This study retrospectively reviewed the demographics, treatment history, and clinical outcomes for all patients with acral melanoma treated with ipilimumab from two academic centers between February 2006 and June 2013. Ipilimumab was found to have activity that appears equivalent to unselected melanoma proving it to be a viable treatment option for this melanoma subpopulation.
Keywords: Melanoma, Acral, Ipilimumab, CTLA-4, CKIT, Immune therapy
Abstract
Background.
Ipilimumab improves overall survival (OS) in advanced melanoma. Acral melanoma is an uncommon clinical subtype of this disease associated with poor prognosis. The clinical activity of ipilimumab has not been well-defined in advanced acral melanoma.
Methods.
We retrospectively reviewed the demographics, treatment history, and clinical outcomes for all patients with acral melanoma treated with ipilimumab from two academic centers between February 2006 and June 2013. Using Cox proportional hazards models, we assessed for factors that correlated with OS.
Results.
A total of 35 patients with acral melanoma received ipilimumab. Melanomas arose on volar surfaces (n = 28) and subungual sites (n = 7); stage M1c disease was present in 54%, and 45% had elevated serum lactate dehydrogenase (LDH). Best response by RECIST 1.1 criteria was complete response in 1 patient, partial response in 3, and stable disease (SD) in 4 for an objective response rate (ORR) of 11.4% and a clinical benefit rate (ORR + SD) at 24 weeks of 22.9%. Median progression-free survival was 2.5 months (95% confidence interval [CI]: 2.3–2.7 months); median OS was 16.7 months (95% CI: 10.9–22.5 months). Normal LDH and absolute lymphocyte count ≥1,000 at 7 weeks predicted longer OS. Immune-related adverse events (irAEs) were noted in 16 patients including 7 with grade 3/4 irAEs (20%).
Conclusion.
Ipilimumab is clinically active in acral melanoma with similar ORR and OS compared with unselected melanoma populations. Ipilimumab remains a viable therapeutic option for patients with advanced acral melanoma.
Implications for Practice:
Ipilimumab is a commonly used immune therapy that improves survival in metastatic melanoma. The clinical activity of ipilimumab in certain rare melanoma subtypes, such as uveal or mucosal melanomas, is suboptimal. Acral melanoma is another unusual subtype of this disease that arises on the palms, soles, and nailbeds. In this study of 35 patients with acral melanoma from 2 centers, ipilimumab was found to have activity that appears equivalent to unselected melanoma (response rate of 11.4%, median overall survival of 16.7 months). Ipilimumab remains a viable treatment option for this melanoma subpopulation.
Introduction
Ipilimumab was the first agent to demonstrate improved overall survival in advanced melanoma in a randomized phase III trial [1, 2]. This agent is a monoclonal antibody to cytotoxic T lymphocyte antigen-4 (CTLA-4), which activates antitumor immunity by inhibiting this key immune regulatory checkpoint. Despite low response rates by classically determined methods, approximately 20% of patients survive 3–5 years after starting therapy, nearly twice that of historical controls [1, 3, 4]. Recently, retrospective reviews have shown that ipilimumab has modest clinical activity in uveal and mucosal melanomas, uncommon subtypes of this disease [5–8]. Although these subgroups have not been directly compared with cutaneous melanoma, the objective response rate and median overall survival appears inferior to unselected, largely cutaneous, melanoma populations.
Acral melanoma is another uncommon, atypical form of melanoma that arises on the volar (palms and soles) and subungual (nailbeds) surfaces. Despite comprising only 2%–10% of all melanomas, acral melanoma is the most frequent subtype in patients of African, Middle Eastern, and Asian descent [9–11]. Acral melanoma confers a worse overall prognosis compared with cutaneous melanomas in Western series (largely in the pre-ipilimumab/BRAF inhibitor era), although this negative effect is not clear in Asian studies [9, 12–16]. The spectrum and frequency of genetic alterations in this subtype is distinct from cutaneous melanoma; KIT mutations occur more commonly (15%–20% vs. 1%), BRAFV600 mutations arise less often (15% vs. 45%–50%), and NRAS mutations are found at similar incidence (15%–20%) [17, 18]. Accordingly, no genotype-specific treatments are available for many patients, thus making ipilimumab and other immune therapies the principal therapeutic strategy for patients with advanced acral melanoma. In addition, the frequency of mutations overall in acral melanoma is much less than in cutaneous melanoma [19]. Defining the role and clinical activity of ipilimumab in this unique clinical and molecular subset of this disease is critical.
We conducted a retrospective analysis of 35 patients from two centers with locally advanced or metastatic acral melanoma who received ipilimumab in a clinical trial or as a commercially available therapy. Here, we report the clinical efficacy and toxicity of ipilimumab in this patient population.
Methods
Patients
After institutional review board approval was obtained, we identified patients with acral melanoma who received ipilimumab from existing, prospectively collected institutional databases. Acral melanoma was clinically defined as melanomas arising on the volar (palmar or plantar) or subungual surfaces. Each patient sample was reviewed by pathologists at the treating institutions confirming the diagnosis of acral melanoma. Clinical data were collected from patients treated at Memorial Sloan-Kettering Cancer Center (MSKCC; n = 27) and Vanderbilt Ingram Cancer Center (VICC; n = 8). All patients who received at least one dose of ipilimumab between May 2006 and June 2013 were included. Clinical data collected included age, gender, site of primary tumor, tumor genotyping, American Joint Committee on Cancer (AJCC) melanoma stage at diagnosis [20] and at the onset of advanced/metastatic disease, and timing and pattern of first recurrence (i.e., in-transit, regional lymph nodes, distant metastases). Laboratory values at the start of ipilimumab and in response to treatment were collected, including lactate dehydrogenase (LDH) and absolute lymphocyte count (ALC). Tumor genotyping was performed by SNaPshot (VICC) or by polymerase chain reaction or Sequenom-based screening (MSKCC) (supplemental online Tables 1, 2). Patients treated in a clinical trial or with commercially available therapy were included and received ipilimumab at doses of either 3 or 10 mg/kg. Information regarding prior and subsequent therapies was also collected. All patient data were deidentified for analysis.
Efficacy Assessment
Objective responses (complete response [CR] or partial response [PR]) were assessed by Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 [21] and confirmed by an independent radiologist at each site or had been previously confirmed in a clinical trial. The clinical benefit rate was defined as patients who experienced CR or PR or had stable disease at 24 weeks. Progression-free survival (PFS) was calculated as the time from starting ipilimumab to progression as confirmed by the independent radiologist or in a clinical trial. Overall survival (OS) was calculated in the method of Kaplan and Meier as the time from ipilimumab initiation to death for any reason.
To assess factors contributing to the duration of overall survival, we performed multivariate Cox proportional hazards analyses adjusting for age and gender for the following variables: baseline LDH, baseline ALC, ALC at 7 weeks, site of primary tumor (volar vs. subungual), metastatic stage (AJCC M1c vs. others), and length of time between diagnosis and first recurrence. LDH was stratified as higher or lower than the institutional upper limit of normal; ALC was stratified as higher or lower than 1,000 cells per microliter. The length of time to recurrence was stratified as greater than or less than 1 year. Statistical significance in the multivariable analysis was defined as p < .05. Survival models were performed using R version 3.1.1; Kaplan-Meier curves were generated with SPSS version 22 (SPSS software; IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/).
Toxicity Assessment
Immune related adverse events (irAEs) were assessed through review of clinician notes and clinical trial adverse event collection forms. Grades were assigned based on Common Terminology Criteria for Adverse Events version 4.0.
Results
Clinical Characteristics
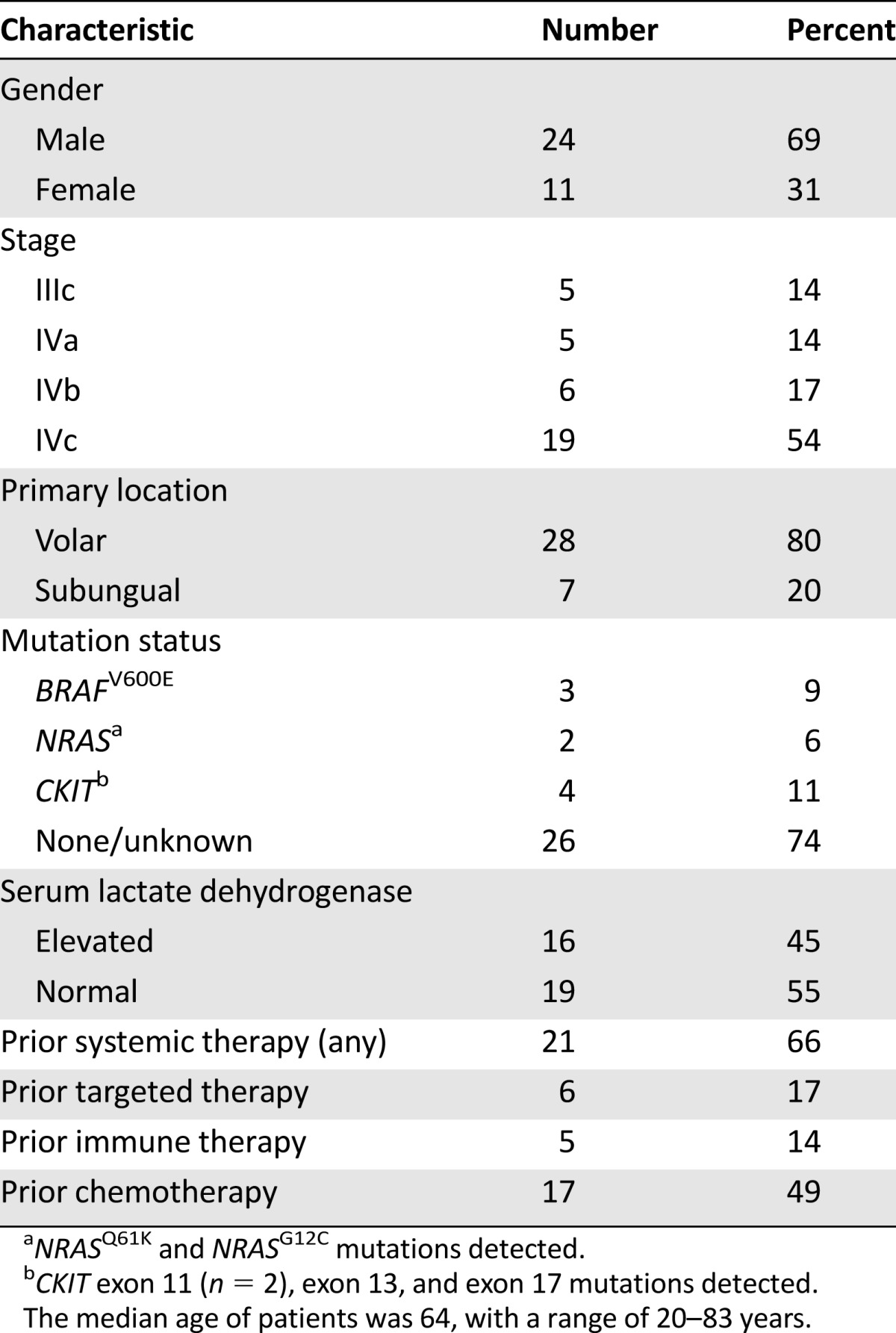
Of 35 patients treated, 28 were Caucasian, 6 were of African descent, and 1 was Hispanic (Table 1). Primary tumors arose on the volar surfaces in 28 patients, and 7 had subungual primaries. Of 31 patients whose tumors were genotyped, 3 had identified mutations in BRAFV600E, 2 had identified mutations in NRAS, and 4 had identified mutations in KIT. Stage IVc disease was present in 19 patients (55%); serum LDH was elevated in 45%. The median number of doses of ipilimumab administered was 4; 33 patients received 3 mg/kg dosing, and 2 received 10 mg/kg. Thirteen patients received therapy in a clinical trial (37%), whereas the remainder received commercially available ipilimumab.
Table 1.
Baseline characteristics
The median number of prior systemic therapies was one. In total, 4 patients previously underwent an isolated limb infusion, 15 patients received cytotoxic chemotherapy prior to ipilimumab, and 5 patients received high-dose interleukin-2 (IL-2) or an anti-PD-1 (programmed death-1)/PD-L1 antibody. Subsequent to ipilimumab therapy, four patients were reinduced with ipilimumab, and five patients received other immune-based therapies (e.g., anti-PD-1). Seven patients received targeted therapy; all three patients harboring BRAFV600 mutations received BRAF inhibitors. Two of four patients with a KIT mutation received KIT inhibitors (one with imatinib and one with dasatinib); two other patients received other experimental targeted agents.
Clinical Efficacy
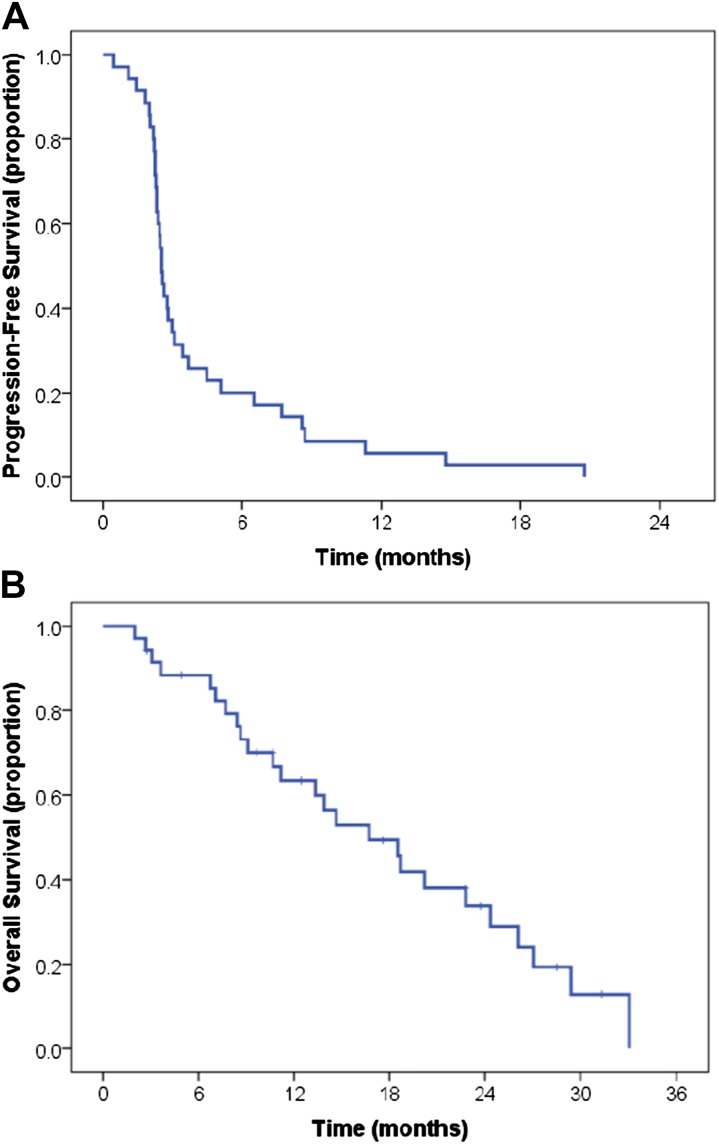
We assessed the objective response rate; the best response experienced at any time included one patient with a CR (2.9%), 3 with PRs (8.6%), and 4 with stable disease (11.4%). The objective response rate was 11.4%, and the clinical benefit rate (CR + PR + stable disease) was 22.9% (Table 2). All patients with objective responses received 3 mg/kg ipilimumab; 1 patient who received 10 mg/kg ipilimumab had stable disease as the best response. The median PFS from initiation of ipilimumab was 2.5 months (95% confidence interval: 2.3–2.7 months), and all patients had experienced disease progression at last follow-up (Fig. 1A). The median OS was 16.7 months (95% confidence interval: 10.9–22.5 months) (Fig. 1B). Altogether, 8 patients (28%) were alive 24 months after therapy initiation, accounting for 6 patients that were alive and censored for follow up prior to 24 months.
Table 2.
Overall response rate and clinical benefit rate from ipilimumab in patients with advanced acral melanoma (RECIST 1.1 criteria)
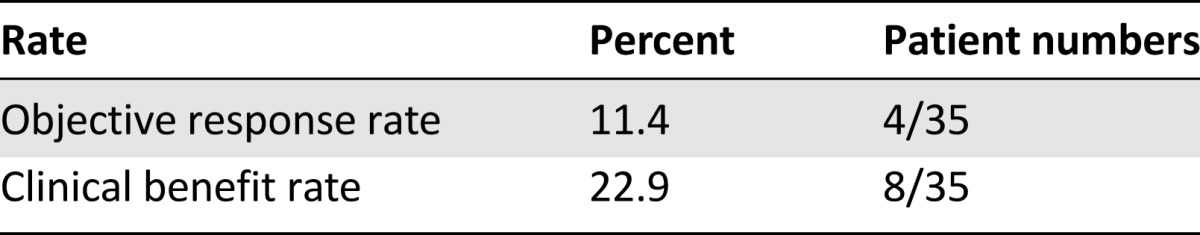
Figure 1.
Progression-free survival (A) and overall survival (B) for study population.
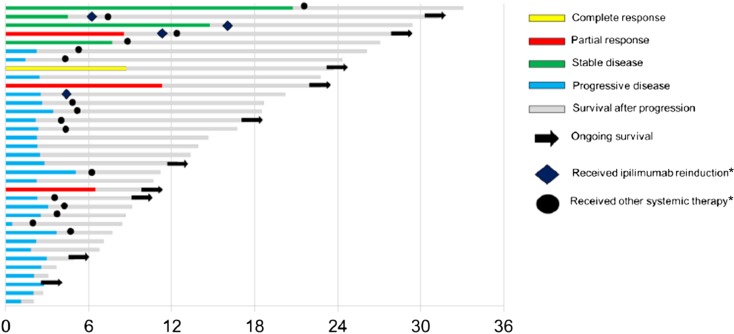
Individual responses to therapy and duration of treatment are displayed in Figure 2. The patient who experienced a CR had largely subcutaneous disease involvement and had rapid disease regression by imaging and physical exam, followed by an isolated recurrence approximately 8 months into therapy. Surgical resection of the progressing lesion was performed, and the patient remains free of disease at last follow-up, approximately 24 months after treatment initiation. There were 4 patients who experienced partial responses lasting from 4 to 9 months. An additional 4 patients had stable disease lasting greater than 6 months as their best response. Of note, 1 additional patient who had disease progression by RECIST criteria experienced stable disease defined by immune-related response criteria that lasted >9 months [22]. Finally, 4 patients received an additional 4 cycles of ipilimumab reinduction, and 16 received other melanoma therapies following progression on ipilimumab.
Figure 2.
Best response, progression-free interval, and overall survival for individual patients. *, Received at any point following ipilimumab (not to scale).
To investigate factors that influenced overall survival, we performed multivariable Cox regression analysis adjusted for age and gender. Elevated LDH predicted an increased risk of death (hazard ratio, 2.60; 95% CI: 1.01–6.73; p = .048), whereas ALC at 7 weeks >1,000 cells per microliter was associated with decreased risk of death (hazard ratio, 0.17; 95% CI: 0.05–0.56; p = .004). Other factors, including age, gender, site of primary tumor, metastatic stage (stage M1c vs. other), baseline ALC, and length of latency period prior to first recurrence did not influence overall survival in this population.
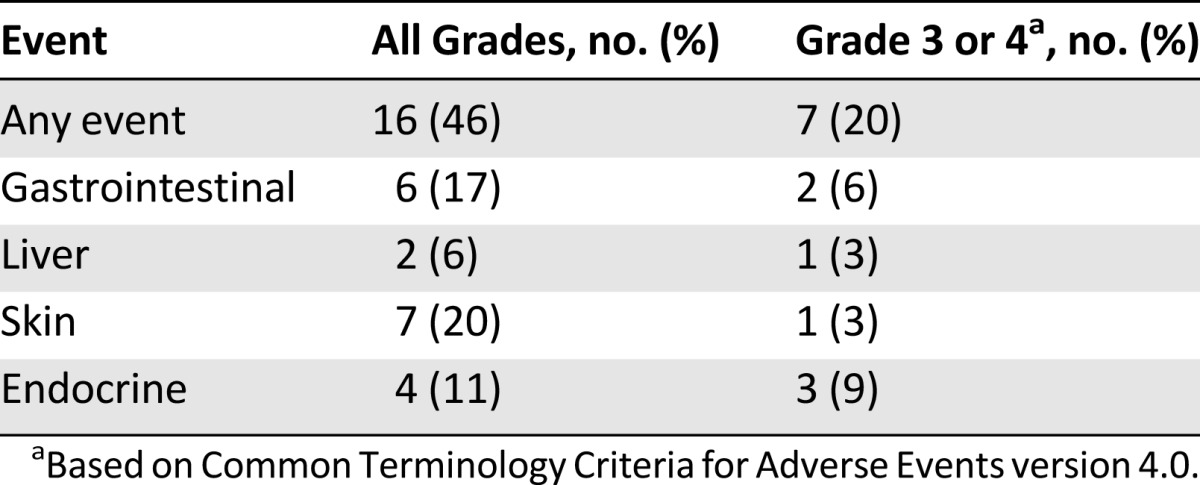
Toxicity
irAEs of any grade occurred in 20 patients (57%; Table 3). Grade 3/4 events occurred in 17% of patients, including colitis (n = 2), hypophysitis (n = 2), hepatotoxicity (n = 1), and skin toxicity (n = 1). Each of these events resolved with corticosteroid administration, although patients with hypophysitis required ongoing hormone replacement with levothyroxine and hydrocortisone. No patients died of irAEs.
Table 3.
Immune-related toxicities from ipilimumab
Discussion
In this retrospective study from two large academic melanoma centers, we found that ipilimumab had clinical efficacy in patients with advanced acral melanoma. To our knowledge, this is the first study of ipilimumab specifically focusing on this subtype of melanoma. We observed that the objective response (11%) and stable disease rates (23%) were comparable to unselected populations [1, 23, 24]. Several patients had prolonged and ongoing clinical benefit; furthermore, the median overall survival was >16 months. Ipilimumab was fairly well tolerated with a similar toxicity profile as demonstrated in previous clinical trials; grade 3 toxicities occurred in 17% of patients.
Acral melanoma is a challenging subgroup of this disease with distinct epidemiologic and genetic features. The atypical location (palms, soles, and nailbeds) and occurrence in populations generally at low risk for melanoma may delay diagnosis and adversely affect prognosis. Although several large, whole-exome sequencing studies have been performed in melanoma, very few of these involved acral melanomas [19, 25]. In these and other studies, however, mutations have been identified in KIT, BRAF, and NRAS in approximately 15% of acral melanomas each [17, 18]. By contrast, in cutaneous melanomas BRAFV600 mutations are present in 40%–50%, and KIT mutations occur in <1%. Acral melanomas harboring BRAF or KIT mutations may respond to commercially available inhibitors of BRAF (vemurafenib, dabrafenib), MEK (trametinib), or KIT (imatinib, nilotinib). The majority of patients, however, do not have available targeted therapy options.
Distinct immunologic features may drive biologic differences in response to immune-based agents as well. Signature C-T substitutions characteristic of ultraviolet light exposure and overall mutational burden occur at distinct (lower) frequencies when compared with cutaneous melanoma [26–29]. Given the association with total somatic mutational burden in melanoma and response to ipilimumab, it might be hypothesized that activity would be inferior in acral melanoma [30]. Furthermore, a recent study suggested that the immune infiltrate is minimal or absent in most acral melanomas [31]. Based on these factors, one could posit that response to immune therapies might be suboptimal.
Nevertheless, immune-based therapies with high-dose IL-2, and more recently ipilimumab and anti-PD-1, have become a mainstay of therapy for patients with acral melanoma (as well as other subtypes). Although additional therapeutic strategies for acral melanoma are still needed, we observed that ipilimumab has clinical activity that appeared comparable with unselected melanoma populations. In the large phase III clinical trials, the objective response rate (ORR) was 11% with a median OS of 10 months in previously treated patients given 3 mg/kg ipilimumab and 15.2% with a median OS of 11.2 months in untreated patients given 10 mg/kg ipilimumab in combination with dacarbazine [1, 2]. The ORR (11.4%) and median OS (16.7 months) in this series was comparable to these prior studies. Further investigation into the specific immune biology of acral melanoma is needed.
In contrast to this study, previous retrospective series have reported inferior outcomes with ipilimumab in other rare subtypes of melanoma. Among 33 patients with mucosal melanoma, 2 patients experienced a response (6.1%), and the median OS was 6.4 months [6]. In uveal melanoma, 2 of 39 patients (5.1%) had an objective response to ipilimumab; the median OS was 9.6 months [5]. Reports from the Italian Expanded Access Program of ipilimumab for patients receiving prior therapies also reported low response rates (12% and 5%) and median OS (6.4 and 6 months) for mucosal and uveal melanoma, respectively [7, 8].
This study has several limitations. Our analysis consisted of patients treated at two large melanoma centers, which may limit the generalizability of our results. In addition, the retrospective nature of the study may introduce bias from patient selection and from other unidentified sources. Patients were predominantly Caucasian; therefore, generalizability to Asian and other non-Western populations is not clear. Otherwise, however, patient characteristics and prior lines of therapy closely approximate other prospective and retrospective clinical studies.
Conclusion
Ipilimumab is an active therapy in acral melanoma with similar efficacy as in unselected patient populations. Although more effective therapies are still needed, ipilimumab is a reasonable and effective treatment option for patients with advanced acral melanoma. Further evaluation of other emerging therapeutics (including agents targeting the PD-1 receptor) in this cohort is needed.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
Research support was provided by NIH Grant K12 CA 0906525 (to D.B.J.), American Cancer Society Melanoma Professorship in Translational Medicine SIMP-09-102-01-CCE (to J.A.S.), a Melanoma Research Alliance Team Science Award (270010) (to C.E.A. and J.A.S.), and an Association of University Radiologists-GE Radiology Research Academic Fellowship award (to R.G.A.). This paper was presented in part at the American Society of Clinical Oncology Annual Meeting on June 1, 2014.
Author Contributions
Conception/Design: Douglas B. Johnson, Jedd D. Wolchok, Jeffrey A. Sosman, Richard D. Carvajal, Charlotte E. Ariyan
Provision of study material or patients: Douglas B. Johnson, Jedd D. Wolchok, Jeffrey A. Sosman, Richard D. Carvajal, Charlotte E. Ariyan
Collection and/or assembly of data: Douglas B. Johnson, Chengwei Peng, Richard G. Abramson, Charlotte E. Ariyan
Data analysis and interpretation: Douglas B. Johnson, Chengwei Peng, Richard G. Abramson, Fei Ye, Shilin Zhao, Charlotte E. Ariyan
Manuscript writing: Douglas B. Johnson, Jeffrey A. Sosman, Richard D. Carvajal, Charlotte E. Ariyan
Final approval of manuscript: Douglas B. Johnson, Chengwei Peng, Richard G. Abramson, Fei Ye, Shilin Zhao, Jedd D. Wolchok, Jeffrey A. Sosman, Richard D. Carvajal, Charlotte E. Ariyan
Disclosures
Richard G. Abramson: AduroBioTech, ICON Medical Imaging (C/A), Association of University Radiologists-GE Radiology Research (RF); Jedd D. Wolchok: Bristol-Myers Squibb (C/A, RF); Jeffrey A. Sosman: Bristol-Myers Squibb, GlaxoSmithKline, Novartis (RF); Richard D. Carvajal: Novartis, AstraZeneca (C/A, RF), Aura Biosciences (C/A), Bristol-Myers Squibb, Merck, Medimmune, Threshold, Verastem, Ignyta, Essenex, Puma, BioMed Valley Discoveries (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: Long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott D, Haanen J, Chen TT, et al. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20) Ann Oncol. 2013;24:2694–2698. doi: 10.1093/annonc/mdt291. [DOI] [PubMed] [Google Scholar]
- 5.Luke JJ, Callahan MK, Postow MA, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: A retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer. 2013;119:3687–3695. doi: 10.1002/cncr.28282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Luke JJ, Bluth MJ, et al. Ipilimumab for patients with advanced mucosal melanoma. The Oncologist. 2013;18:726–732. doi: 10.1634/theoncologist.2012-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maio M, Danielli R, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann Oncol. 2013;24:2911–2915. doi: 10.1093/annonc/mdt376. [DOI] [PubMed] [Google Scholar]
- 8.Del Vecchio M, Di Guardo L, Ascierto PA, et al. Efficacy and safety of ipilimumab 3mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur J Cancer. 2014;50:121–127. doi: 10.1016/j.ejca.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: Incidence and survival patterns in the United States, 1986–2005. Arch Dermatol. 2009;145:427–434. doi: 10.1001/archdermatol.2008.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999–2006. J Am Acad Dermatol. 2011;65(suppl 1):S26–S37. doi: 10.1016/j.jaad.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 11.Piliang MP. Acral lentiginous melanoma. Clin Lab Med. 2011;31:281–288. doi: 10.1016/j.cll.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Bello DM, Chou JF, Panageas KS, et al. Prognosis of acral melanoma: A series of 281 patients. Ann Surg Oncol. 2013;20:3618–3625. doi: 10.1245/s10434-013-3089-0. [DOI] [PubMed] [Google Scholar]
- 13.Chi Z, Li S, Sheng X, et al. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: A study of 522 consecutive cases. BMC Cancer. 2011;11:85. doi: 10.1186/1471-2407-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang JW, Yeh KY, Wang CH, et al. Malignant melanoma in Taiwan: A prognostic study of 181 cases. Melanoma Res. 2004;14:537–541. doi: 10.1097/00008390-200412000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Luk NM, Ho LC, Choi CL, et al. Clinicopathological features and prognostic factors of cutaneous melanoma among Hong Kong Chinese. Clin Exp Dermatol. 2004;29:600–604. doi: 10.1111/j.1365-2230.2004.01644.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuno Y, Ishihara K, Yamazaki N, et al. Clinical and pathological features of cutaneous malignant melanoma: A retrospective analysis of 124 Japanese patients. Jpn J Clin Oncol. 1996;26:144–151. doi: 10.1093/oxfordjournals.jjco.a023198. [DOI] [PubMed] [Google Scholar]
- 17.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 18.Lovly CM, Dahlman KB, Fohn LE, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS One. 2012;7:e35309. doi: 10.1371/journal.pone.0035309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 23.O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: A multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 24.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 25.Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu HT, Zhou QM, Wang F, et al. Identification of anaplastic lymphoma kinase break points and oncogenic mutation profiles in acral/mucosal melanomas. Pigment Cell Melanoma Res. 2013;26:646–653. doi: 10.1111/pcmr.12129. [DOI] [PubMed] [Google Scholar]
- 27.Griewank KG, Murali R, Puig-Butille JA, et al. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J Natl Cancer Inst. 2014;106:pii. doi: 10.1093/jnci/dju246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai J, Kong Y, Si L, et al. Large-scale analysis of pdgfra mutations in melanomas and evaluation of their sensitivity to tyrosine kinase inhibitors imatinib and crenolanib. Clin Cancer Res. 2013;19:6935–6942. doi: 10.1158/1078-0432.CCR-13-1266. [DOI] [PubMed] [Google Scholar]
- 29.Furney SJ, Turajlic S, Stamp G, et al. The mutational burden of acral melanoma revealed by whole-genome sequencing and comparative analysis. Pigment Cell Melanoma Res. 2014;27:835–838. doi: 10.1111/pcmr.12279. [DOI] [PubMed] [Google Scholar]
- 30.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SJ, Lim HJ, Choi YH, et al. The clinical significance of tumor-infiltrating lymphocytes and microscopic satellites in acral melanoma in a korean population. Annals Dermatol. 2013;25:61–66. doi: 10.5021/ad.2013.25.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.