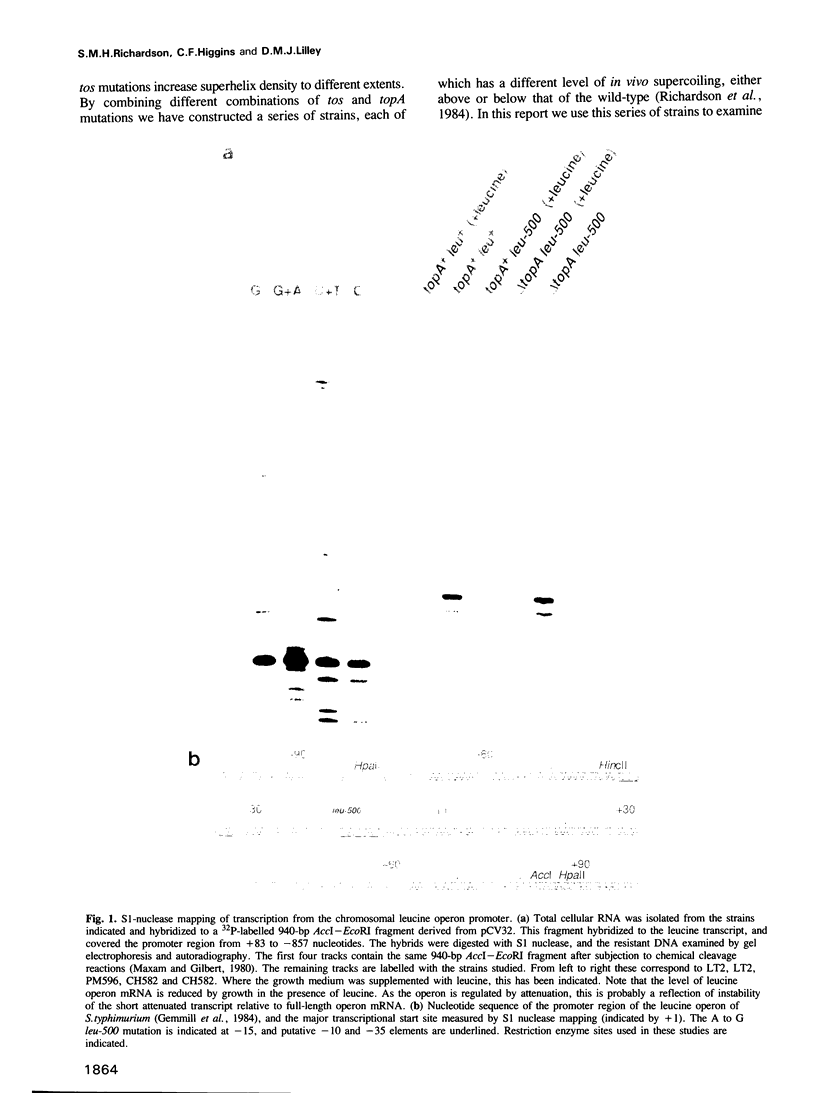
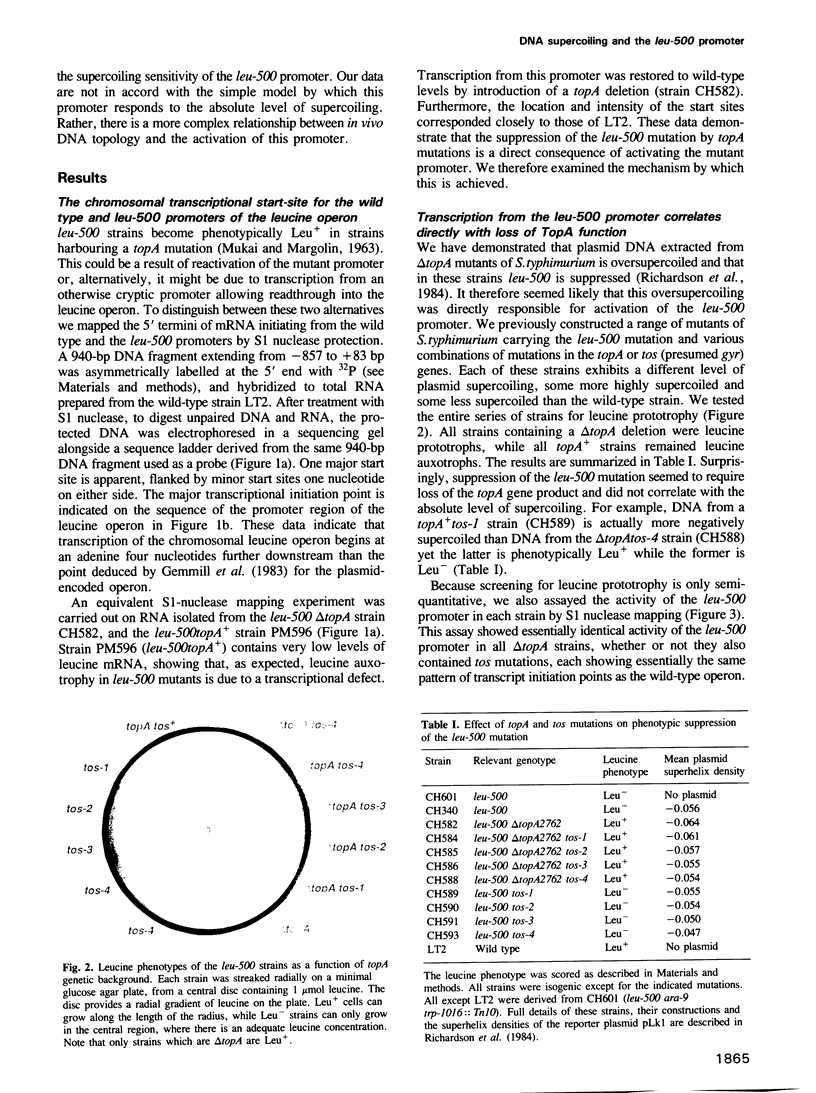
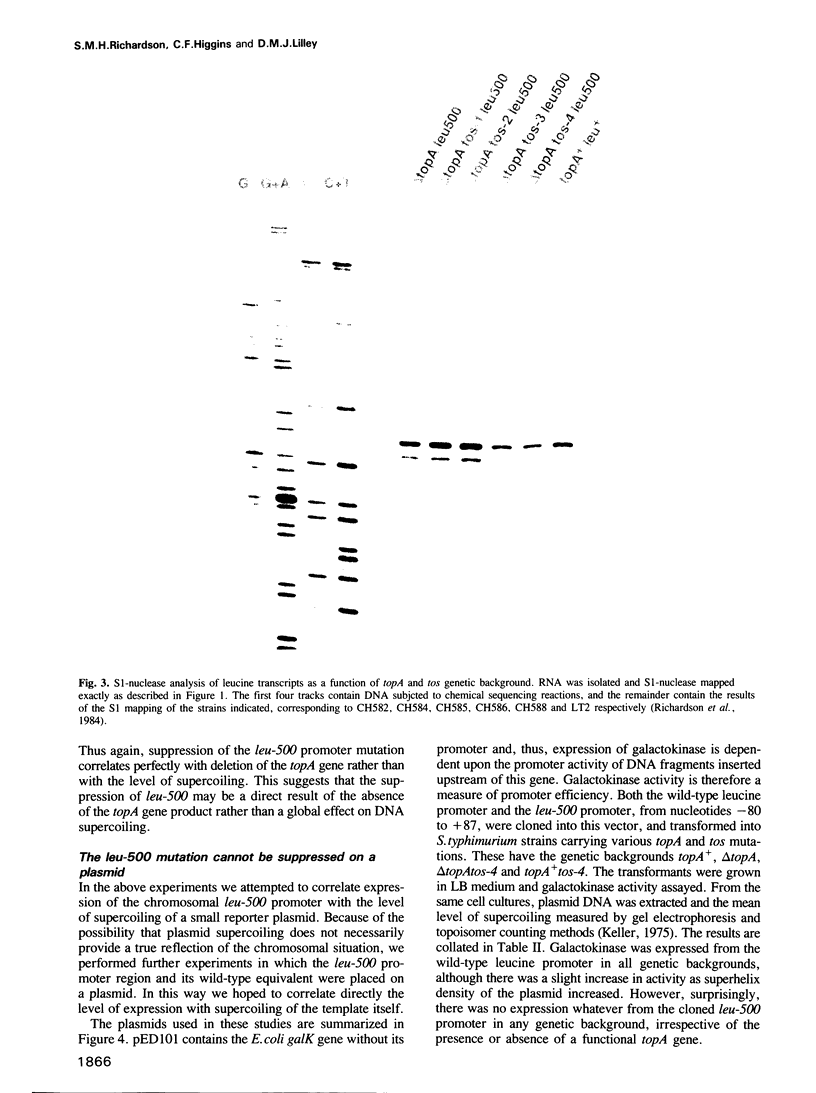
Abstract
DNA supercoiling is an important, but relatively poorly understood factor which influences promoter function. leu-500 is a point mutation in the promoter of the leucine operon of Salmonella typhimurium which confers leucine auxotrophy. It can be phenotypically suppressed by mutations in the topA gene, which encodes topoisomerase I, implicating DNA supercoiling in the regulation of this promoter. We have demonstrated that phenotypic suppression of this mutant promoter is transcriptional, and that topA mutations restore function to the mutant promoter. Transcription from the leu-500 promoter was examined in a series of strains harbouring topA and tos (presumptive gyr) mutations, each of which exhibits a different level of in vivo plasmid supercoiling. Promoter function did not correlate with the level of supercoiling but rather with the presence or absence of a functional topA gene. Furthermore, when cloned onto a multicopy plasmid, the leu-500 promoter failed to function, even in a topA background. Thus, local rather than global changes in DNA topology are implicated in the activation of this promoter.
Full text
PDF
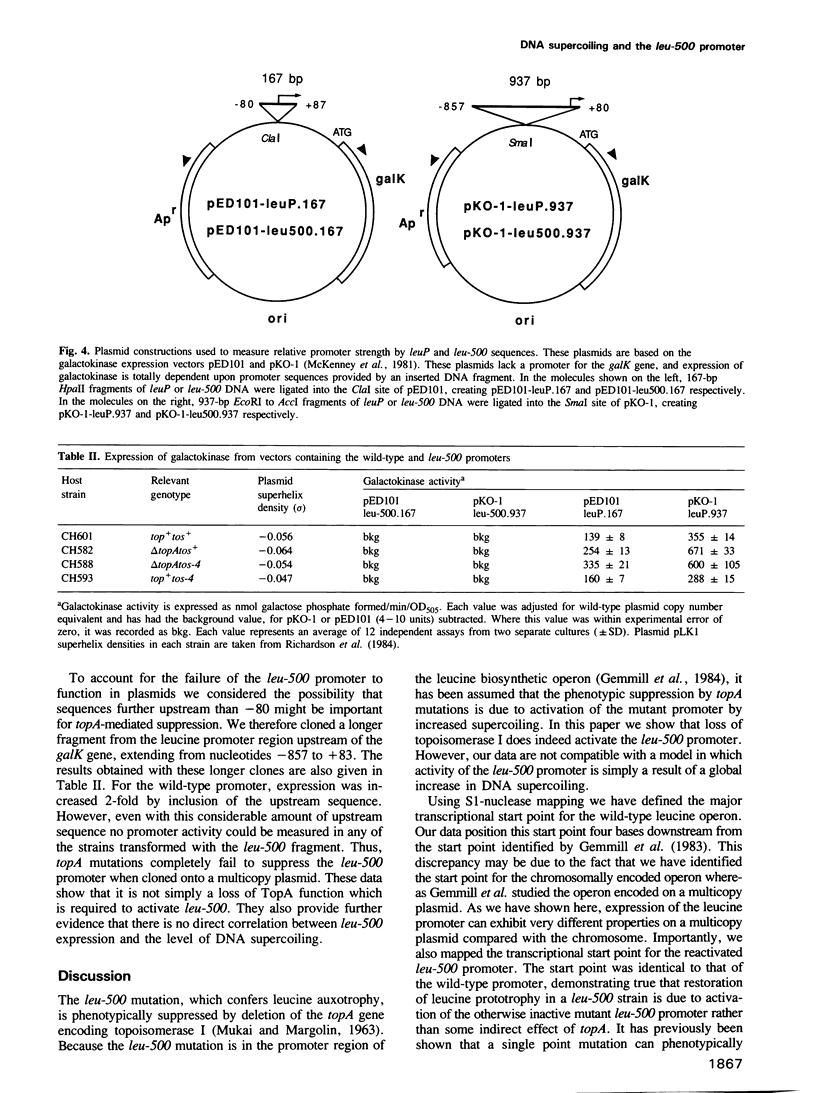


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke V. L., Gralla J. D. Changes in the linking number of supercoiled DNA accompany growth transitions in Escherichia coli. J Bacteriol. 1987 Oct;169(10):4499–4506. doi: 10.1128/jb.169.10.4499-4506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Cozzarelli N. R. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J Mol Biol. 1987 Mar 20;194(2):205–218. doi: 10.1016/0022-2836(87)90369-x. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Gralla J. D. All three elements of the lac ps promoter mediate its transcriptional response to DNA supercoiling. J Mol Biol. 1987 May 5;195(1):89–97. doi: 10.1016/0022-2836(87)90329-9. [DOI] [PubMed] [Google Scholar]
- Broyles S. S., Pettijohn D. E. Interaction of the Escherichia coli HU protein with DNA. Evidence for formation of nucleosome-like structures with altered DNA helical pitch. J Mol Biol. 1986 Jan 5;187(1):47–60. doi: 10.1016/0022-2836(86)90405-5. [DOI] [PubMed] [Google Scholar]
- Buc H., McClure W. R. Kinetics of open complex formation between Escherichia coli RNA polymerase and the lac UV5 promoter. Evidence for a sequential mechanism involving three steps. Biochemistry. 1985 May 21;24(11):2712–2723. doi: 10.1021/bi00332a018. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K. A., Sternglanz R., Reynolds A. E., Wright A. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell. 1982 Nov;31(1):43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- Dorman C. J., Higgins C. F. Fimbrial phase variation in Escherichia coli: dependence on integration host factor and homologies with other site-specific recombinases. J Bacteriol. 1987 Aug;169(8):3840–3843. doi: 10.1128/jb.169.8.3840-3843.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmill R. M., Jones J. W., Haughn G. W., Calvo J. M. Transcription initiation sites of the leucine operons of Salmonella typhimurium and Escherichia coli. J Mol Biol. 1983 Oct 15;170(1):39–59. doi: 10.1016/s0022-2836(83)80226-5. [DOI] [PubMed] [Google Scholar]
- Gemmill R. M., Tripp M., Friedman S. B., Calvo J. M. Promoter mutation causing catabolite repression of the Salmonella typhimurium leucine operon. J Bacteriol. 1984 Jun;158(3):948–953. doi: 10.1128/jb.158.3.948-953.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Glasgow A. C., Simon M. I. Spatial relationship of the Fis binding sites for Hin recombinational enhancer activity. Nature. 1987 Oct 1;329(6138):462–465. doi: 10.1038/329462a0. [DOI] [PubMed] [Google Scholar]
- Jovanovich S. B., Lebowitz J. Estimation of the effect of coumermycin A1 on Salmonella typhimurium promoters by using random operon fusions. J Bacteriol. 1987 Oct;169(10):4431–4435. doi: 10.1128/jb.169.10.4431-4435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. Bacterial chromatin. A new twist to an old story. Nature. 1986 Mar 6;320(6057):14–15. doi: 10.1038/320014a0. [DOI] [PubMed] [Google Scholar]
- Malan T. P., Kolb A., Buc H., McClure W. R. Mechanism of CRP-cAMP activation of lac operon transcription initiation activation of the P1 promoter. J Mol Biol. 1984 Dec 25;180(4):881–909. doi: 10.1016/0022-2836(84)90262-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Menzel R., Gellert M. Fusions of the Escherichia coli gyrA and gyrB control regions to the galactokinase gene are inducible by coumermycin treatment. J Bacteriol. 1987 Mar;169(3):1272–1278. doi: 10.1128/jb.169.3.1272-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R., Gellert M. Regulation of the genes for E. coli DNA gyrase: homeostatic control of DNA supercoiling. Cell. 1983 Aug;34(1):105–113. doi: 10.1016/0092-8674(83)90140-x. [DOI] [PubMed] [Google Scholar]
- Mukai F. H., Margolin P. ANALYSIS OF UNLINKED SUPPRESSORS OF AN O degrees MUTATION IN SALMONELLA. Proc Natl Acad Sci U S A. 1963 Jul;50(1):140–148. doi: 10.1073/pnas.50.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss G. J., Drlica K. DNA supercoiling and suppression of the leu-500 promoter mutation. J Bacteriol. 1985 Nov;164(2):947–949. doi: 10.1128/jb.164.2.947-949.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss G. J., Drlica K. Topoisomerase I mutants: the gene on pBR322 that encodes resistance to tetracycline affects plasmid DNA supercoiling. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8952–8956. doi: 10.1073/pnas.83.23.8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss G. J., Manes S. H., Drlica K. Escherichia coli DNA topoisomerase I mutants: increased supercoiling is corrected by mutations near gyrase genes. Cell. 1982 Nov;31(1):35–42. doi: 10.1016/0092-8674(82)90402-0. [DOI] [PubMed] [Google Scholar]
- Richardson S. M., Higgins C. F., Lilley D. M. The genetic control of DNA supercoiling in Salmonella typhimurium. EMBO J. 1984 Aug;3(8):1745–1752. doi: 10.1002/j.1460-2075.1984.tb02041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs D. L., Mueller R. D., Kwan H. S., Artz S. W. Promoter domain mediates guanosine tetraphosphate activation of the histidine operon. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9333–9337. doi: 10.1073/pnas.83.24.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd K. E., Menzel R. his operons of Escherichia coli and Salmonella typhimurium are regulated by DNA supercoiling. Proc Natl Acad Sci U S A. 1987 Jan;84(2):517–521. doi: 10.1073/pnas.84.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanzey B. Modulation of gene expression by drugs affecting deoxyribonucleic acid gyrase. J Bacteriol. 1979 Apr;138(1):40–47. doi: 10.1128/jb.138.1.40-47.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searles L. L., Wessler S. R., Calvo J. M. Transcription attenuation is the major mechanism by which the leu operon of Salmonella typhimurium is controlled. J Mol Biol. 1983 Jan 25;163(3):377–394. doi: 10.1016/0022-2836(83)90064-5. [DOI] [PubMed] [Google Scholar]
- Sternglanz R., DiNardo S., Voelkel K. A., Nishimura Y., Hirota Y., Becherer K., Zumstein L., Wang J. C. Mutations in the gene coding for Escherichia coli DNA topoisomerase I affect transcription and transposition. Proc Natl Acad Sci U S A. 1981 May;78(5):2747–2751. doi: 10.1073/pnas.78.5.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucksis M., Depew R. E. Identification and localization of a gene that specifies production of Escherichia coli DNA topoisomerase I. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2164–2168. doi: 10.1073/pnas.78.4.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucksis M., Golub E. I., Zabel D. J., Depew R. E. Escherichia coli and Salmonella typhimurium supX genes specify deoxyribonucleic acid topoisomerase I. J Bacteriol. 1981 Aug;147(2):679–681. doi: 10.1128/jb.147.2.679-681.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse-Dinh Y. C. Regulation of the Escherichia coli DNA topoisomerase I gene by DNA supercoiling. Nucleic Acids Res. 1985 Jul 11;13(13):4751–4763. doi: 10.1093/nar/13.13.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wood D. C., Lebowitz J. Effect of supercoiling on the abortive initiation kinetics of the RNA-I promoter of ColE1 plasmid DNA. J Biol Chem. 1984 Sep 25;259(18):11184–11187. [PubMed] [Google Scholar]
- Yamamoto N., Droffner M. L. Mechanisms determining aerobic or anaerobic growth in the facultative anaerobe Salmonella typhimurium. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2077–2081. doi: 10.1073/pnas.82.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. L., Heller K., Gellert M., Zubay G. Differential sensitivity of gene expression in vitro to inhibitors of DNA gyrase. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3304–3308. doi: 10.1073/pnas.76.7.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. S., Furano A. V. Regulation of the synthesis of E. coli elongation factor Tu. Cell. 1981 Jun;24(3):695–706. doi: 10.1016/0092-8674(81)90096-9. [DOI] [PubMed] [Google Scholar]