Abstract
Lessons Learned
This study is a rare example of effective doses of both targeted agents being both administered and tolerated.
This combination should not be used in melanoma.
Background.
Sorafenib and bortezomib affect BCL family member expression. We previously demonstrated that bortezomib augmented sorafenib-mediated cytotoxicity in melanoma cell lines in vitro. We aimed to combine sorafenib 400 mg b.i.d. with increasing doses of weekly bortezomib.
Methods.
Patients with metastatic melanoma were enrolled in dose-escalation cohorts to determine the maximum tolerated dose (MTD) of sorafenib (twice daily) in combination with bortezomib (weekly for 3 of 4 weeks). The MTD was defined as the highest dose level at which less than 33% of patients exhibited a dose-limiting toxicity (DLT). Efficacy, as measured by 6-month progression-free survival and response rate per RECIST, was documented.
Results.
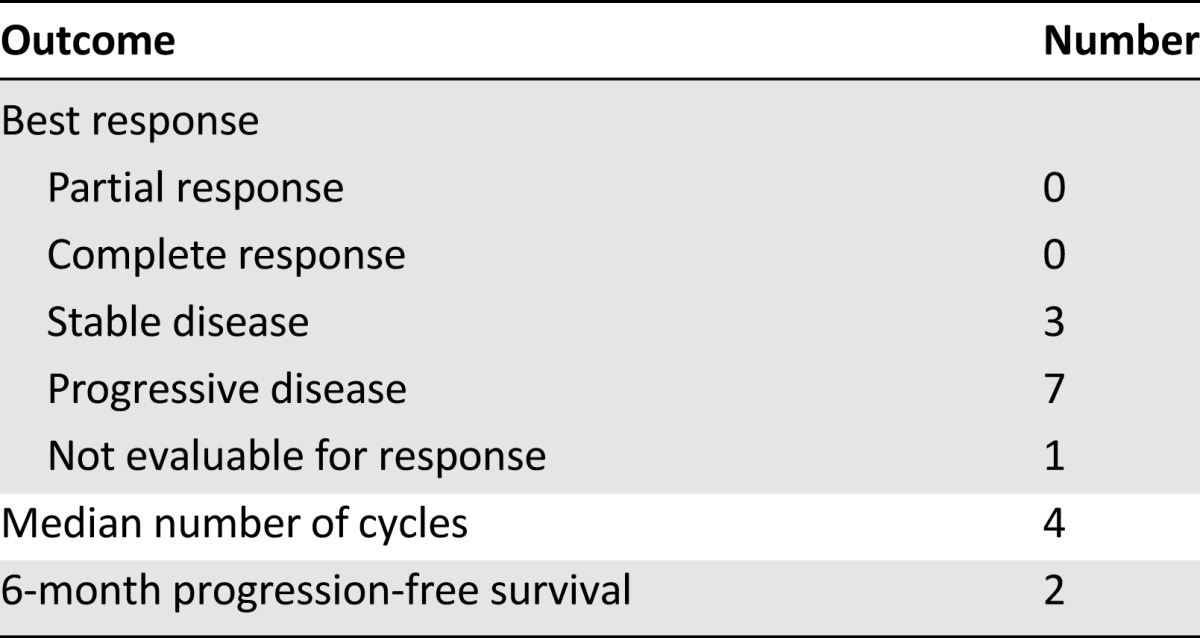
Eleven patients were enrolled at three dose levels. DLTs (fatigue and rash) were seen in two of three patients at the highest dose level. Five patients were enrolled for sorafenib 400 mg b.i.d. and bortezomib 1.0 mg/m2 weekly for 3 of every 4 weeks; none had DLTs, and this dose level was defined as the MTD. Of 10 evaluable patients, no responses were seen. Two of 11 patients (18%) remained progression free for longer than 6 months.
Conclusion.
The combination of sorafenib and bortezomib is safe but not active in patients with melanoma.
Author Summary
Discussion
The combination of sorafenib and bortezomib had been tested in a phase I trial with the MTD determined to be sorafenib 200 mg b.i.d. and bortezomib 1 mg/m2 on days 1, 4, 8, and 11 of a 21-day cycle [1]. Although these two agents had been tested in combination previously, we designed our study to maximize exposure to sorafenib by treating patients with weekly bortezomib, a regimen that had shown effectiveness in patients with myeloma with a favorable toxicity profile, compared with days 1, 4, 8, and 11 of a 21-day cycle [1–3] (Table 1). In this phase I dose-escalation study, we identified sorafenib 400 mg b.i.d. with weekly bortezomib 1.0 mg/m2 for 3 of 4 weeks as the MTD. Although a dose-escalation cohort was planned, given the lack of clinical activity at or above the MTD (no responses, 1 of 8 with stable disease at 24 weeks) and the increasing therapeutic options for patients with metastatic melanoma, the study was closed after determination of the MTD.
Table 1.
Clinical efficacy of the combination of sorafenib plus bortezomib
The standard of care for melanoma has changed dramatically over the past 5 years when this study was conceived, given the emergence of effective BRAF-targeted therapy and immune checkpoint inhibitors, leading to approval of 6 agents (BRAF inhibitors vemurafenib and dabrafenib, the MEK inhibitor trametinib, the anti-CTLA4 antibody ipilimumab, and the anti-PD1 antibodies pembrolizumab and nivolumab) by the U.S. Food and Drug Administration since 2011 [4–11]. Still, the majority of patients diagnosed with metastatic melanoma will die of their disease, and novel therapies and combination regimens are needed. Dual targeting of growth factor pathways and apoptosis is an approach that is building momentum, and four phase I clinical trials (NCT02110355, NCT01989585, NCT01897116, NCT02097225) based on compelling preclinical data have been opened recently. Our study serves as an early example of this type of approach. It is hoped that more potent and specific growth factor inhibitors—such as selective BRAF and/or MEK inhibitors for BRAF-mutant melanoma or pan-RAF, MEK, or ERK inhibitors for BRAF wild-type melanoma in combination with agents that target specific cell-survival mechanisms (BCL-2 family, inhibitor of apoptosis proteins, HDM2, heat shock protein 90, autophagy)—will be an effective therapeutic strategy.
Supplementary Material
Footnotes
Access the full results at: Sullivan-15-105.theoncologist.com
ClinicalTrials.gov Identifier: NCT01078961
Sponsor(s): Dana-Farber/Harvard Cancer Center
Principal Investigator: Ryan J. Sullivan
IRB Approved: Yes
For Further Reading: Jason J. Luke, F. Stephen Hodi. Ipilimumab, Vemurafenib, Dabrafenib, and Trametinib: Synergistic Competitors in the Clinical Management of BRAF Mutant Malignant Melanoma. The Oncologist 2013;18:717–725.
Implications for Practice: Ipilimumab, vemurafenib, dabrafenib, and trametinib have recently significantly advanced the management of patients with BRAF mutant melanoma. Clinical trials that would guide the use of combinations and/or sequencing of these drugs are currently in progress or being developed. Until those data are available, we suggest that patients with good performance status be treated with immunotherapy prior to consideration of kinase inhibitors such as vemurafenib, dabrafenib, and trametinib. This recommendation is based on the potential time required for induction of an antitumor immune response by ipilimumab, the modest durability of clinical benefit by kinase inhibitors and the observation that a not-insignificant proportion of patients treated initially with kinase inhibitors are unable to later complete ipilimumab induction due to clinical decline.
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


