Abstract
Despite advances in antiemetic therapy, chemotherapy-induced nausea and vomiting (CINV) remains the most feared and expected side effect of chemotherapy. Optimization of antiemetic therapy is important because CINV can lead to reduced quality of life, increased use of health care resources, and compromised treatment adherence. The evidence illustrates how antiemetic recommendations have evolved and raises ongoing issues and controversies in the management of CINV.
Despite advances in antiemetic therapy, chemotherapy-induced nausea and vomiting (CINV) remains the most feared and expected side effect of chemotherapy [1]. Optimization of antiemetic therapy is important because CINV can lead to reduced quality of life, increased use of health care resources, and compromised treatment adherence. The combination of anthracycline and cyclophosphamide is used extensively in breast cancer patients and traditionally has been classified as being moderately emetogenic [2]. However, in 2011, the American Society of Clinical Oncology reclassified the combination of an anthracycline with cyclophosphamide as highly emetogenic, given its propensity to induce CINV, particularly nausea, in breast cancer patients [3]. Since then, local [4], national [3, 5], and international [6] antiemetic guidelines have emphasized the use of aprepitant in combination with 5-hydroxytryptamine-3 (5-HT3) antagonists and corticosteroids in this population. Not surprisingly, aprepitant-containing regimens have become the standard recommendation at many institutions, and aprepitant use has soared. Has this optimism from both patients and physicians significantly improved patient care?
Despite the significant improvements in CINV reported in the aprepitant trials, control of CINV—and nausea in particular—remains suboptimal in patients receiving anthracycline and cyclophosphamide-based regimens [7, 8]. In the nontrial setting, ∼58%–71% of patients will have nausea and/or vomiting despite “optimal” antiemetic prescribing (C. Hernandez-Torres, personal communication) [9]. What is the cause of this discrepancy? It is likely a combination of factors including health care staff underestimating the incidence of CINV [10], suboptimal adherence to antiemetic guidelines [9], lack of an optimal antiemetic regimen [11], clinical trials overestimating the benefit of antiemetics [7], and, quite simply, current trial endpoints that do not fully reflect patient experience. We feel this is an opportune moment to review the evidence to illustrate how antiemetic recommendations have evolved and to raise ongoing issues and controversies in the management of CINV, especially in the breast cancer population.
As all oncologists are aware, there has been significant progress in CINV control. Historically, antiemetic regimens were developed using cisplatin (>50 mg/m2) as the benchmark because it causes CINV in almost all patients not receiving antiemetic prophylaxis. Based on the proposed pathophysiology of CINV, control of emesis has also been divided into acute (0–24 hours), delayed (>24–120 hours), and overall (0–120 hours) periods following chemotherapy [12], with antiemetic combinations being specifically chosen depending on their efficacy during these different time points. Following the results from antiemetic trials for patients receiving cisplatin-based chemotherapy (Table 1), most current antiemetic guidelines recommend a three-drug regimen consisting of aprepitant (days 1–3), 5-HT3 receptor antagonist (day 1), and dexamethasone (days 1–3 or 4) [3, 4, 6]; however, there are several areas to highlight regarding these trials. Although data for the aprepitant-based regimen from the cisplatin trials has shown significant improvement in control of chemotherapy-induced vomiting and modest improvement in controlling chemotherapy-induced nausea, closer examination of the data strongly suggests that that the same three-drug regimen with aprepitant does not necessarily confer the same benefits for patients receiving anthracycline and cyclophosphamide.
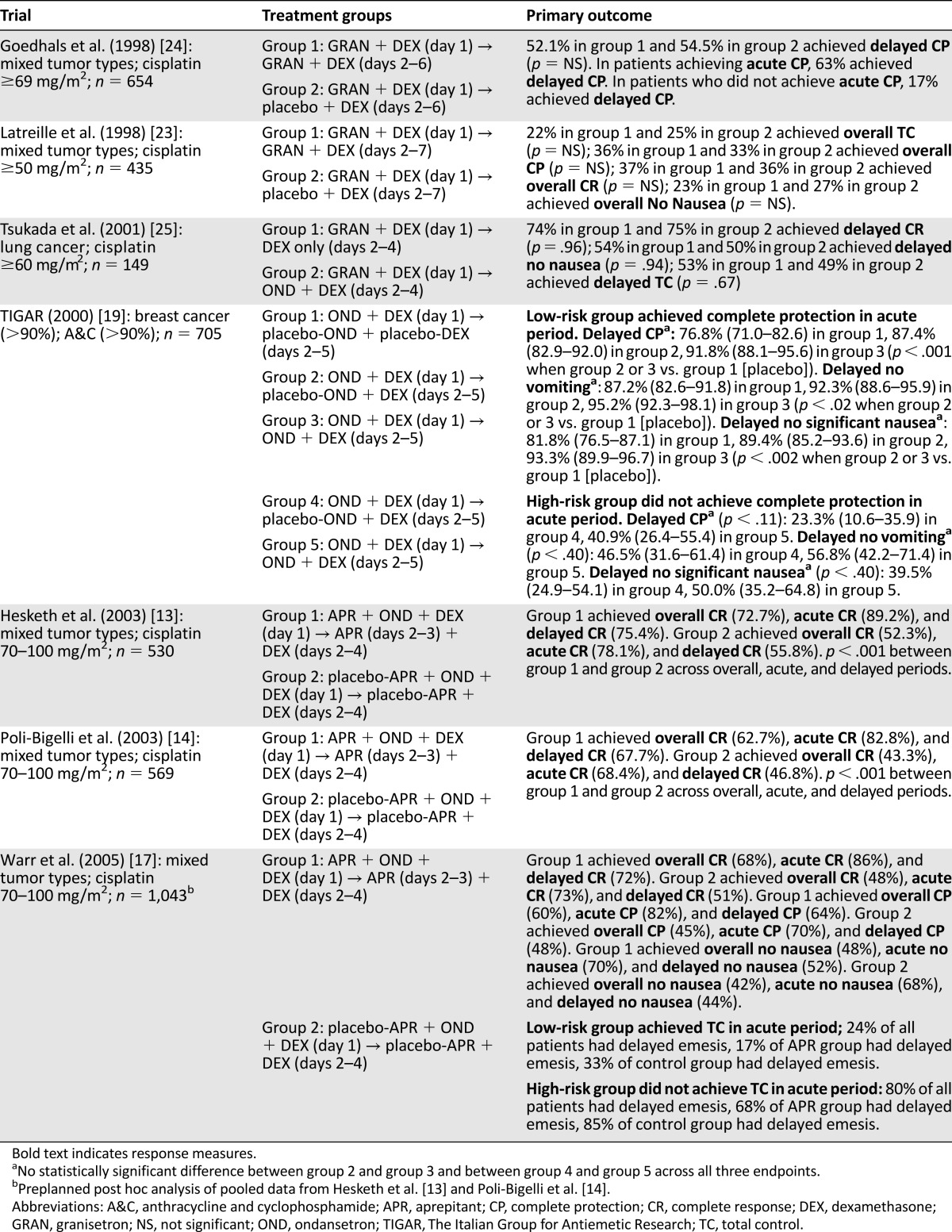
Table 1.
Summary of clinical trials published prior to the use of aprepitant in patients receiving anthracycline and cyclophosphamide
First, based on two multicenter trials in patients receiving cisplatin, use of an aprepitant-based regimen, as described above, improved CINV control during the acute period [13, 14] beyond that seen with regimens composed of 5-HT3 antagonist and dexamethasone only [15, 16]. Second, a pooled analysis of these two aprepitant trials in the cisplatin population showed an improvement of delayed vomiting and delayed nausea, albeit the latter effect being small (48% vs. 42%), in patients receiving cisplatin when added to dexamethasone given on days 2–4, regardless of CINV control in the acute period [17]. This subtlety is important because good control of CINV in the acute period in and of itself leads to better control of CINV in the delayed period [18, 19]. Third, ongoing controversy exists about the optimal regimen for CINV in the delayed period [20–22]. Trials in the late 1990s and early 2000s demonstrated that in the delayed period, for patients receiving cisplatin or anthracycline and cyclophosphamide, the addition of a 5-HT3 antagonist to dexamethasone was not superior to dexamethasone alone [19, 23–25]. In addition, the efficacy of 5-HT3 antagonists as a single agent for the control of delayed CINV is modest at best [20, 26, 27].
Following from the cisplatin trials, the same aprepitant-containing regimen was then evaluated in breast cancer patients receiving anthracyclines and cyclophosphamide. A large double-blind randomized controlled trial showed that an aprepitant-containing regimen provided superior overall complete response (i.e., no vomiting and no use of rescue antiemetics) compared with the control group (Table 2) [7]. Despite earlier data showing that the use of 5-HT3 antagonist in the delayed period is suboptimal, this pivotal trial used ondansetron instead of dexamethasone in the delayed period in the control group [7]. Moreover, although there was a trend showing that aprepitant provided better delayed complete response, the difference was not significant. In addition, contrary to the results from the pooled analysis of the cisplatin trials [17] aprepitant in the anthracycline trials did not confer an additional benefit for the two separate exploratory endpoints of no significant nausea (61% vs. 56%) and no nausea (33% vs. 33%). Subsequent studies in patients receiving anthracycline with cyclophosphamide regimens reaffirmed that aprepitant provided superior overall complete response, but they all used the same, likely suboptimal, control regimen [8, 28, 29]. In these studies, aprepitant also did not demonstrate a benefit for the control of nausea. Furthermore, a large systematic review published by the Agency for Healthcare Research and Quality Technology Assessment Program showed that although a regimen containing aprepitant, a 5-HT3 antagonist, and dexamethasone provided superior complete protection (no vomiting, no rescue, minimal nausea) and complete response in patients receiving cisplatin chemotherapy, there was no difference in complete protection with or without aprepitant in patients receiving moderately emetogenic chemotherapy (MEC), including patients receiving anthracycline and cyclophosphamide. However, there was significant advantage in complete response for patients receiving aprepitant in the MEC population, suggesting again that NK1 antagonists are not effective in controlling nausea in this population [30]. Another review focusing on chemotherapy-induced nausea drew the same conclusion [31]. Despite our hopes that NK1 antagonists will be the solution for CINV, the data do not strongly support the use of these costly drugs in the breast cancer population.
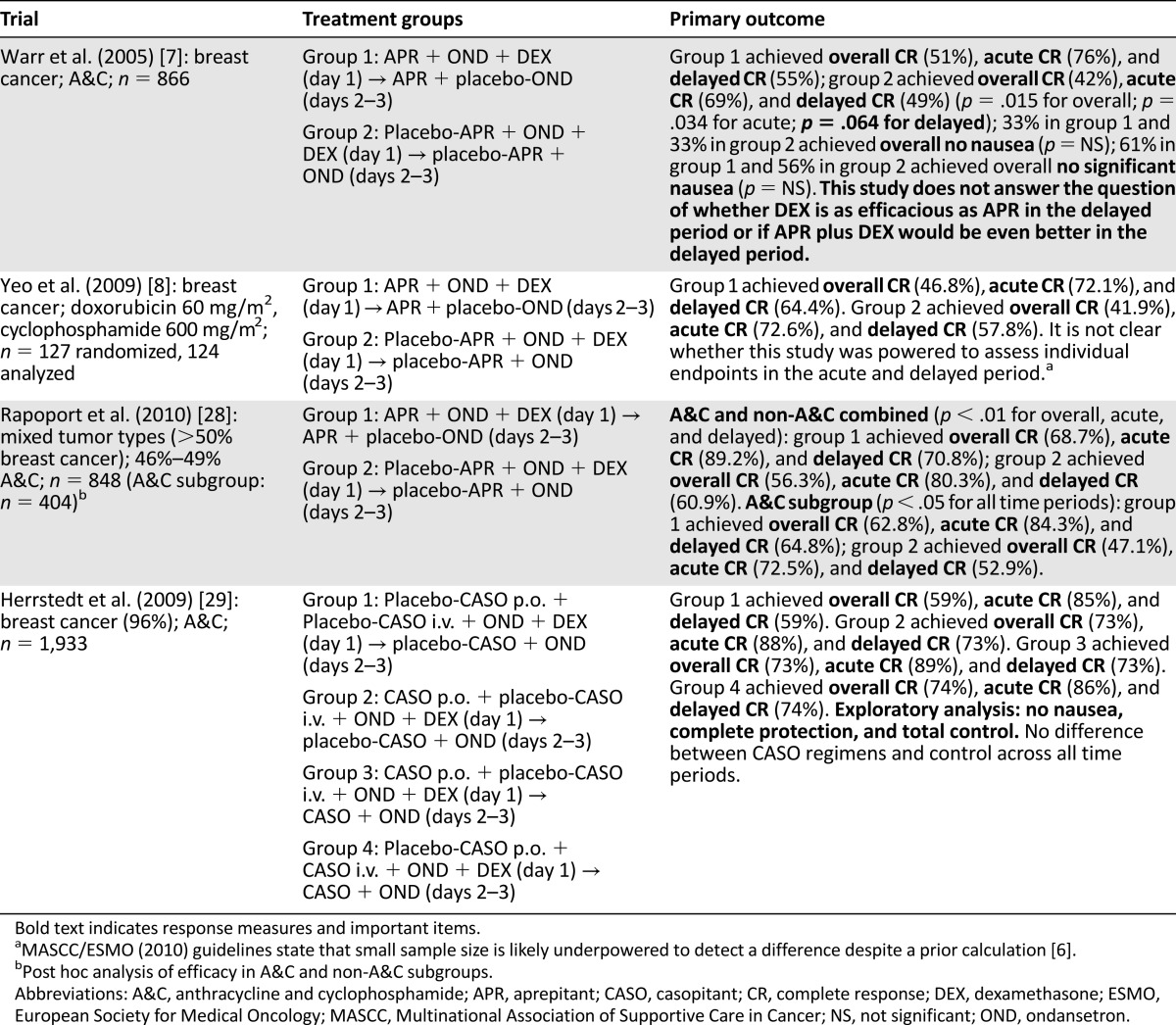
Table 2.
Summary of clinical trials testing aprepitant in breast cancer patients receiving anthracycline and cyclophosphamide
Clearly, there is an unmet need that current antiemetic guidelines must address. Despite these important trials, why is nausea control still such an issue? One reason that trials likely overestimate the benefit of antiemetic regimens is because of the large number of potentially confusing terms used to define CINV endpoints. This confusion arises from the need to measure control of both vomiting and nausea across the acute, delayed, and overall periods. Clinical trials, for example, have used a number of composite endpoints that represent various combinations of vomiting, nausea, both nausea and vomiting, or the use of rescue medications (Table 3). In an attempt to determine the optimal antiemetic regimen to prevent CINV in breast cancer patients receiving anthracycline and cyclophosphamide and to assess whether the randomized trial evidence supports current guideline recommendations of aprepitant use, we performed a systematic review that incorporated network meta-analysis [11]. In the 30 randomized controlled trials that met our criteria, 15 trial endpoints were used (Table 4). These included overall total control (6 trials), overall complete protection (6 trials), and overall complete response (15 trials). This made cross-trial comparisons challenging, and the results suggest that despite the rather firm recommendations of guideline groups, we still do not really know what the optimal antiemetic regimen is for our patients [32].
Table 3.
Definition of chemotherapy-induced nausea and vomiting outcomes used in randomized controlled trials
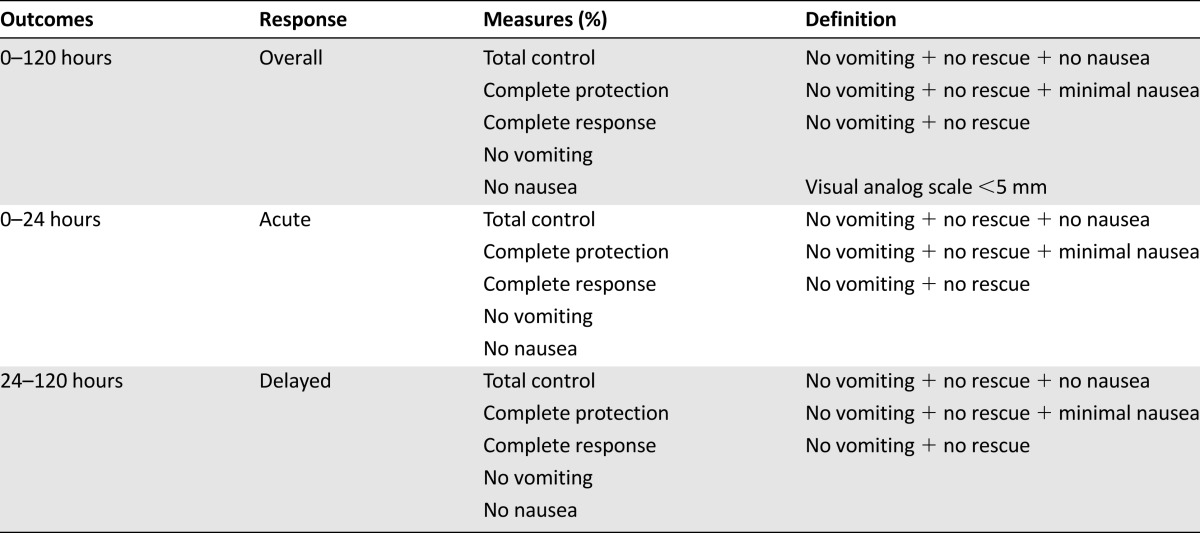
Table 4.
Summary of reported chemotherapy-induced nausea and vomiting outcomes across randomized controlled trials

Another confounding and more concerning issue is that the outcomes used in clinical trials might not reflect the patients’ actual experience of CINV. The use of composite endpoints is favored in clinical trials because a single dichotomous endpoint could be used in an attempt to summarize the patient’s experience of CINV. Few studies have used nausea as a primary endpoint [21]. Although there is value in using an endpoint such as total control (i.e., no vomiting, no rescue, and no nausea), nausea should also be measured and reported as a separate outcome because it is a subjective, patient-reported symptom, whereas vomiting is an objective and measurable phenomenon. Due to its subjective nature, the most reliable index is to measure “no nausea” rather than “no significant nausea,” as is used in endpoints such as complete protection. Several assessment tools exist to measure nausea as a single endpoint (e.g., visual analog scale [VAS]) or as part of a multiple symptom assessment tool (e.g., Edmonton Symptom Assessment System [ESAS], Functional Living Index-Emesis [FLIE], Functional Assessment of Chronic Illness Therapy [FACIT]). Because we were unable to find any literature that examined which CINV endpoints patients felt were the most important, we performed a survey of 168 patients with early stage breast cancer who had received anthracycline and cyclophosphamide-based chemotherapy to assess their experience of CINV and their perception of different CINV assessment tools (C. Hernandez-Torres, personal communication). Our survey was a 26-item questionnaire that asked patients to rate their nausea using five different scores based on the most common ones used in clinical trials. These included the VAS, the Likert scale, a scale that combined the Likert scale and descriptors of functional capacity, a modified version of the Common Terminology Criteria for Adverse Events 4.0, and a scale that combined the VAS and the Likert scale. The responses across the different scales were concordant most of the time. Patients consistently ranked nausea over vomiting as a “most feared side effect from chemotherapy.” In this survey, despite the use of guideline-recommended antiemetic regimens, 71% experienced nausea and 26% experienced vomiting. Of particular note regarding those composite endpoints that contained “use of rescue medications,” only 57% of those patients with nausea or vomiting took any rescue medication and did so only when the emesis was severe. Consequently, “use of rescue medication” as a surrogate measure for nausea control is inappropriate because it significantly underestimates nausea. Not surprisingly, patients strongly favored a CINV end point that included the absence of both nausea and vomiting.
In conclusion, there has been a significant improvement in CINV over past decades, and that is a testament to the randomized trials that have been performed; however, it is not the time to be complacent. Ultimately, all our patients deserve improved control of CINV. Where will progress come from? CINV remains important, and we need to continue to perform large randomized trials. We need to standardize CINV study endpoints and ensure that all existing studies allow open access to their data so that this process can begin. Standardization of data collection and reporting would allow objective cross-trial comparisons and meta-analyses to better inform treatment guidelines and health policy. We also have to accept that in this era of so-called personalized medicine, a cookie-cutter approach to prescribing antiemetics is not appropriate. We need better tools to stratify a patient’s CINV risk based on validated predictive tools [33, 34] to optimize care (NCT01913990). Clinical trials of more affordable, generic drugs, specifically olanzapine but also gabapentin, have shown some promising data and might offer better control of CINV without the need for more expensive, newer agents [35, 36]. Drugs that were recommended in the older guidelines [15] such as metoclopramide show some benefit compared with 5-HT3 antagonists in the delayed period when given with dexamethasone but have fallen to the wayside because of a low risk of extrapyramidal side effects [37]. The current evidence does not support the use of other agents such as cannabinoids and ginger for prevention or treatment of CINV [31]. Standardizing outcomes to what patients feel is important will improve their quality of life, leading to appropriate trial designs for future regimens and, hopefully, improving the cost-effectiveness of antiemetic treatment.
Footnotes
For Further Reading: Matti Aapro, Alexandra Carides, Bernardo L. Rapoport et al. Aprepitant and Fosaprepitant: A 10-Year Review of Efficacy and Safety. The Oncologist 2015;20:450–458.
Implications for Practice: Aprepitant (and its prodrug fosaprepitant) is a neurokinin-1 receptor antagonist approved more than a decade ago for the prevention of chemotherapy-induced nausea and vomiting (CINV). Its alternative mechanism of action complements traditional antiemetic drugs, enhancing control of CINV. This review examined safety and efficacy data for aprepitant and fosaprepitant accumulated since the first regulatory approval and explores recommendations in current guidelines for their use. The review serves as a useful reminder for the practitioner that aprepitant and fosaprepitant are valuable additions to the therapeutic armamentarium for the prevention of CINV. Future perspectives on potential uses of aprepitant and fosaprepitant for indications other than CINV are discussed.
Author Contributions
Conception/Design: Terry L. Ng, Brian Hutton, Mark Clemons
Provision of study material or patients: Terry L. Ng, Brian Hutton, Mark Clemons
Collection and/or assembly of data: Terry L. Ng, Brian Hutton, Mark Clemons
Data analysis and interpretation: Terry L. Ng, Brian Hutton, Mark Clemons
Manuscript writing: Terry L. Ng, Brian Hutton, Mark Clemons
Final approval of manuscript: Terry L. Ng, Brian Hutton, Mark Clemons
Disclosures
Mark Clemons: Eisai Pharmaceuticals (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. The Oncologist. 2014;19:127–134. doi: 10.1634/theoncologist.2013-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103–109. doi: 10.1200/JCO.1997.15.1.103. [DOI] [PubMed] [Google Scholar]
- 3.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [published correction appears in J Clin Oncol 2014;32:2117]. J Clin Oncol 2011;29:4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antiemetic report: Clinical evidence for recommendations. Available at https://www.cancercare.on.ca/CCO_DrugFormulary/Pages/FileContent.aspx?fileId=288895. Accessed October 26, 2014.
- 5.Ettinger DS, Armstrong DK, Barbour S, et al. Antiemesis. J Natl Compr Canc Netw. 2012;10:456–485. doi: 10.6004/jnccn.2012.0047. [DOI] [PubMed] [Google Scholar]
- 6.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: Results of the Perugia consensus conference. Ann Oncol. 2010;21(suppl 5):v232–v243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 7.Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23:2822–2830. doi: 10.1200/JCO.2005.09.050. [published correction appears in J Clin Oncol 2005;14:5851]. J Clin Oncol 2005;23:2822–2830. [DOI] [PubMed] [Google Scholar]
- 8.Yeo W, Mo FKF, Suen JJS, et al. A randomized study of aprepitant, ondansetron and dexamethasone for chemotherapy-induced nausea and vomiting in Chinese breast cancer patients receiving moderately emetogenic chemotherapy. Breast Cancer Res Treat. 2009;113:529–535. doi: 10.1007/s10549-008-9957-9. [DOI] [PubMed] [Google Scholar]
- 9.Aapro M, Molassiotis A, Dicato M, et al. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): The Pan European Emesis Registry (PEER) Ann Oncol. 2012;23:1986–1992. doi: 10.1093/annonc/mds021. [DOI] [PubMed] [Google Scholar]
- 10.Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261–2268. doi: 10.1002/cncr.20230. [DOI] [PubMed] [Google Scholar]
- 11.Ng TL, Clemons M, Kuchuk I et al. Anti-emetic choice for breast cancer patients receiving anthracycline-based chemotherapy: Using Network Meta-analyses to Drive Optimal Care. Cancer Res 2013;73(suppl 24):P3-09-02.
- 12.Hesketh PJ, Van Belle S, Aapro M, et al. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer. 2003;39:1074–1080. doi: 10.1016/s0959-8049(02)00674-3. [DOI] [PubMed] [Google Scholar]
- 13.Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–4119. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 14.Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090–3098. doi: 10.1002/cncr.11433. [DOI] [PubMed] [Google Scholar]
- 15.Gralla RJ, Osoba D, Kris MG, et al. Recommendations for the use of antiemetics: Evidence-based, clinical practice guidelines. J Clin Oncol. 1999;17:2971–2994. doi: 10.1200/JCO.1999.17.9.2971. [DOI] [PubMed] [Google Scholar]
- 16.Prevention of chemotherapy- and radiotherapy-induced emesis: Results of Perugia Consensus Conference. Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC). Ann Oncol. 1998;9:811–819. [PubMed] [Google Scholar]
- 17.Warr DG, Grunberg SM, Gralla RJ, et al. The oral NK(1) antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: Pooled data from 2 randomised, double-blind, placebo controlled trials. Eur J Cancer. 2005;41:1278–1285. doi: 10.1016/j.ejca.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Delayed emesis induced by moderately emetogenic chemotherapy: Do we need to treat all patients? The Italian Group for Antiemetic Research. Ann Oncol. 1997;8:561–567. [PubMed] [Google Scholar]
- 19.Dexamethasone alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. The Italian Group for Antiemetic Research. N Engl J Med. 2000;342:1554–1559. doi: 10.1056/NEJM200005253422102. [DOI] [PubMed] [Google Scholar]
- 20.Hickok JT, Roscoe JA, Morrow GR, et al. 5-Hydroxytryptamine-receptor antagonists versus prochlorperazine for control of delayed nausea caused by doxorubicin: A URCC CCOP randomised controlled trial. Lancet Oncol. 2005;6:765–772. doi: 10.1016/S1470-2045(05)70325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roscoe JA, Heckler CE, Morrow GR, et al. Prevention of delayed nausea: A University of Rochester Cancer Center Community Clinical Oncology Program study of patients receiving chemotherapy. J Clin Oncol. 2012;30:3389–3395. doi: 10.1200/JCO.2011.39.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roila F, Ruggeri B, Ballatori E, et al. Aprepitant versus dexamethasone for preventing chemotherapy-induced delayed emesis in patients with breast cancer: A randomized double-blind study. J Clin Oncol. 2014;32:101–106. doi: 10.1200/JCO.2013.51.4547. [DOI] [PubMed] [Google Scholar]
- 23.Latreille J, Pater J, Johnston D, et al. Use of dexamethasone and granisetron in the control of delayed emesis for patients who receive highly emetogenic chemotherapy. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1998;16:1174–1178. doi: 10.1200/JCO.1998.16.3.1174. [DOI] [PubMed] [Google Scholar]
- 24.Goedhals L, Heron JF, Kleisbauer JP, et al. Control of delayed nausea and vomiting with granisetron plus dexamethasone or dexamethasone alone in patients receiving highly emetogenic chemotherapy: A double-blind, placebo-controlled, comparative study. Ann Oncol. 1998;9:661–666. doi: 10.1023/a:1008256115221. [DOI] [PubMed] [Google Scholar]
- 25.Tsukada H, Hirose T, Yokoyama A, et al. Randomised comparison of ondansetron plus dexamethasone with dexamethasone alone for the control of delayed cisplatin-induced emesis. Eur J Cancer. 2001;37:2398–2404. doi: 10.1016/s0959-8049(01)00326-4. [DOI] [PubMed] [Google Scholar]
- 26.Roila F, Warr D, Clark-Snow RA, et al. Delayed emesis: Moderately emetogenic chemotherapy. Support Care Cancer. 2005;13:104–108. doi: 10.1007/s00520-004-0700-8. [DOI] [PubMed] [Google Scholar]
- 27.Geling O, Eichler H-G. Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol. 2005;23:1289–1294. doi: 10.1200/JCO.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: A randomized, double-blind study. Support Care Cancer. 2010;18:423–431. doi: 10.1007/s00520-009-0680-9. [DOI] [PubMed] [Google Scholar]
- 29.Herrstedt J, Apornwirat W, Shaharyar A, et al. Phase III trial of casopitant, a novel neurokinin-1 receptor antagonist, for the prevention of nausea and vomiting in patients receiving moderately emetogenic chemotherapy. J Clin Oncol. 2009;27:5363–5369. doi: 10.1200/JCO.2009.21.8511. [DOI] [PubMed] [Google Scholar]
- 30.McDonagh M, Peterson K, Thakurta S. Consideration of evidence on antiemetic drugs for nausea and vomiting associated with chemotherapy or radiation therapy in adults. Technology assessment report. Available at http://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id74ta.pdf. Accessed January 23, 2015. [PubMed]
- 31.Navari RM. Treatment of chemothearpy-induced nausea. Community Oncol. 2012;9:20–26. [Google Scholar]
- 32.Ng TL, Clemons M, Hutton B, et al. Aprepitant versus dexamethasone to prevent delayed emesis after chemotherapy. J Clin Oncol. 2014;32:2184–2185. doi: 10.1200/JCO.2014.55.3503. [DOI] [PubMed] [Google Scholar]
- 33.Bouganim N, Dranitsaris G, Hopkins S, et al. Prospective validation of risk prediction indexes for acute and delayed chemotherapy-induced nausea and vomiting. Curr Oncol. 2012;19:e414–e421. doi: 10.3747/co.19.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dranitsaris G, Bouganim N, Milano C, et al. Prospective validation of a prediction tool for identifying patients at high risk for chemotherapy-induced nausea and vomiting. J Support Oncol. 2013;11:14–21. doi: 10.1016/j.suponc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A randomized phase III trial. J Support Oncol. 2011;9:188–195. doi: 10.1016/j.suponc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Cruz FM, de Iracema Gomes Cubero D, Taranto P, et al. Gabapentin for the prevention of chemotherapy-induced nausea and vomiting: A pilot study. Support Care Cancer. 2012;20:601–606. doi: 10.1007/s00520-011-1138-4. [DOI] [PubMed] [Google Scholar]
- 37.Navari RM. Pathogenesis-based treatment of chemotherapy-induced nausea and vomiting—two new agents. J Support Oncol. 2003;1:89–103. [PubMed] [Google Scholar]
- 38.Aapro M, Fabi A, Nole F, et al. Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol. 2010;21:1083–1088. doi: 10.1093/annonc/mdp584. [DOI] [PubMed] [Google Scholar]
- 39.Herrstedt J, Muss HB, Warr DG, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. Cancer. 2005;104:1548–1555. doi: 10.1002/cncr.21343. [DOI] [PubMed] [Google Scholar]
- 40.Vardy J, Pond G, Dodd A, et al. A randomized double-blind placebo-controlled cross-over trial of the impact on quality of life of continuing dexamethasone beyond 24 h following adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2012;136:143–151. doi: 10.1007/s10549-012-2205-3. [DOI] [PubMed] [Google Scholar]
- 41.Herrington JD, Jaskiewicz AD, Song J. Randomized, placebo-controlled, pilot study evaluating aprepitant single dose plus palonosetron and dexamethasone for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. Cancer. 2008;112:2080–2087. doi: 10.1002/cncr.23364. [DOI] [PubMed] [Google Scholar]
- 42.Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: A double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10:115–124. doi: 10.1016/S1470-2045(08)70313-9. [DOI] [PubMed] [Google Scholar]
- 43.Celio L, Frustaci S, Denaro A, et al. Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: A randomized, multicenter, phase III trial. Support Care Cancer. 2011;19:1217–1225. doi: 10.1007/s00520-010-0941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campora E, Baldini E, Rubagotti A, et al. Double-blind randomized trial of lorazepam versus placebo with methylprednisolone for control of emesis due to non-cisplatin containing chemotherapy. J Chemother. 1990;2:336–339. doi: 10.1080/1120009x.1990.11739039. [DOI] [PubMed] [Google Scholar]
- 45.Lajolo PP, de Camargo B, del Giglio A. Omission of day 2 of antiemetic medications is a cost saving strategy for improving chemotherapy-induced nausea and vomiting control: Results of a randomized phase III trial. Am J Clin Oncol. 2009;32:23–26. doi: 10.1097/COC.0b013e318178e4fe. [DOI] [PubMed] [Google Scholar]
- 46.Mabro M, Granisétron PK. Comparative trial of oral granisetron and intravenous ondansetron in patients receiving chemotherapy for breast cancer. Study Group of Granisetron. Bull Cancer. 1999;86:295–301. [PubMed] [Google Scholar]
- 47.Bosnjak SM, Nesković-Konstantinović ZB, Radulović SS, et al. High efficacy of a single oral dose of ondansetron 8 mg versus a metoclopramide regimen in the prevention of acute emesis induced by fluorouracil, doxorubicin and cyclophosphamide (FAC) chemotherapy for breast cancer. J Chemother. 2000;12:446–453. doi: 10.1179/joc.2000.12.5.446. [DOI] [PubMed] [Google Scholar]
- 48.Janinis J, Giannakakis T, Athanasiades A, et al. A randomized open-label parallel-group study comparing ondansetron with ondansetron plus dexamethasone in patients with metastatic breast cancer receiving high-dose epirubicin. A Hellenic Cooperative Oncology Group study. Tumori. 2000;86:37–41. doi: 10.1177/030089160008600107. [DOI] [PubMed] [Google Scholar]
- 49.Campora E, Giudici S, Merlini L, et al. Ondansetron and dexamethasone versus standard combination antiemetic therapy. A randomized trial for the prevention of acute and delayed emesis induced by cyclophosphamide-doxorubicin chemotherapy and maintenance of antiemetic effect at subsequent courses. Am J Clin Oncol. 1994;17:522–526. [PubMed] [Google Scholar]
- 50.Dexamethasone, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. N Engl J Med. 1995;332:1–5. doi: 10.1056/NEJM199501053320101. [DOI] [PubMed] [Google Scholar]
- 51.Herrington JD, Kwan P, Young RR, et al. Randomized, multicenter comparison of oral granisetron and oral ondansetron for emetogenic chemotherapy. Pharmacotherapy. 2000;20:1318–1323. doi: 10.1592/phco.20.17.1318.34894. [DOI] [PubMed] [Google Scholar]
- 52.Koo WH, Ang PT. Role of maintenance oral dexamethasone in prophylaxis of delayed emesis caused by moderately emetogenic chemotherapy. Ann Oncol. 1996;7:71–74. doi: 10.1093/oxfordjournals.annonc.a010483. [DOI] [PubMed] [Google Scholar]
- 53.Lofters WS, Pater JL, Zee B, et al. Phase III double-blind comparison of dolasetron mesylate and ondansetron and an evaluation of the additive role of dexamethasone in the prevention of acute and delayed nausea and vomiting due to moderately emetogenic chemotherapy. J Clin Oncol. 1997;15:2966–2973. doi: 10.1200/JCO.1997.15.8.2966. [DOI] [PubMed] [Google Scholar]
- 54.Pater JL, Lofters WS, Zee B, et al. The role of the 5-HT3 antagonists ondansetron and dolasetron in the control of delayed onset nausea and vomiting in patients receiving moderately emetogenic chemotherapy. Ann Oncol. 1997;8:181–185. doi: 10.1023/a:1008247830641. [DOI] [PubMed] [Google Scholar]
- 55.Kaizer L, Warr D, Hoskins P, et al. Effect of schedule and maintenance on the antiemetic efficacy of ondansetron combined with dexamethasone in acute and delayed nausea and emesis in patients receiving moderately emetogenic chemotherapy: A phase III trial by the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1994;12:1050–1057. doi: 10.1200/JCO.1994.12.5.1050. [DOI] [PubMed] [Google Scholar]
- 56.Wenzell CM, Berger MJ, Blazer MA, et al. Pilot study on the efficacy of an ondansetron- versus palonosetron-containing antiemetic regimen prior to highly emetogenic chemotherapy. Support Care Cancer. 2013;21:2845–2851. doi: 10.1007/s00520-013-1865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25:1328–1333. doi: 10.1093/annonc/mdu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kosaka Y, Sengoku N, Minatani N et al. Final result of randomised controlled phase II study of the efficiency of palonosetron, aprepitant, and dexamethasone for day 1 with or without dexamethasone on days 2 and 3 [abstract P3-09-03]. Presented at: San Antonio Breast Cancer Symposium; December 10–14, 2013; San Antonio, TX. [Google Scholar]


