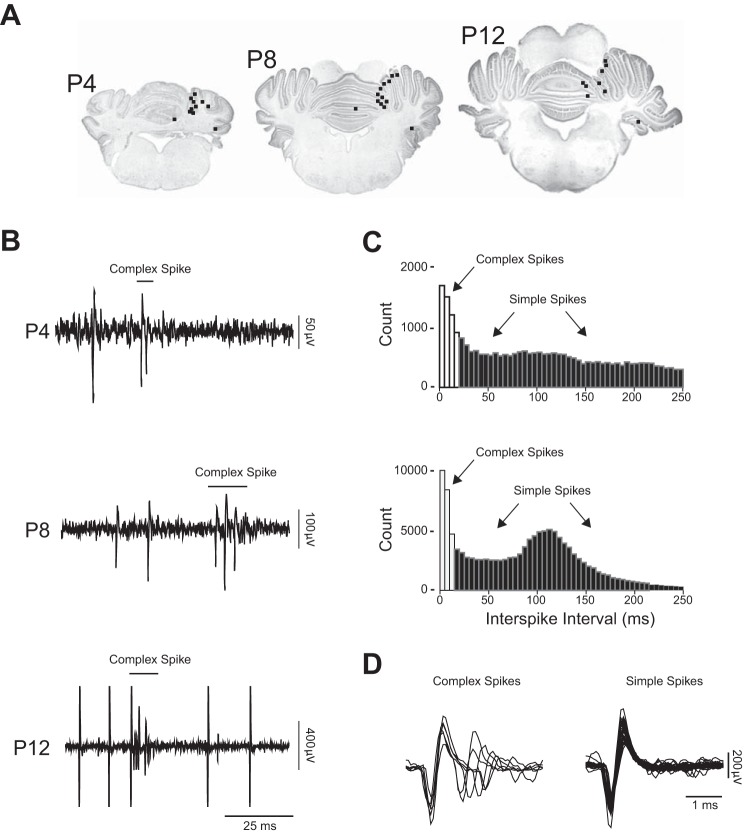
After recording, pups were overdosed with pentobarbital sodium (1.5 mg/g) and transcardially perfused with phosphate-buffered saline followed by 4% paraformaldehyde. The recording site was visualized on 80-μm sections under a microscope with fluorescent illumination at 5–10× magnification (Leica Microsystems, Buffalo Grove, IL). Recording site locations were determined after Nissl staining using a calibrated reticle (Fig. 1A).
Fig. 1.
A: coronal sections depicting locations of Purkinje cell recordings in postnatal day (P)4, P8, and P12 rats. The black squares denote multisite recording locations for individual subjects. B: illustrative recordings of Purkinje cell activity in P4, P8, and P12 rats. Complex spikes are indicated; the rest are simple spikes. C: frequency histograms of interspike interval (ISI) for P4 (top) and P8 (bottom) units. At P4 and P8, multispike events with ISIs ≤20 ms or ≤15 ms, respectively, were designated as complex spikes (white bars) based on previous studies at these ages (Puro and Woodward 1977a; Sokoloff et al. 2014). All other unit activity was designated as simple spikes (black bars). D: at P12, the shapes of the waveforms were used to distinguish complex from simple spikes.
Data Analysis
Spike sorting and burst analysis.
Spike sorting was performed using template matching with Spike2 software (Cambridge Electronic Design, Cambridge, UK). The distributions of waveforms comprising each template were analyzed using principal components analysis; waveforms > 3.5 SDs outside of each distribution were excluded. In P4 and P8 subjects (n = 21 and 26 units, respectively), complex and simple spikes were distinguished using a burst script custom-written in Spike2. Multi-spike events with interspike intervals ≤20 ms and ≤15 ms were identified as complex spikes and were treated as single events for P4 and P8 pups, respectively. Complex and simple spikes for each unit were then analyzed separately (Fig. 1, B and C).
At P12 (n = 20 units), complex spikes are more adultlike in form than are those at earlier ages (Crepel 1971; Puro and Woodward 1977a). Therefore, to identify complex spikes at this age, spikes were sorted into individual units using a 3- to 5-ms window for each template. Then a second threshold was applied to identify the subsequent depolarizations characteristic of complex spikes (Fig. 1D).
Identification of behavioral state.
As previously described (Karlsson et al. 2005; Mohns and Blumberg 2010; Sokoloff et al. 2014), sleep and wake periods were defined based on nuchal EMG activity in conjunction with scored behavior. Each EMG record was rectified and smoothed (tau = 0.001 s) and the mean amplitude of high muscle tone and atonia was calculated from five representative 1-s EMG segments. Based on the midpoint between the two, the EMG signal was dichotomized into periods of high tone (indicative of wake) and atonia (indicative of sleep). Wake was characterized by high muscle tone, often accompanied by bouts of high-amplitude limb movements. AS was characterized by myoclonic twitches occurring against a background of muscle atonia (Blumberg and Seelke 2010; Seelke and Blumberg 2008). Twitches were defined as brief EMG events with amplitudes exceeding at least 3× the mean EMG amplitude during atonia, as described previously (Sokoloff et al. 2014).
Analysis of state dependency.
Mean unit firing rates were determined across all periods of wake and AS for each pup. To assess whether units were significantly more active during one behavioral state or the other, independent pairwise comparisons of mean firing rates during wake and AS were assessed using the Wilcoxon matched-pairs signed-ranks test (SPSS, IBM, Armonk, NY). For any unit, firing rates that exceeded three times the standard deviation were excluded as outliers. To test for age-related differences in mean firing rates, a single-factor ANOVA was used. A Chi-Square test was used to assess age-related differences in the number of state-dependent units. For all tests, including those described below, alpha was set at 0.05 and a Bonferroni post hoc was used when significant main effects were found. Mean firing rates across ages are presented as mean ± SE.
Twitch-related event correlations.
To determine the relationship between complex and simple spike activity and twitching, we constructed twitch-triggered perievent histograms over a 500-ms window using 10-ms bins centered on twitch onset. We performed these analyses separately using twitches from the nuchal and hindlimb muscles. As described previously (Tiriac et al. 2014), we tested statistical significance by jittering twitch events 1,000 times over a 500-ms window using PatternJitter (Amarasingham et al. 2012; Harrison and Geman 2009). Then, using a custom-written Matlab program, we generated upper and lower confidence bands (P ≤ 0.01 for each confidence band) using a method that corrects for multiple comparisons (see Amarasingham et al. 2011). For each unit, after histograms were separately constructed for nuchal and hindlimb muscle twitches, we selected the histogram that was significant and, if both were significant, that exhibited the stronger relationship with complex or simple spike activity. We then pooled these data to produce perievent histograms composed of significant twitch-dependent units at each age; we performed a final jitter analysis on these pooled data. To test for age-related differences in percent time of AS, rate of twitching per minute of AS and rate of twitching per minute of total time, a single-factor ANOVA was used. A Chi-Square test was used to assess age-related differences in the number of twitch-dependent units. For all tests, including those described below, alpha was set at 0.05 and a Bonferroni post hoc was used when significant main effects were found.
Complex and simple spike variability and rhythmicity.
Variability and rhythmicity for complex and simple spike unit activity were calculated and analyzed using the methods of Arancillo and colleagues (2015). Briefly, variability in complex and simple spike activity was measured using the coefficient of variance (CV1) to assess the average variability in interspike intervals (ISIs) by calculating the standard deviation (SD) of ISIs and dividing it by mean ISI:
Rhythmicity was measured for complex and simple spike unit activity using CV2 and a rhythmicity index (RI). In contrast to CV1, CV2 measures moment-to-moment variability in spike activity by comparing differences in adjacent ISIs at time t and t+1, thereby providing an estimate of either tonic or rhythmic burst-like activity, as opposed to highly irregular patterns of neural activity (Holt et al. 1996):
Rhythmicity was measured by calculating a rhythmicity index (Arancillo et al. 2015; Lang et al. 1997; Sugihara et al. 1995). Using Spike2, autocorrelations of complex and simple spike activity were visualized in 5-ms bins over a 1-s window for the entire recording period (i.e., including both sleep and wake). Using the equation below, we first calculated baseline activity over the autocorrelation window:
Thresholds for determining peaks and valleys were calculated by adding and subtracting from baseline the SD of spike counts over the period of −400 to −500 ms and 400 to 500 ms. For complex spike rhythmicity, the first peak was recorded as the bin with the highest number of spikes in the autocorrelogram within the window from 50 to 500 ms based on previously reported oscillation frequencies (see De Zeeuw et al. 2008). Because oscillation frequencies of simple spikes have been reported to be faster (Arancillo et al. 2015), for simple spike rhythmicity the first peak was recorded within a 15- to 500-ms window. Successive valleys and peaks were identified when autocorrelogram bin values fell below or exceeded the calculated thresholds, or if the difference between the preceding peak or valley exceeded twice the SD. Furthermore, subsequent peaks and valleys had to fall within the time range:
where tn is the bin (in ms) of the nth valley or peak and t1 is the bin for the first peak in the autocorrelation. Finally,
in which bin count represents the spike count of either a peak or valley. Oscillation frequency was subsequently calculated by calculating the inverse of the millisecond value of the bin identified for the first peak (t1−1). The rhythmicity index and oscillation frequency were not calculated for autocorrelations lacking obvious peaks, or rhythm indexes ≤ 0.01. Differences in CV1, CV2, rhythmicity index, and oscillation frequency were tested using the Kruskall-Wallace (H) nonparametric test with post hoc pairwise comparisons across age with significance values adjusted for multiple comparisons.
RESULTS
Purkinje cell activity (P4: 21 units; P8: 26 units; P12: 20 units) was recorded from medial regions of cerebellar cortex including vermis (lobules V–VI), Simplex, and Crus I and II (Fig. 1A). There were significant increases with age in mean firing rates for both complex and simple spikes [complex spikes: F(2,64) = 34.4, P < 0.001; simple spikes: F(2,64) = 28.6, P < 0.001]. For complex spikes, P8 rats exhibited a significant increase in overall mean firing rate compared with P4 rats (P4: 0.19 ± 0.03 Hz; P8: 0.64 ± 0.1 Hz; P < 0.05), and P12 rats exhibited a significantly higher complex spike firing rate (1.66 ± 0.2 Hz) in relation to both younger ages (P4: P < 0.001; P8: P < 0.001). For simple spikes, P12 rats again exhibited a significantly higher firing rate in relation to both younger ages (P4: 1.77 ± 0.3 Hz; P8: 4.56 ± 0.5 Hz; P12: 11.84 ± 1.6 Hz; P values < 0.001). Firing rates at P4 and P8 were not significantly different (P = 0.1). The increased firing rate at P12 is consistent with previous studies and reflects developmental changes intrinsic to Purkinje cells (McKay and Turner 2005; Saywell et al. 2014) and also changes in their patterns of innervation over this postnatal period (Hashimoto et al. 2009; Puro and Woodward 1977b, Shimono et al. 1976).
Purkinje Cell Activity Is Modulated by Behavioral State
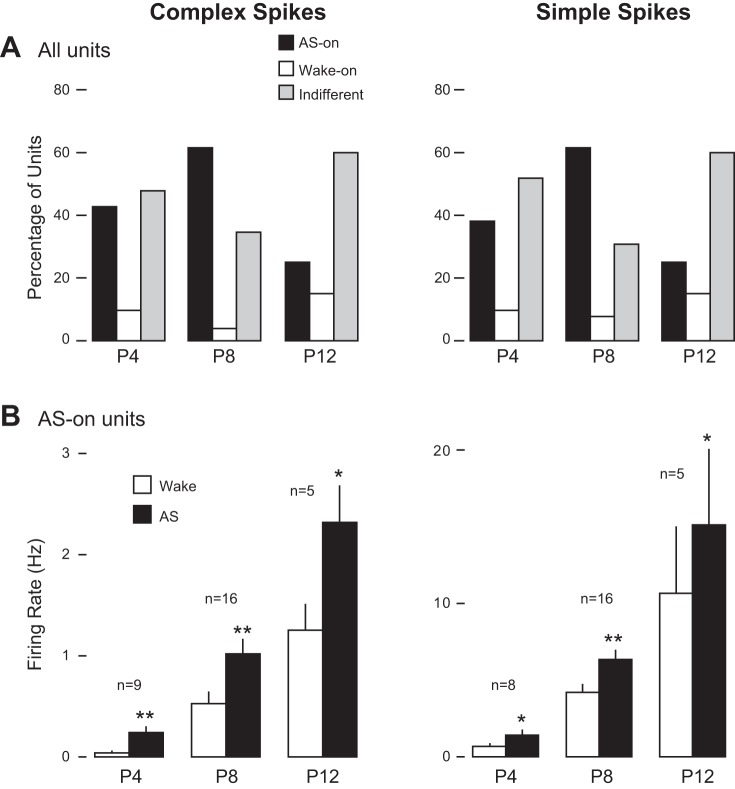
Based on the firing rates of Purkinje cells, we classified complex and simple spikes as AS-on, Wake-on, or state indifferent. The percentage of units that fell within each category varied across age (Fig. 2A), resulting in significant differences in state dependency of Purkinje cell activity [complex spikes: X2(2, N = 30) = 6.2, P = 0.045; simple spikes: X2(2, N = 29) = 6.7, P = 0.035]. At P8, state dependency for both complex and simple spikes was strongest with 16 units being classified at AS-on (61.5%). At P4 and P12 for complex spikes, only 9 (42.8%) and 5 (25%) units, respectively, exhibited significant AS-on activity. Similarly, at P4 and P12 for simple spikes, only 8 (38.1%) and 5 (25%) units, respectively, exhibited significant AS-on activity. Importantly, for both complex and simple spikes at all three ages fewer than 15% of Purkinje cells were classified as Wake-on. Finally, Fig. 2B presents mean firing rates of AS-on units across behavioral states at each age for both complex and simple spikes showing that even as firing rates increase across age, significantly more neural activity is observed during AS. Similar to our previous findings in other brain areas (thalamus: Tiriac et al. 2012; motor cortex: Tiriac et al. 2014; hippocampus: Mohns and Blumberg 2008) Purkinje cells exhibit elevated firing rates during active sleep by the end of the first postnatal week.
Fig. 2.
State dependency of complex and simple spikes. A: percentage of units showing significant increases in firing rate during active sleep (AS) in relation to wake (AS-on) or wake in relation to AS (Wake-on); the remaining units were indifferent. At P8 only, for both complex and simple spikes, the majority of units were AS-on. B: mean firing rates for AS-on units during AS and wake at each age. Mean + SE. *P < 0.05 in relation to Wake. **P < 0.001 in relation to Wake.
Twitch-Dependent Purkinje Cell Activity
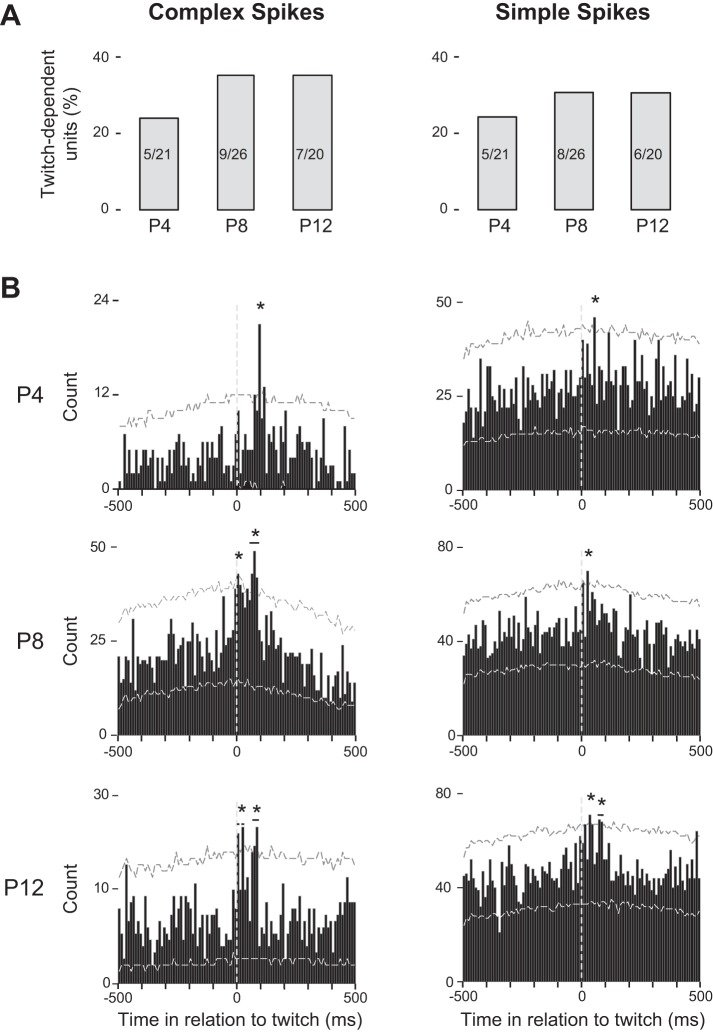
Because twitches are discrete events, they can be used as triggers in perievent histograms to assess associated neural activity. In our previous study examining twitch-related Purkinje cell activity at P6 (Sokoloff et al. 2014), we found that both complex and simple spike activity increased significantly after twitches. Here, at all three ages and as shown in Fig. 3A, we again observed significant twitch-dependent increases in complex and simple spike activity within 100 ms of either nuchal or hindlimb twitches. There was no significant change in the percentage (24–35%) of units that exhibited twitch-dependent increases in firing rate across development [complex spikes: X2(2, N = 21) = 1.1, P = 0.58; simple spikes: X2(2, N = 19)= 0.74, P = 0.69].
Fig. 3.
Twitch-dependent complex and simple spike activity. A: percentage of units with significant twitch-dependent activity (number of units indicated within bars) at P4, P8, and P12. B: perievent histograms for data pooled across units exhibiting significant twitch-dependent complex and simple spike activity. The trigger twitch for each plot (at 0 ms, vertical dashed lines) could be a twitch of either the nuchal or hindlimb muscle, whichever exhibited the strongest relationship with unit activity. See materials and methods for details. Upper and lower confidence bands (horizontal dashed lines, P = 0.01 for each band) are shown, and asterisks highlight regions of statistical significance.
Perievent histograms of twitch-dependent activity for complex and simple spikes, pooled across units exhibiting significant twitch-dependent activity at each age, are presented in Fig. 3B. For P8 and P12 rats, complex spikes exhibited two significant peaks after a twitch, the first at 0–30 ms and the second at 60–90 ms; in contrast, complex spikes at P4 exhibited only a single significant peak at 100–110 ms after a twitch. In our earlier study at P6, we also observed multiple peaks in complex spike activity after twitches (Sokoloff et al. 2014). For simple spikes at P4 and P8, a single significant peak in activity occurred at 50–60 and 20–30 ms after a twitch, respectively. At P12, and similar to complex spikes, simple spikes exhibited two significant peaks in activity after a twitch, the first at 30–40 ms and the second at 70–90 ms.
These data demonstrate substantial twitch-dependent Purkinje cell activity across early development. However, to assess the opportunity for twitching to influence cerebellar development across age, we need to interpret the present findings within the context of age-related changes in the rate of twitching. We assessed rate of twitching in two ways. First, the number of nuchal muscle twitches per minute of AS decreased by 28% between P4 and P8 and by another 69% between P8 and P12 [P4: 31.08 ± 3.3 twitches/min; P8: 22.31 ± 3.16 twitches/min; P12: 7.0 ± 1.33 twitches/min; F(2,38) = 15.7, P < 0.0001]; the decrease from P8 to P12 was significant (P < 0.05), but that between P4 and P8 was not. Second, the number of twitches per minute of total recording time decreased by 17% between P4 and P8 and by another 77% between P8 and P12 [P4: 20.32 ± 2.18 twitches/min; P8: 16.82 ± 9.38 twitches/min; P12: 3.83 ± 0.75 twitches/min; F(2,38) = 15.64, P < 0.0001]; again, the decrease from P8 to P12 was significant (P < 0.05). In contrast, the percentage of time spent in AS increased nonsignificantly between P4 and P8 and decreased significantly between P8 and P12 [P4: 66.66 ± 3.5%; P8: 76.48 ± 2.6%; P12: 54.00 ± 9.7%; F(2,38) = 13.72, P < 0.0001]; importantly, the magnitude of the decrease in AS between P8 and P12 was relatively modest compared with the decreased rate of twitching.
Complex and Simple Spike Rhythmicity
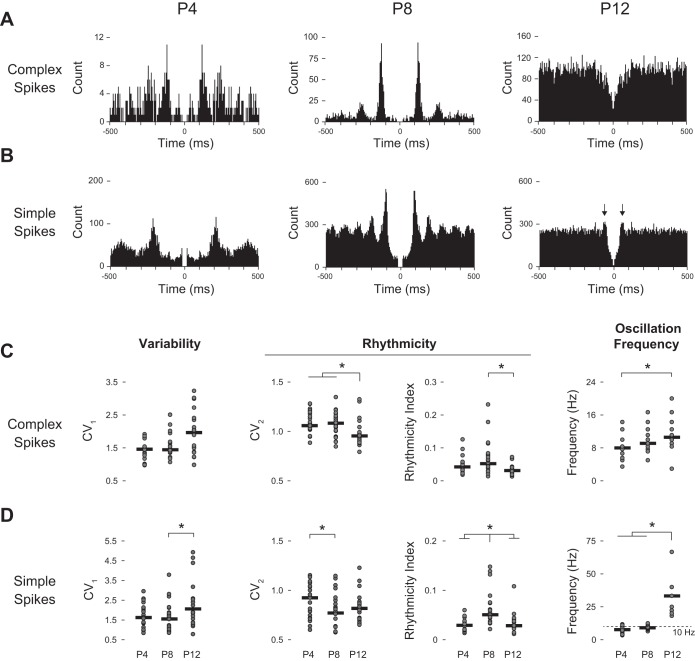
We previously reported that complex spike activity in P6 rats can be highly rhythmic (Sokoloff et al. 2014), consistent with studies in adults showing rhythmicity in both complex spikes and inferior olive neurons (De Zeeuw et al. 2008; Yarom and Cohen 2002). Figure 4 shows representative autocorrelations of complex spike (Fig. 4A) and simple spike (Fig. 4B) activity at each age. Figure 4C presents variability and rhythmicity measures for complex spikes at each age. Complex spike ISIs did not exhibit any differences in variability across age (CV1, Fig. 4C, left panel). For measures of rhythmicity (Fig. 4C, middle panels), CV2 differed significantly across ages (H = 9.45, P < 0.01). Specifically, P12 rats had a significantly lower CV2 in relation to both P4 and P8 rats (P < 0.05). The rhythmicity index also differed across ages (H = 7.18; P < 0.05); in this instance, however, P8 rats had a larger index than P12 rats (P < 0.05). These findings suggest that although the rhythmicity of complex spikes is less variable at P12, the rhythm itself is stronger at P8. Finally, oscillation frequency also differed across ages (Fig. 4C, right panel; H = 6.58, P < 0.05) with P12 rats exhibiting faster oscillations in relation to P4 rats (P < 0.05; median oscillation frequency: P4 = 8.0 Hz; P8 = 9.1 Hz; P12 = 10.6 Hz).
Fig. 4.
Representative autocorrelograms (5-ms bins) of complex spike (A) and simple spike rhythmicity (B) from P4, P8, and P12 subjects. Scatterplots of variability and rhythmicity of complex spike (C) and simple spike activity (D) across all three ages. Presented are analyses of the overall variability of activity (CV1), two measures of rhythmicity (CV2, rhythmicity index), and oscillation frequency. See materials and methods for descriptions of these analyses. Median values denoted by black horizontal bar. Arrows indicate peak in simple spike autocorrelations at P12. *Significant differences between or among indicated age groups, P < 0.05.
Figure 4D presents variability and rhythmicity measures for simple spikes at each age. Simple spike ISIs did show a difference in variability across ages (Fig. 4D, left panel; H = 7.79, P < 0.05), with P8 rats showing significantly less variability in ISI that P12 rats (P < 0.05). For measures of rhythmicity (Fig. 4D, middle panels), CV2 differed significantly across ages (H = 6.97, P < 0.05), with P8 rats having a significantly lower CV2 than P4 rats (P < 0.05). The rhythmicity index also differed across ages (H = 16.59; P < 0.001) with P8 rats showing a significantly larger index than both P4 and P12 rats (P < 0.005). Finally, as with complex spikes, oscillation frequency differed across ages (Fig. 4D, right panel; H = 32.60, P < 0.001) with P12 rats exhibiting strikingly faster oscillations in relation to P4 and P8 rats (P < 0.001; median oscillation frequency: P4 = 7.6 Hz; P8 = 9.1 Hz; P12 = 33.33 Hz); this qualitative increase in oscillation frequency at P12 is attributable to single short-latency peaks (see arrows in Fig. 4B, right panel).
DISCUSSION
In a previous study of Purkinje cell activity in P6 rats (Sokoloff et al. 2014), we found strong evidence of state dependency, twitch dependency, and rhythmicity. Here, we aimed to place those findings within the broader context of the rapid and dramatic cerebellar circuit changes that take place between P4 and P12 (Crepel 1974; Puro and Woodward 1977a; Shimono et al. 1976; see Fig. 5). We predicted that across this 8-day interval, complex and simple spikes would exhibit patterns of activity reflecting these developmental changes. As detailed below, the present findings are in accord with this prediction.
Fig. 5.
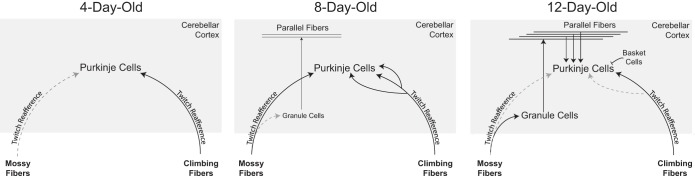
Changes in cerebellar circuit development in rats over the first two postnatal weeks. At P4, Purkinje cells receive weak climbing fiber innervation and direct innervation from mossy fibers. By P8, granule cells have begun to migrate to the internal granular layer, but direct mossy fiber innervation of Purkinje cells still predominates. Also, multiple climbing fibers innervate Purkinje cell somas. By P12, climbing fiber synapse elimination and translocation have begun, and the direct mossy fiber innervation of Purkinje cells is regressing as mossy fibers establish connections with granule cells. In turn, the granule cells are establishing innervation of Purkinje cells via the parallel fiber system. In addition, newly developed interneurons within the molecular layer (e.g., basket cells) modulate the activity of Purkinje cells through an inhibitory connection. AS-dependent and rhythmic Purkinje cell activity is most pronounced at P8. Rhythmicity may be most pronounced at P8 due to 1) direct innervation of Purkinje cells by mossy fibers conveying rhythmic activity from precerebellar nuclei and 2) polyneuronal innervation by climbing fibers. Finally, at all three ages, reafferent input from myoclonic twitches are transmitted through climbing and mossy fibers to the cerebellar cortex.
Given how little is known about the development of cerebellar activity, it is worthwhile to compare the present findings with those of previous functional and anatomical studies. First, we observed age-related increases in both complex and simple spike firing rates, consistent with previous reports of Purkinje cell activity in anesthetized infant rats (Crepel 1971; McKay and Turner 2005; Puro and Woodward 1977a,b). Second, we found no evidence of molecular layer interneuron activity or granule cell activity until P12, consistent with anatomical investigations of the cerebellar cortical circuit (Altman 1972b,c; Shimono et al. 1976; see discussion below). Third, we observed pronounced AS-dependent Purkinje cell activity at P8, consistent with our own previous findings at P6 (Sokoloff et al. 2014); however, AS dependency in the present study was less prominent at both P4 and P12. Despite the cerebellum's well-established role in sensorimotor integration, very few units at any age exhibited wake-dependent increases in activity even though wake-related movements are typically more robust than myoclonic twitches. This lack of wake-related neural activity has been observed in other brain areas (see Tiriac et al. 2014).
Based on our previous findings at P6, as well as evidence that twitches are ideally suited to promote activity-dependent development of neural systems (Blumberg et al. 2013; Tiriac et al. 2014), we proposed that myoclonic twitches are an important source of activity to the developing cerebellum (Sokoloff et al. 2014). The present results strengthen the case for that proposal. At all three ages, both complex and simple spikes activity for approximately one-third of the recorded units increased significantly within 100 ms of twitching in either the nuchal or hindlimb muscle. These relatively short latencies are consistent with those reported for anesthetized pups in response to peripheral limb stimulation (Puro and Woodward 1977a,b). Also, as we found previously at P6 (Sokoloff et al. 2014), twitch-dependent complex spikes at P8 and P12 rats exhibited double peaks. Although much of this twitch-dependent activity likely results from reafference from the sensory periphery, preliminary data suggest that the shortest-latency complex spikes are produced by a corollary discharge from the red nucleus conveyed through the inferior olive (Del Rio-Bermudez et al. 2015).
Although the proportion of Purkinje cells exhibiting twitch-related activity at P8 and P12 did not change (see Fig. 3), the rate of nuchal muscle twitching decreased substantially between those two ages. This is consistent with a previous study showing high rates of nuchal muscle twitching at P2-6 followed by a sharp decline over the next week (Marcano-Reik et al. 2010). Thus the opportunity for twitching to effect activity-dependent changes in cerebellar development decreases substantially over the second postnatal week.
Independent of twitching, there was substantial rhythmicity exhibited by complex and simple spikes that was especially prominent at P8. With respect to complex spikes, the 10-Hz rhythmicity that is sometimes (but not always) reported in adult animals has been attributed to rhythmic firing of neuronal populations in the inferior olive (e.g., Kitazawa and Wolpert 2005; Sasaki et al. 1989; Van Der Giessen et al. 2008). There have also been reports of simple spike rhythmicity in adult mice and cats (e.g., Arancillo et al. 2015; Cheron et al. 2005; Goossens et al. 2001; McCarley and Hobson 1972). Specifically, the simple spike rhythmicity observed here at P8 is similar to that recorded in adult rats from the lateral reticular nucleus, a major source of mossy fibers to the cerebellum (Xu et al. 2013). In contrast the high-frequency simple spike oscillation observed at P12 resembles that reported in 2-wk-old mice (Arancillo et al. 2015).
We propose, therefore, that the simple spike rhythmicity observed here at P8 results from the direct conveyance of rhythmic activity from the lateral reticular nucleus (and perhaps other precerebellar nuclei) via mossy fibers to Purkinje cells. Accordingly, the dramatic increase in the oscillation frequency at P12 could result from the loss of these direct connections and the interposition of the parallel fiber system between mossy fibers and Purkinje cells (Kalinovsky et al. 2011; Kuwako et al. 2014). Interestingly, the explanation for the emergence and disappearance of complex spike rhythmicity may be different from that for simple spike rhythmicity. Specifically, at P8, strong rhythmicity may reflect a peak in polyneuronal innervation by multiple climbing fibers on Purkinje cell somas (Hashimoto et al. 2009; Watanabe and Kano 2011); this notion is supported by the observation that complex spike rhythmicity in adults is not consistently observable from individual Purkinje cells (Yarom and Cohen 2002). Regardless of its origin, what is perhaps most striking about the pronounced complex spike rhythmicity at P8 is how closely its oscillation frequency corresponds with that of simple spikes (Fig. 4, C and D; right panels); this correspondence is lacking at P4 and regular rhythms have largely disappeared by P12. Thus there appears to be a very narrow window when climbing and mossy fiber inputs provide strong rhythmic input to the Purkinje cell soma. We suggest that such rhythmic activity provides a means of converging somatotopically related climbing and mossy fiber inputs to the developing cerebellum (Huang et al. 2013; Singer 1993; Whittington et al. 1995).
Limitations
The activity of Purkinje cells is easily differentiated from that of other cell types based on histological verification of electrode placement and the shape of the waveform. Also, at P4 and P8, basket cells are not fully functional (Altman 1972b) and few granule cells have migrated and become functionally incorporated into the cerebellar circuit (Altman 1972c). However, because complex spikes are immature at these ages (Crepel 1971; Puro and Woodward 1977a), it is possible that some complex spikes were inappropriately classified here as simple spikes, particularly at P4 (see Fig. 1C; Puro and Woodward 1977a). Regardless, because the patterns of state dependency for complex and simple spikes were similar at each age, such possible misclassifications are unlikely to have altered our conclusions in any significant way.
Instead of a coherent somatotopic representation, as exhibited by other sensorimotor areas, the cerebellar cortex comprises numerous discontinuous maps (i.e., “fractured somatotopy”; Shambes et al. 1978). Although these maps have been well described in adults, little is known about their development. Our preliminary results investigating the development of these fractured maps using exafferent stimulation and twitch-related reafference indicate that Purkinje cells, as well as neurons in the interpositus nucleus, are responsive to evoked stimulation of specific muscle groups (e.g., the forelimb) and that such cells are also specifically responsive to twitch-related feedback arising from those same muscle groups (unpublished observations). In this context, it is worth noting that when we have recorded from brain areas with known somatotopy (e.g., the hindlimb-responsive region of sensorimotor cortex; Tiriac et al. 2014), all of the units have exhibited clear and precise twitch-dependent activity consistent with that somatotopy. Accordingly, given the current state of knowledge of fractured somatotopy in the developing cerebellum, it will be important in future studies to record from additional muscles throughout the body, including extraocular and whisker muscles (Seelke et al. 2005; Tiriac et al. 2012). Such recordings may reveal that twitches play an even greater role in driving cerebellar activity than the present results suggest.
Even if twitching is a key player in activity-dependent cerebellar development, it is possible that AS-dependent aspects of Purkinje cell activity, independent of twitching, also play a role. Therefore, future studies should consider the possible state-dependent contributions of serotonergic and noradrenergic neuromodulators to Purkinje cell activity (Rahimi-Balaei et al. 2015). Such neuromodulation could also help to explain the relative lack of Wake-on Purkinje cell activity observed here.
Non-Purkinje-Cell Activity
Consistent with previous anatomical and neurophysiological investigations of the development of the cerebellar cortex (Altman 1972b,c; Crepel 1974; Pouzat and Hestrin 1997; Shimono et al. 1976), we observed little or no neural activity within the molecular or granule cell layers at P4 or P8. At P12, however, based on histological assessment of electrode placement and firing properties (Ruigrok et al. 2011), we recorded neural activity that did not exhibit the characteristics of Purkinje cells (n = 11 units; data not shown). In one P12 subject, we recorded simultaneously from a Purkinje cell and an adjacent cell with properties reminiscent of basket cells. Specifically, this putative basket cell became active exclusively during wake, at which time the previously active Purkinje cell became inactive. This reciprocal activation pattern could reflect the known inhibitory influence of basket cells on Purkinje cells (Andersen et al. 1964).
Sensitive Periods and Neurodevelopmental Disorders
In developing systems, sensitive periods occur at times of rapid change and are characterized by heightened responsiveness to perturbation and, in the nervous system, increased neural plasticity (Knudsen 2004; Stockard 1921; Thomas and Johnson 2008). In the brain, sensitive periods have perhaps been most extensively studied in cerebral cortex (e.g., Hensch 2005; Hooks and Chen 2007), but they have also been discussed with respect to various aspects of cerebellar development (e.g., Kakizawa et al. 2000; Sherrard et al. 2013). The observation here of rapid developmental changes in patterns of neural activity within cerebellar cortex at the end of the first postnatal week, corresponding with established rapid changes in neural circuitry, suggests the presence of a sensitive period. Whether the cerebellar system is especially sensitive to perturbation during this period remains to be determined.
The identification of sensitive periods within the cerebellum could have important implications for understanding the developmental origins of motor and cognitive disorders (Wang et al. 2014). Importantly, in addition to its well-known role in sensorimotor processing, the cerebellum is receiving increasing attention as a contributor to cognition (Ito 2008; White and Sillitoe 2013). For example, genetic disorders resulting in altered cerebellar development (e.g., Dandy-Walker malformation) result in both motor dysfunction and cognitive delay (Millen and Gleeson 2008). Similarly, cerebellar dysfunction has been implicated in autism spectrum disorder, bipolar disorder, and schizophrenia (Ito 2008; Johnson et al. 2015; Kern 2002; Martin and Albers 1995; Wang et al. 2014).
With respect to autism, cerebellar injury at birth is the second greatest risk factor (Wang et al. 2014). Citing this fact, and the precise and bidirectional somatotopic connections between the cerebellum and neocortex, Wang and colleagues (2014) proposed that disruptions of cerebellar function during sensitive periods of development, whatever the cause, could produce negative cascading effects on cerebellar-neocortical communication and associated cognitive processes. Consistent with what is known about the timing of cerebellar development in humans, Wang and colleagues (2014) further suggested that such negative effects could impact humans prenatally as well as long into the early postnatal period. In line with this suggestion and the present findings, we further propose that prolonged or severe sleep restriction or disruption could be one such cause of early cerebellar dysfunction. For example, even small decreases in air temperature can suppress AS-related twitching in infant rats (Blumberg and Stolba 1996; Sokoloff and Blumberg 1998), which could be one of many factors contributing to the emergence of autism-like symptoms in human infants raised in severely deprived conditions (Nelson et al. 2011). Therefore, in light of the rapid transformation in cerebellar circuitry and associated changes in Purkinje cell activity at the end of the first postnatal week in rats, and the modulation of that activity by sleep-related processes, the present findings suggest the existence of a sensitive period in this system when sleep disruption could have particularly profound and long-lasting effects on sensorimotor and cognitive development.
GRANTS
This work was supported by funds awarded to M. S. Blumberg from the National Institute of Child Health and Human Development (R37-HD-81168 and R01-HD-63071).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.S. and M.S.B. conception and design of research; G.S., A.M.P., and D.M. performed experiments; G.S., A.M.P., D.M., and M.S.B. analyzed data; G.S. and M.S.B. interpreted results of experiments; G.S. and M.S.B. prepared figures; G.S., A.M.P., and M.S.B. drafted manuscript; G.S., A.M.P., D.M., and M.S.B. edited and revised manuscript; G.S. and M.S.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank B. Uitermarkt and A. Fanning for assistance in data collection and A. Tiriac and C. Del Rio Bermudez for comments on an earlier draft of this manuscript. We also thank Drs. M. Arancillo and R. Sillitoe for helpful discussions about, and assistance with, the rhythmicity analyses.
REFERENCES
- Altman J. Postnatal development of the cerebellar cortex I. The external germinal layer and the transitional molecular layer. J Comp Neurol 145: 353–398, 1972a. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol 145: 399–464, 1972b. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex III. Maturation of the components of the granular layer. J Comp Neurol 145: 465–514, 1972c. [DOI] [PubMed] [Google Scholar]
- Amarasingham A, Harrison MT, Hatsopoulos NG, Geman S. Conditional modeling and the jitter method of spike re-sampling. J Neurophysiol 107: 517–531, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarasingham A, Harrison MT, Hatsopoulos NG, Geman S. Conditional modeling and the jitter method of spike re-sampling: Supplement. arXiv:1111.4296[stat.ME], 2011. Available at: http://arxiv.org/abs/1111.4296 [Google Scholar]
- Andersen P, Eccles JC, Voorhoeve PE. Postsynaptic inhibition of cerebellar Purkinje cells. J Neurophysiol 27: 1138–1153, 1964. [DOI] [PubMed] [Google Scholar]
- Andjus PR, Zhu L, Cesa R, Carulli D, Strata P. A change in the pattern of activity affects the developmental regression of the Purkinje cell polyinnervation by climbing fibers in the rat cerebellum. Neuroscience 121: 563–572, 2003. [DOI] [PubMed] [Google Scholar]
- Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci 6: 297–311, 2005. [DOI] [PubMed] [Google Scholar]
- Arancillo M, White JJ, Lin T, Stay TL, Sillitoe RV. In vivo analysis of Purkinje cell firing properties during postnatal mouse development. J Neurophysiol 113: 578–591, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Curr Biol 23: R532–R537, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AMH. The form and function of infant sleep: From muscle to neocortex. In: The Oxford Handbook of Developmental Behavioral Neuroscience, edited by Blumberg MS, Freeman JH, Robinson SR. New York: Oxford Univ. Press, 2010. [Google Scholar]
- Blumberg MS, Sokoloff G, Tiriac A, Del Rio-Bermudez C. A valuable and promising method for recording brain activity in behaving newborn rodents. Dev Psychobiol 57: 506–517, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Stolba M. Thermogenesis, myoclonic twitching, and ultrasonic vocalization in neonatal rats during moderate and extreme cold exposure. Behav Neurosci 110: 305–314, 1996. [DOI] [PubMed] [Google Scholar]
- Carillo J, Nishiyama N, Nishiyama H. Dendritic translocation establishes the winner in cerebellar climbing fiber synapse elimination. J Neurosci 33: 7641–7653, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Servais L, Wagstaff J, Dan B. Fast cerebellar oscillation associated with ataxia in a mouse model of Angelman syndrome. Neuroscience 130: 631–637, 2005. [DOI] [PubMed] [Google Scholar]
- Crepel F. Maturation of climbing fiber responses in the rat. Brain Res 35: 272–276, 1971. [DOI] [PubMed] [Google Scholar]
- Crepel F. Excitatory and inhibitory processes acting upon cerebellar Purkinje cells during maturation in the rat; influence of hypothyroidism. Exp Brain Res 20: 403–420, 1974. [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J, Delhaye-Bouchard N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. Dev Neurobiol 7: 567–578, 1976. [DOI] [PubMed] [Google Scholar]
- Del Rio-Bermudez C, Sokoloff G, Blumberg MS. Sensorimotor processing in the newborn rat red nucleus during active sleep. J Neurosci 35: 8322–8332, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child 48: 757–767, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw CI, Hoebeek FE, Schonnewille M. Causes and consequences of oscillations in the cerebellar cortex. Neuron 58: 655–658, 2008. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentagothai J. The Cerebellum As a Neuronal Machine. Berlin: Springer-Verlag, 1967. [Google Scholar]
- Ellis RS. Norms for some structural changes in the cerebellum from birth to old age. J Comp Neurol 32: 1–33, 1920. [Google Scholar]
- Goossens J, Daniel H, Rancillac A, van der Steen J, Oberdick J, Crépel F, De Zeeuw CI, Frens MA. Expression of protein kinase C inhibitor blocks cerebellar long-term depression without affecting Purkinje cell excitability in alert mice. J Neurosci 21: 5813–5823, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Dev Psychobiol 3: 267–280, 1970. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ichikawa R, Kitamura K, Watanabe M, Kano M. Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron 63: 106–118, 2009. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Ito R, Kitamura N, Namba K, Hisano Y. Epha4 controls the midline crossing and contralateral axonal projections of inferior olive neurons. J Comp Neurol 520: 1702–1720, 2012. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber input during synapse elimination of the developing cerebellum. Neuron 38: 785–796, 2003. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Postnatal development and synapse elimination of climbing fiber to Purkinje cell projection in the cerebellum. Neurosci Res 53: 221–228, 2005. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Synapse elimination in the developing cerebellum. Cell Mol Life Sci 70: 4667–4680, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MT, Geman S. A rate and history-preserving resampling algorithm for neural spike trains. Neural Comput 21: 1244–1258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6: 877–888, 2005. [DOI] [PubMed] [Google Scholar]
- Holt GR, Softky WR, Koch C. Comparison of discharge variability in vitro and in vivo in cat visual cortex neurons. J Neurophysiol 75: 1806–184, 1996. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron 56: 312–326, 2007. [DOI] [PubMed] [Google Scholar]
- Huang CC, Sugino K, Shima Y, Guo C, Bai S, Mensh BD, Nelson SB, Hantman AW. Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. Elife 2: e00400, 2013. doi: 10.7554/eLife.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci 9: 304–313, 2008. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Follmer RL, Oguz I, Warren LA, Christensen GE, Fiedorowicz JG, Magnotta VA, Wemmie JA. Brain abnormalities in bipolar disorder detected by quantitative T1ρ mapping. Mol Psychiatry 20: 201–206, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol 2: 216–239, 1969. [DOI] [PubMed] [Google Scholar]
- Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci 20: 4954–4951, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinovsky A, Bouktouche F, Blazeski R, Bornmann C, Suzuki N, Mason CA, Scheiffele P. Development of axon-target specificity of ponto-cerebellar afferents. PLoS Biol 9: e1001013, 2011. doi: 10.1371/journal.pbio.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biol 3: 891–901, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK. The possible role of the cerebellum in autism/PDD: disruption of a multisensory feedback loop. Med Hypotheses 59: 255–260, 2002. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 432: 758–761, 2004. [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Wolpert DM. Rhythmicity, randomness and synchrony in climbing fiber signals. Trends Neurosci 28: 611–619, 2005. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cog Neurosci 16: 1412–1425, 2004. [DOI] [PubMed] [Google Scholar]
- Kuwako KI, Nishimoto Y, Okano HJ, Okano H. Cadherin-7 regulates mossy fiber connectivity in the cerebellum. Cell Reports 9: 311–323, 2014. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Sugihara I, Llinás. Differential roles of apamin- and charybdotoxin-sensitive K+ conductances in the generation of inferior olive rhythmicity in vivo. J Neurosci 17: 2825–2838, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini MC, Ward MS, Zhang Q, Lieberman MD, Mason CA. The stop signal revised: Immature cerebellar granule neurons in the external germinal layer arrest pontine mossy fiber growth. J Neurosci 26: 6040–6051, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcano-Reik AJ, Prasad T, Weiner JA, Blumberg MS. An abrupt developmental shift in callosal modulation of sleep-related spindle bursts coincides with the emergence of excitatory-inhibitory balance and a reduction of somatosensory cortical plasticity. Behav Neurosci 124: 600–611, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Albers M. Cerebellum and schizophrenia: a selective review. Schizophr Bull 21: 241–250, 1995. [DOI] [PubMed] [Google Scholar]
- Mason CA, Gregory E. Postnatal maturation of cerebellar mossy and climbing fibers: transient expression of dual features on single axons. J Neurosci 4: 1715–1735, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Simple spike firing patterns of cat cerebellar Purkinje cells in sleep and waking. Electroen Clin Neuro 33: 471–483, 1972. [DOI] [PubMed] [Google Scholar]
- McKay BE, Turner RW. Physiological and morphological development of the rat cerebellar Purkinje cell. J Physiol 567: 829–850, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni T, Uesaka N, Okuno H, Hirai H, Deisseroth K, Bito H, Kano M. Arc/Arg3.1 is a postsynaptic mediator of activity-dependent synapse elimination in the developing cerebellum. Neuron 78: 1024–1035, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen KJ, Gleeson JG. Cerebellar development and disease. Curr Opin Neurobiol 18: 12–19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohns EJ, Blumberg MS. Synchronous bursts of neuronal activity in the developing hippocampus: modulation by active sleep, and association with emerging gamma and theta rhythms. J Neurosci 28: 10134–10144, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohns EJ, Blumberg MS. Neocortical activation of the hippocampus during sleep in infant rats. J Neurosci 30: 3438–3449, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Miyazaki T, Kitamura K, Hashimoto K, Yanagawa Y, Obata K, Sakimura K, Watanabe M, Kano M. GABAergic inhibition regulates developmental synapse elimination in the cerebellum. Neuron 74: 384–396, 2012. [DOI] [PubMed] [Google Scholar]
- Nelson CA 3rd, Bos K, Gunnar MR, Sonuga-Barke EJS. The neurobiological toll of early human deprivation. Monogr Soc Res Child Dev 76: 127–146, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeh F, Ackerley R, Bjaalie JG, Apps R. Pontine maps linking somatosensory and cerebellar cortices are in register with climbing fiber somatotopy. J Neurosci 25: 5680–5690, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson P, Waldenström A, Fåhraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature 424: 72–75, 2003. [DOI] [PubMed] [Google Scholar]
- Pouzat C, Hestrin S. Developmental regulation of Basket/Stellate cell→Purkinje cell synapses in the cerebellum. J Neurosci 17: 9104–9112, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro DG, Woodward DJ. Maturation of evoked climbing fiber input to rat cerebellar Purkinje cells (I). Exp Brain Res 28: 85–100, 1977a. [DOI] [PubMed] [Google Scholar]
- Puro DG, Woodward DJ. Maturation of evoked mossy fiber input to rat cerebellar Purkinje cells (II). Exp Brain Res 28: 427–441, 1977b. [DOI] [PubMed] [Google Scholar]
- Rahimi-Balaei M, Afsharinezhad P, Bailey K, Buchok M, Yeganeh B, Marzban H. Embryonic stages in cerebellar afferent development. Cereb Ataxias 2: 7, 2015. doi: 10.1186/s40673-015-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok TJH, Hensbroek RA, Simpson JI. Spontaneous activity signatures of morphologically identified interneurons in the vestibulocerebellum. J Neurosci 31: 712–724, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Bower JM, Llinás R. Multiple Purkinje cell recording in rodent cerebellar cortex. Eur J Neurosci 1: 572–586, 1989. [DOI] [PubMed] [Google Scholar]
- Saywell V, Cioni JM, Ango F. Developmental gene expression profile of axon guidance cues in Purkinje cells during cerebellar circuit formation. Cerebellum 13: 307–317, 2014. [DOI] [PubMed] [Google Scholar]
- Seelke AMH, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep 31: 691–699, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Karlsson KÆ, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. Eur J Neurosci 22: 911–920, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol 15: 116–140, 1978. [DOI] [PubMed] [Google Scholar]
- Sherrard RM, Letellier M, Lohof AM, Mariani J. Formation and reformation of climbing fibre synapses in the cerebellum: a similar story? Cerebellum 12: 319–321, 2013. [DOI] [PubMed] [Google Scholar]
- Shimono T, Shoichiro N, Sasaki K. Electrophysiological study on the postnatal development of neuronal mechanisms in the rat cerebellar cortex. Brain Res 108: 279–294, 1976. [DOI] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol 55: 349–374, 1993. [DOI] [PubMed] [Google Scholar]
- Sokoloff G, Blumberg MS. Active sleep in cold-exposed infant Norway rats and Syrian golden hamsters: the role of brown adipose tissue thermogenesis. Behav Neurosci 112: 695–706, 1998. [DOI] [PubMed] [Google Scholar]
- Sokoloff G, Uitermarkt BD, Blumberg MS. REM sleep twitches rouse nascent cerebellar circuits: implications for sensorimotor development. Dev Neurobiol 2014. March 28. doi: 10.1002/dneu.22177 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockard C. Developmental rate and structural expression: an experimental study of twins, “double monsters” and single deformities, and the interaction among embryonic organs during their origin and development. Am J Anat 28: 115–277, 1921. [Google Scholar]
- Sugihara I, Lang EJ, Llinás R. Serotonin modulation of inferior olivary oscillations and synchronicity: A multiple-electrode study in the rat cerebellum. Eur J Neurosci 7: 521–534, 1995. [DOI] [PubMed] [Google Scholar]
- Thomas MSC, Johnson MH. New advances in understanding sensitive periods in brain development. Curr Dir Psychol Sci 17: 1–5, 2008. [Google Scholar]
- Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Curr Biol 22: 2075–2080, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Del Rio-Bermudez C., Blumberg MS. Self-generated movements with “unexpected” sensory consequences. Curr Biol 24: 2136–2141, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Giessen RS, Koekkoek SK, van Dorp S, De Gruijl JR, Cupido A, Khosrovani S, Dortland B, Wellershaus K, Degen J, Deuchars J, Fuchs EC, Monyer H, Willecke K, De Jeu MTG, De Zeeuw CI. Role of olivary electrical coupling in cerebellar motor learning. Neuron 58: 599–612, 2008. [DOI] [PubMed] [Google Scholar]
- Wang SSH, Kloth AD, Badura A. The cerebellum, sensitive periods, autism. Neuron 83: 518–532, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Kano M. Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur J Neurosci 34: 1697–1710, 2011. [DOI] [PubMed] [Google Scholar]
- White JJ, Sillitoe RV. Development of the cerebellum: from gene expression patterns to circuit maps. Wiley Interdiscip Rev Dev Biol 2: 149–164, 2013. doi: 10.1002/wdev.65. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JGR. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373: 612–615, 1995. [DOI] [PubMed] [Google Scholar]
- Xu W, Jones S, Edgley SA. Event time representation in cerebellar mossy fibres arising from the lateral reticular nucleus. J Physiol 591: 1045–1062, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarom Y, Cohen D. The olivocerebellar system as a generator of temporal patterns. Ann NY Acad Sci 978: 122–134, 2002. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Rakic P. Differentiation of Purkinje cells and their relationship to other components of developing cerebellar cortex in man. J Comp Neurol 167: 27–48, 1976. [DOI] [PubMed] [Google Scholar]