This study collects the results of an online survey of breast cancer survivors who were current tamoxifen or aromatase inhibitor users. Participants reported whether they were bothered by each of 47 symptoms during the past month and whether they thought each symptom was related to endocrine therapy (ET). Attention to these symptoms may reduce symptom burden and improve quality of life, potentially improving ET adherence and optimizing survival.
Keywords: Tamoxifen, Aromatase inhibitors, Breast cancer, Symptoms
Abstract
Background.
Adherence to adjuvant endocrine therapy (ET) influences breast cancer survival. Because ET side effects are frequently cited as reasons for nonadherence, understanding how perceptions and motivations in relation to ET are associated with symptom attribution can help promote timely symptom management.
Materials and Methods.
Participants were 2,086 breast cancer survivors recruited through the Army of Women registry who were current tamoxifen or aromatase inhibitor (AI) users. Participants reported whether they were bothered by each of 47 symptoms during the past month and whether they thought each symptom was related to taking ET. Frequencies of overall symptoms and symptoms attributed and misattributed to ET were calculated, and linear regression was used to assess sociodemographics, emotions, and illness perceptions as predictors of symptoms attributed to ET.
Results.
Women attributed a mean of 8.9 symptoms and misattributed a mean of 1.5 symptoms to ET. In the multivariable analysis, younger age, a more recent diagnosis, AI use (vs. tamoxifen), anxiety, depressive symptoms, more ET-related negative emotions, more concern about long-term ET use, and greater perceived ET necessity were independently associated with attribution of more symptoms to ET. More perceived ET necessity was associated with correctly attributing symptoms to ET, whereas higher depressive symptoms and more concern about ET use were associated with misattribution of symptoms to ET.
Conclusion.
Given that many women perceive a range of symptoms as a consequence of ET, attention to these symptoms may reduce symptom burden and improve quality of life, potentially improving ET adherence and optimizing survival.
Implications for Practice:
Many breast cancer survivors on endocrine therapy (ET) experience a range of side effects while taking ET. Targeting potentially modifiable factors associated with attributing a greater number of symptoms to ET, including perceived need for ET, concerns about long-term ET use, negative emotions toward ET, and symptoms of anxiety and depression, may reduce symptom burden and improve quality of life.
Introduction
In light of substantial evidence that tamoxifen and aromatase inhibitors (AIs) decrease breast cancer recurrence and mortality [1, 2], recently updated guidelines recommend 10 years of adjuvant endocrine therapy (ET) in women with hormone receptor-positive breast cancer [3]. Despite significant implications for survival, ensuring that breast cancer survivors adhere to the prescribed duration of therapy remains challenging, with the prevalence of nonadherence (i.e., failure to take medication as prescribed) as high as 59% for tamoxifen and 50% for aromatase inhibitors reported in some studies [4]. Nonpersistence, or discontinuation, is also of concern, with studies demonstrating that 31%–73% of women stop therapy early [4].
Side effects from tamoxifen and AIs can negatively affect both health-related and psychosocial quality of life (QOL) and are one of the most commonly cited reasons for nonadherence and discontinuation of ET [5–9]. Side effects generally increase patient concerns about taking therapy and thereby reduce adherence [10]. In one study, among women who stopped taking AIs, more than 70% did so because of musculoskeletal side effects [8]. In another study, two thirds of women who stopped their adjuvant ET early cited side effects as the reason for doing so [11]. Although most prior research has evaluated the association between symptoms reported during treatment and ET compliance, a recent analysis found that women who reported a high symptom burden before starting treatment with AIs were more likely to have stopped their ET within the first year of treatment [12].
Because bothersome side effects can contribute to both nonadherence and nonpersistence to therapy, identifying who is mostly likely to attribute their symptoms to ET, together with a better understanding of how perceptions and motivations about ET are associated with symptom burden, can help promote effective and timely symptom management, targeting those most burdened by their ET. Furthermore, women who stop treatment early are more apt to attribute side effects to their ET than women who are compliant with treatment [11]. In this study, we aim to describe symptom burden and symptom attribution patterns in a large sample of breast cancer survivors, as well as assess the relationship of sociodemographic characteristics, emotions, and perceptions surrounding ET with symptoms attributed to this therapy.
Materials and Methods
Participants
Upon approval by the relevant institutional review boards, participants were recruited via e-mail from the Dr. Susan Love Research Foundation’s Love/Avon Army of Women (AOW) research registry. The AOW is a registry of 362,314 individuals (at the point of study recruitment) who have joined as potential participants for research pertaining to breast cancer; approximately 14% of the women in the registry have a history of breast cancer. AOW participants are recruited from scientific conferences, social media, private and public events, partnerships with other organizations, and other media outlets. All studies conducted as part of the AOW must be funded and institutional review board-approved.
In January of 2012, a “call-to-action” e-mail was sent to registry participants. The e-mail described endocrine therapies and stated the study’s purpose as gathering information “to understand women’s thoughts, feelings, and behaviors relevant to taking endocrine therapies.” Women who were interested in participating in the study were asked to affirm that they met the following eligibility criteria: (a) at least 18 years of age with a history of breast cancer; (b) currently taking, or has taken within the past 12 months, one of the following medications: Nolvadex (tamoxifen), Arimidex (anastrozole), Aromasin (exemestane), or Femara (letrozole); (c) has Internet access and is willing to complete an online survey; and (d) lives in the United States. Upon confirmation of eligibility, women were automatically routed to the online survey. Of the estimated 51,000 women with breast cancer who were emailed an invitation, 2,341 met the eligibility criteria and completed the online survey. Of those, 2,086 reported that they were currently on ET.
Measures
Sociodemographic and Medical Characteristics
Age, ethnic background, education, marital status, and perceived financial status (enough money for special things; little spare money for special things; money to pay bills only because you have to cut back; difficulty paying bills) were self-reported. Clinical factors including current ET type (tamoxifen, anastrozole, exemestane, and letrozole), duration of current ET use, patterns of ET use (e.g., switching between therapies and reasons for switching), breast cancer stage, surgery, chemotherapy, radiotherapy, and time from diagnosis were also self-reported.
Psychological and Emotional Factors
Symptoms of anxiety and depression were measured with the 14-item Hospital Anxiety and Depression Scale (HADS) [13], with higher scores on the HADS representing more symptoms. Women were asked to rate their worry about recurrence on a 0–10 scale (0 = not at all; 10 = a great deal). Additional items measured on a 0–10 scale were adapted from the Brief Illness Perception Questionnaire [14, 15] and included concern about their breast cancer diagnosis and treatment (0 = not at all concerned; 10 = extremely concerned) and emotional impact of their breast cancer diagnosis and treatment (0 = not at all affected emotionally; 10 = extremely affected emotionally).
Symptoms
Symptoms were assessed with the Breast Cancer Prevention Trial Symptom Scales, which has sound psychometric properties in breast cancer patients [16]. Items accounting for side effects specifically related to AIs (e.g., bone pain) and other general symptoms (e.g., constipation) were added. Participants reported whether they were bothered by each of 47 symptoms during the past 4 weeks and, if they were bothered by a particular symptom, whether they thought it was related to their ET or not related to their ET. The two resulting scales consisted of the sum of the endorsed symptom total each woman did or did not attribute to endocrine therapy. If a respondent did not endorse a response for a particular item, this was scored as not endorsing the symptom as bothersome. Respondents who did not answer any of the 47 symptom attribution questions (n = 51) were excluded from all analyses.
Other Endocrine Therapy-Related Measures
Two items based on the necessity-concerns framework [10] measured perceived therapy necessity: (a) How much do you feel your endocrine therapy can help reduce your risk of breast cancer recurring? (0 = not at all; 10 = a great deal) and (b) How much do you feel that you need your current endocrine medication for your breast cancer? (0 = I don’t need it at all; 10 = it is absolutely essential for me). An additional question assessed long-term use concern: How concerned are you about the long-term use of your current endocrine medication? (0 = not at all; 10 = extremely concerned).
Negative and positive emotions about ET were adapted from items for affective properties of attitudes [17]. Respondents endorsed the degree to which five positive emotions (happy, calm, enthusiastic, comforted, accepting) and five negative emotions (sad, annoyed, tense, reluctant, angry) described their feelings toward endocrine therapy (i.e., does not describe, slightly describes, definitely describes). Positive and negative emotion scales were generated by summing the endorsed items (each item scored as follows: does not describe = 1, slightly describes = 2, definitely describes = 3) corresponding to each of these dimensions.
Statistical Analysis
Descriptive statistics, including means, medians, and frequency distributions, were used to characterize the study population, including sociodemographics and treatment history, as well as to describe the prevalence and types of symptoms attributed to ET. Linear regression models were fit to evaluate the relationship between symptom attribution (dependent variable was number of symptoms attributed to ET) and the following factors: age, time since diagnosis, ET type (aromatase inhibitor vs. tamoxifen), anxiety, depression, emotions and perceptions toward ET, emotional impact of breast cancer, and worries and perceptions about breast cancer recurrence. Because time on drug and time since diagnosis were correlated (r = .49), we did not include time on drug in the regression analyses because of collinearity.
As a secondary analysis, we grouped symptoms that have been documented as side effects [18–23] (i.e., hot flashes, night sweats, vaginal dryness, muscle stiffness, joint pain, low sexual enjoyment, pain with intercourse, bone pain, genital irritation, vaginal discharge, vaginal bleeding) separately from symptoms likely unrelated or not specific to ET (i.e., unhappiness with appearance, headache, feeling irritable, constipation, decreased range of motion in arm, dizziness, palpitations/irregular heartbeat, arm swelling, nightmares, diarrhea, breathing problems, increased appetite, abdominal pain, chest pain, tremor, vomiting, anxiety/nervousness, depressed mood). To examine whether certain perceptions and characteristics might differ between women who misattribute side effects and those who do not, we fit separate logistic regression models to evaluate which factors were associated with (a) misattribution of at least one symptom among all women and (b) attribution of at least one documented symptom among women who did not misattribute any symptoms. A third category of symptoms categorized as “possibly” related to ET (i.e., lack of interest in sex, general aches and pains, sleep problems, tiredness, difficulty concentrating, forgetfulness, easily distracted, difficulty with bladder control when laughing and crying, difficulty with bladder control at other times, breast tenderness, breast pain, breast sensitivity, lack of energy, weight gain, hair loss/thinning, nausea, skin rash, reduced appetite) were not considered in this secondary analysis.
Although both anxiety and depression were among the 47 side effects women were asked to report about, we excluded them from the symptom count (e.g., dependent variable) of all regression analyses because anxiety and depression as measured by the HADS were included as independent variables in all models. Women for whom relevant covariate data were missing (n = 382) were also excluded from regression analyses. Sample sizes vary somewhat across analyses because of nonresponse on specific items. All analyses were conducted in SAS version 9.4 (SAS Institute, Inc., Cary, NC, http://www.sas.com).
Results
Study Sample Characteristics
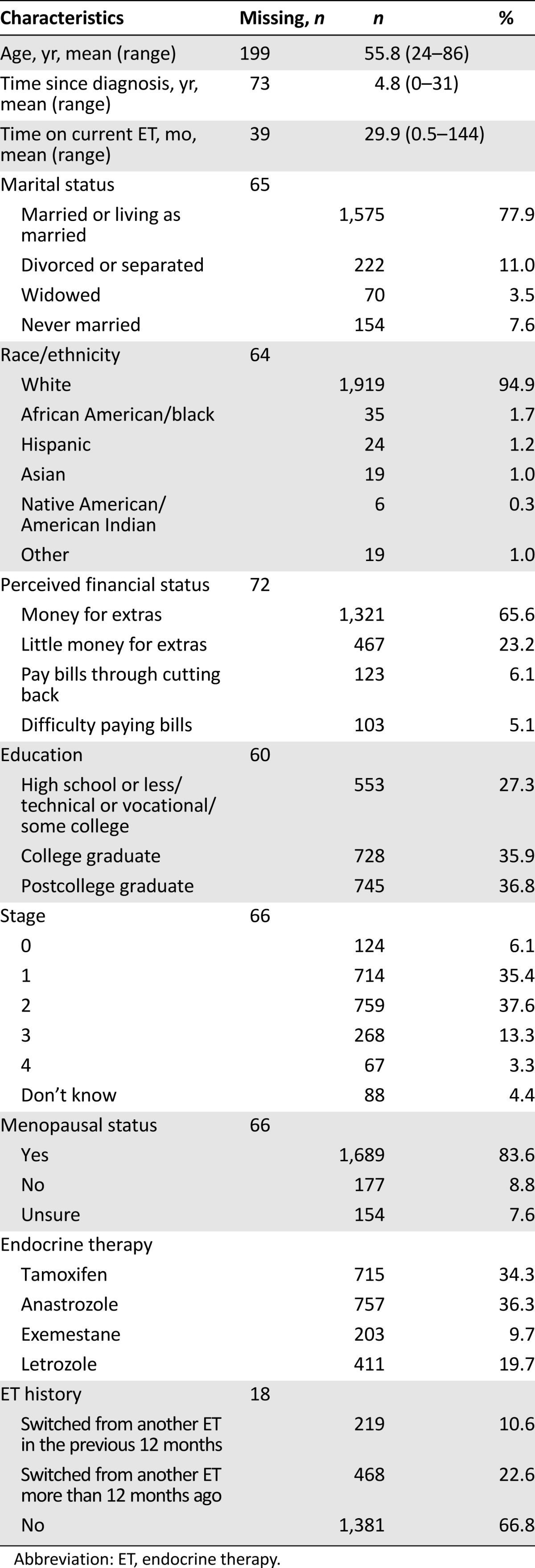
Table 1 includes sociodemographic and treatment information for the study sample. Respondents were predominantly white non-Hispanic, were partnered, and had at least a college education. Regarding perceived financial standing, approximately two thirds of women said they had enough money for special things after paying the bills, 23% said they had enough money to pay the bills but little extra, and 11% said they were able to pay bills but had to cut back (6%) or had difficulty paying the bills (5%). Mean age was 55.8 years, and 79% had stage 0, I, or II disease. Approximately one third of women were current tamoxifen users, with the remainder of women reporting taking an AI. At the time of survey completion, mean duration of current ET use was 29.9 months (range: 0.5–144). Although two thirds of women did not report changing ET regimens, approximately 11% said they had switched from another ET in the prior 12 months, and 23% said they had switched more than 12 months ago. Among the women who switched and provided a reason for doing so (n = 668), 17% reported switching to decrease the risk of the cancer coming back, 46% said they switched to decrease the side effects, and 37% said they switched for “some other reason.”
Table 1.
Participant characteristics (n = 2,086)
Symptom Prevalence and Attribution Patterns
Women reported a mean total of 14.2 (range: 0–43) symptoms and attributed a mean of 8.9 (range: 0–43) symptoms to ET. When we limited the attribution analysis to the 11 side effects that have been documented in several studies to be commonly associated with ET, women reported a mean of 3.7 of these symptoms (range: 0–10). More than half of women (53%) misattributed at least one symptom to ET, with a mean of 1.5 (range: 0–17) symptoms misattributed.
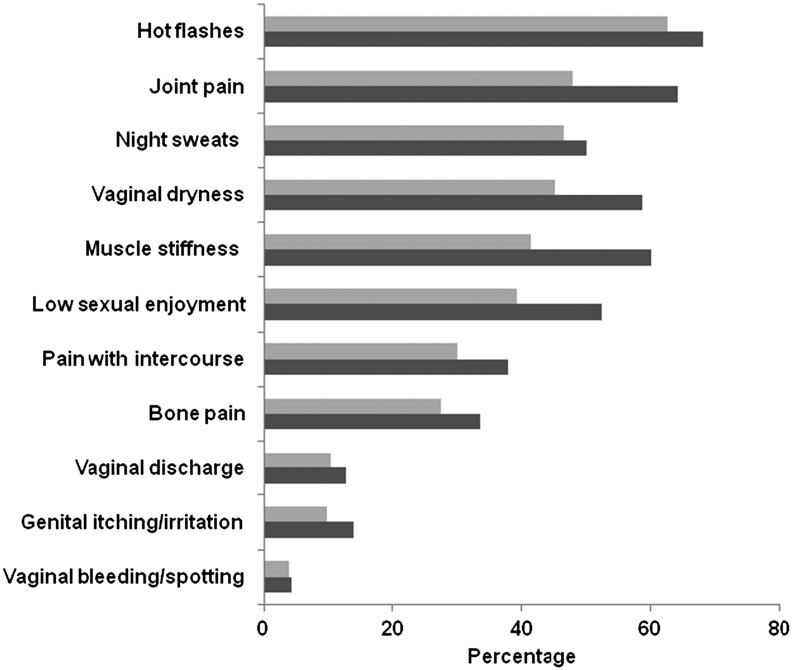
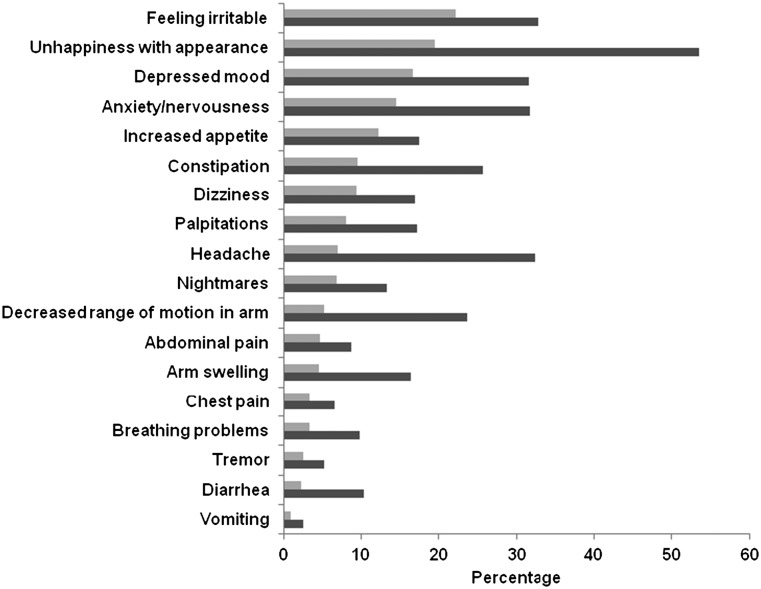
Figure 1 details the frequency of reporting any type of symptom relative to the frequency of attributing a symptom to ET among documented symptoms. Experiencing hot flashes was the most frequent symptom reported, as well as the most frequently attributed symptom, with 68% of all women reporting that they had bothersome hot flashes and 63% attributing them to their ET. Joint pain (48%), night sweats (47%), vaginal dryness (45%), and muscle stiffness (41%) were also commonly attributed to ET. Irritability (22%), unhappiness with appearance (19%), and depressed mood (17%) were the most common symptoms misattributed to ET (Fig. 2).
Figure 1.
Prevalence of documented endocrine therapy (ET) symptoms (n = 2,035).  , symptoms attributed to endocrine therapy;
, symptoms attributed to endocrine therapy;  , any symptoms reported.
, any symptoms reported.
Figure 2.
Prevalence of misattributed endocrine therapy (ET) symptoms (n = 2,035).  , symptoms misattributed to endocrine therapy;
, symptoms misattributed to endocrine therapy;  , any symptoms reported.
, any symptoms reported.
Factors Associated With ET Symptom Attribution
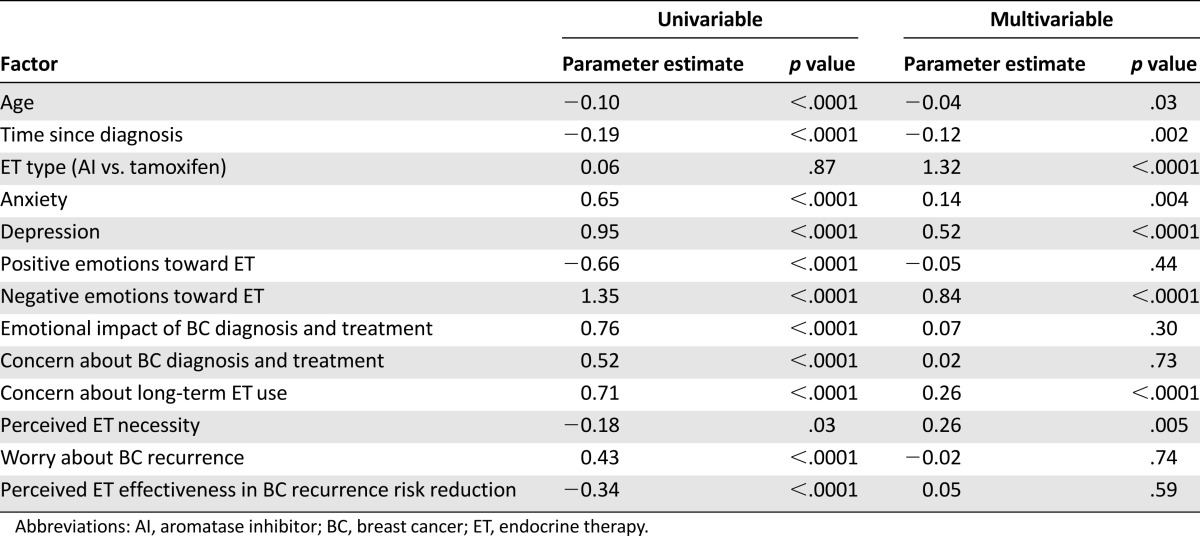
Table 2 includes the results of the analysis that examined age, time since diagnosis, ET type, anxiety, depressive symptoms, and ET- and breast cancer-associated emotions and perceptions in relation to the number of symptoms attributed to ET. In the multivariable analysis, younger age, a more recent diagnosis, AI (vs. tamoxifen) use, as well as more symptoms of anxiety and depression, more negative emotions toward ET, more concern about long-term ET use, and greater perceived necessity of ET, all were independently associated with greater symptom attribution to ET.
Table 2.
Factors associated with more symptoms attributed to ET (n = 1,653)
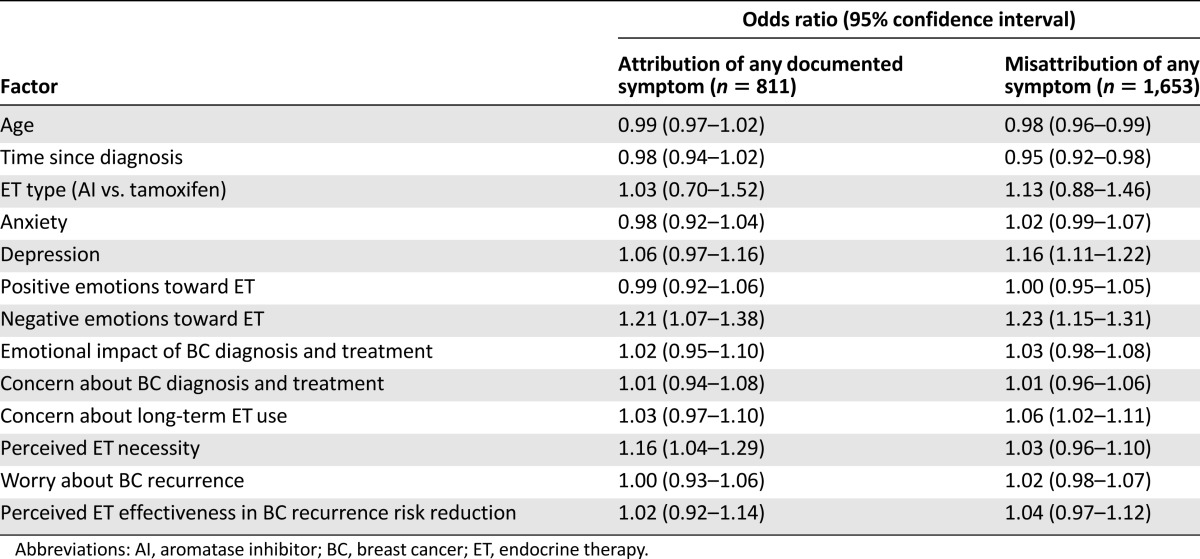
In the analyses of factors associated with the classification of symptom as documented vs. misattributed (Table 3), higher perceived necessity of ET was associated with higher odds of correctly attributing at least one of the documented symptoms to ET. Reporting more depressive symptoms and more concern about long-term ET use were both associated with higher odds of misattributing at least one symptom; more time since diagnosis and older age were associated with lower odds of misattribution. More ET-related negative emotions were associated with higher odds of correct attribution and misattribution; other results were also similar between the two groups.
Table 3.
Multivariable analysis of factors associated with attribution of documented ET symptoms (among women who did not misattribute any symptoms) vs. misattribution of any symptom (among all women)
Discussion
Because women who perceive their symptoms as a consequence of ET might be less likely to adhere to treatment, there is potential value in distinguishing between symptoms attributed to ET, symptoms misattributed to ET, and symptoms that might be prevalent but are infrequently perceived or attributed as being related to ET. Other research has examined factors associated with symptom burden in ET users, including one study in which older women (aged 75 years and older vs. aged 55–64 years) and women with lower levels of emotional distress were less likely to report side effects of tamoxifen [24] and another study that found no association between AI side effect burden and fear of recurrence after adjusting for several breast cancer-related health beliefs and perceptions [15]. Little information is available, however, about patterns of symptom attribution in breast cancer survivors on ET.
We found that several potentially modifiable factors were related to the degree of symptom attribution. Higher perceived ET necessity and ET-related negative emotions were associated with more attributed symptoms and might indicate that this group of patients, despite a high symptom burden attributed to ET, chooses to remain on treatment because they recognize the importance of ET for survival. Additionally, women who had higher levels of anxiety and depressive symptoms and more concern about long-term ET use attributed a greater overall number of symptoms to their ET. If targeted appropriately, these factors are potentially modifiable and suggest an important role for providers in effectively communicating that adherence to ETs for the duration of treatment is an essential part of ensuring optimal breast cancer outcomes. Educational tools can potentially be useful in this setting by helping women understand the risks and benefits of ET, as well as by providing accurate information regarding side effects associated with ET [25]. Our analysis of a subsample of women (n = 1,371) from the present research who completed a self-reported ET adherence measure 2 weeks after the initial assessment revealed high rates of adherence [26]. Whereas symptoms that women attributed and did not attribute to ET did not predict adherence over and above the contribution of other potentially modifiable factors (e.g., perceived ET necessity, ET-related negative emotions, perceived quality of the relationship with the oncologist), these are promising factors to target in interventions [26].
Many of the most common symptoms reported by study participants have been documented as possible or probable ET side effects. However, 53% of the sample also misattributed at least one symptom to their ET despite a lack of evidence from randomized controlled trials that these symptoms are more common in women on ET compared with placebo controls [18–23]. Although some women attributed them to their ET, complaints like headaches, gastrointestinal problems, and other nonspecific side effects are frequently reported by individuals who have an unremarkable health history [27–29]. Interestingly, we found that women who had more depressive symptoms and ET-related negative emotions had higher odds of symptom misattribution, which is supported by findings of other research that symptom misattribution is related to greater emotional distress [30].
Importantly, we also identified younger age, less time since diagnosis, and taking an AI (vs. tamoxifen) as independently associated with a greater number of symptoms attributed to ET. This information can potentially be used to focus on subsets of the population most at risk for experiencing and perceiving their symptoms as caused by ET. By identifying those women who are more likely to attribute symptoms to the drug, such as younger women, an opportunity exists to promote interventions that help women cope with bothersome symptoms. A wide variety of pharmacological, behavioral, and psychoeducational interventions have been studied in breast cancer survivors, and many have been found to be highly effective in improving the most common side effects, including hot flashes, vaginal dryness, sexual dysfunction, sleep, emotional, and cognitive problems [31–37]. Exercise has also been associated with better QOL outcomes in breast cancer survivors [38] and can help improve bothersome muscle and joint pain symptoms often associated with AI use; pharmacologic options to ameliorate these symptoms include nonsteroidal anti-inflammatories and COX-2 inhibitors [39, 40]. Additional strategies for managing hot flashes and night sweats include dressing in layers, using cold packs, and lowering ambient temperatures. Several nonhormonal options also exist to reduce vaginal dryness and help with dyspareunia, including vaginal lubricants and moisturizers [41]. Sharing such strategies proactively with patients early in the course of their ET may reduce their symptom burden.
Our analysis has several limitations. This was a cross-sectional survey, and the direction of any significant associations must be interpreted cautiously. The sample was recruited from a large, national web-based registry of women who volunteered to be contacted for research purposes and therefore generalizability might be limited. Furthermore, participants might have been experiencing a larger symptom burden and therefore might have been more likely to participate in the study than women experiencing fewer side effects from ET. In turn, our study might be overestimating the prevalence of certain ET symptoms. Conversely, our analyses only included current ET users and therefore possibly excluded women experiencing the worst side effects who might be the most likely to stop ET early and be off therapy for several years. Future studies to capture the experiences of those high-risk women are warranted, given that their perceptions and emotions about ET and breast cancer risk might differ from women who remain on ET despite having symptoms.
Conclusion
Notwithstanding these limitations, our findings that many women attribute a range of symptoms to their ET suggest that implementation of effective approaches to modify negative perceptions and emotions toward ET, together with recognition of and attention to symptoms, even when they are not necessarily caused by ET, may improve symptom burden and associated QOL for breast cancer survivors.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This work was supported by NIH Grant 5 R25 CA057711 (to S.M.R.) and by the Breast Cancer Research Foundation (to A.L.S.).
Footnotes
For Further Reading: Vishal Saggar, Shenhong Wu, Maura N. Dickler et al. Alopecia With Endocrine Therapies in Patients With Cancer. The Oncologist 2013;18:1126–1134.
Implications for Practice: Whereas the frequency of alopecia in the context of cytotoxic chemotherapies has been well described, its incidence with endocrine therapies (i.e., anti-estrogens, aromatase inhibitors) has not been systematically described. This lack of knowledge precludes comprehensive therapeutic decision-making, appropriate pretherapy counseling, and the establishment of interventions for patients who experience alopecia. Moreover, this lack of knowledge has negated the importance of alopecia and its associated psychosocial impact, hindering research endeavors toward its prevention, management, and the identification of individuals at risk. The data presented here reveal that alopecia is a common and likely underreported adverse event of treatment with endocrine therapies for cancer. Data also showed a higher relative risk of alopecia for those treated with selective estrogen receptor modulators than for those treated with aromatase inhibitors. This knowledge represents a step toward a heightened awareness of this condition, which may have an impact on patient adherence and persistence.
Author Contributions
Conception/Design: Annette L. Stanton, Keith J. Petrie, Ann H. Partridge
Provision of study material or patients: Annette L. Stanton, Keith J. Petrie, Ann H. Partridge
Collection and/or assembly of data: Annette L. Stanton, Keith J. Petrie, Ann H. Partridge
Data analysis and interpretation: Shoshana M. Rosenberg, Annette L. Stanton, Keith J. Petrie, Ann H. Partridge
Manuscript writing: Shoshana M. Rosenberg, Annette L. Stanton, Keith J. Petrie, Ann H. Partridge
Final approval of manuscript: Shoshana M. Rosenberg, Annette L. Stanton, Keith J. Petrie, Ann H. Partridge
Disclosures
The authors indicated no financial relationships.
References
- 1.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res Treat. 2012;134:459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunfeld EA, Hunter MS, Sikka P, et al. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns. 2005;59:97–102. doi: 10.1016/j.pec.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Friese CR, Pini TM, Li Y, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138:931–939. doi: 10.1007/s10549-013-2499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon R, Latreille J, Matte C, et al. Adherence to adjuvant endocrine therapy in estrogen receptor-positive breast cancer patients with regular follow-up. Can J Surg. 2014;57:26–32. doi: 10.1503/cjs.006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30:936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortimer JE. Managing the toxicities of the aromatase inhibitors. Curr Opin Obstet Gynecol. 2010;22:56–60. doi: 10.1097/GCO.0b013e328334e44e. [DOI] [PubMed] [Google Scholar]
- 10.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–567. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 11.Bowles EJ, Buist DS, Chubak J, et al. Endocrine therapy initiation from 2001 to 2008 varies by age at breast cancer diagnosis and tumor size. J Oncol Pract. 2012;8:113–120. doi: 10.1200/JOP.2011.000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidwell KM, Harte SE, Hayes DF, et al. Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer. 2014;120:2403–2411. doi: 10.1002/cncr.28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 14.Broadbent E, Petrie KJ, Main J, et al. The brief illness perception questionnaire. J Psychosom Res. 2006;60:631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Corter AL, Findlay M, Broom R, et al. Beliefs about medicine and illness are associated with fear of cancer recurrence in women taking adjuvant endocrine therapy for breast cancer. Br J Health Psychol. 2013;18:168–181. doi: 10.1111/bjhp.12003. [DOI] [PubMed] [Google Scholar]
- 16.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97:448–456. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 17.Crites S, Fabrigar L, Petty R. Measuring the affective and cognitive properties of attitudes: Conceptual and methodological issues. Pers Soc Psychol Bull. 1994;20:619–634. [Google Scholar]
- 18.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer: 96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 19.Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–1048. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 21.Day R, Ganz PA, Costantino JP, et al. Health-related quality of life and tamoxifen in breast cancer prevention: A report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–2669. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 22.Powles TJ, Ashley S, Tidy A, et al. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 23.Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 24.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: Predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19:322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 25.Heisig SR, Shedden-Mora MC, von Blanckenburg P, et al. Informing women with breast cancer about endocrine therapy: Effects on knowledge and adherence. Psychooncology. 2015;24:130–137. doi: 10.1002/pon.3611. [DOI] [PubMed] [Google Scholar]
- 26.Stanton AL, Petrie KJ, Partridge AH. Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat. 2014;145:525–534. doi: 10.1007/s10549-014-2961-3. [DOI] [PubMed] [Google Scholar]
- 27.Hannay DR. Symptom prevalence in the community. J R Coll Gen Pract. 1978;28:492–499. [PMC free article] [PubMed] [Google Scholar]
- 28.Eriksen HR, Ihlebaek C. Subjective health complaints. Scand J Psychol. 2002;43:101–103. doi: 10.1111/1467-9450.00274. [DOI] [PubMed] [Google Scholar]
- 29.Petrie KJ, Faasse K, Crichton F, et al. How common are symptoms?: Evidence from a New Zealand national telephone survey. BMJ Open. 2014;4:e005374. doi: 10.1136/bmjopen-2014-005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faasse K, Petrie KJ. The nocebo effect: Patient expectations and medication side effects. Postgrad Med J. 2013;89:540–546. doi: 10.1136/postgradmedj-2012-131730. [DOI] [PubMed] [Google Scholar]
- 31.Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: A randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2011;29:3862–3868. doi: 10.1200/JCO.2010.33.1298. [DOI] [PubMed] [Google Scholar]
- 32.Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: A randomised controlled trial. Lancet. 2000;356:2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 33.Bordeleau L, Pritchard K, Goodwin P, et al. Therapeutic options for the management of hot flashes in breast cancer survivors: An evidence-based review. Clin Ther. 2007;29:230–241. doi: 10.1016/j.clinthera.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: Systematic review and meta-analysis. JAMA. 2006;295:2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 35.Mann E, Smith MJ, Hellier J, et al. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): A randomised controlled trial. Lancet Oncol. 2012;13:309–318. doi: 10.1016/S1470-2045(11)70364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savard J, Simard S, Ivers H, et al. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer: Part I. Sleep and psychological effects. J Clin Oncol. 2005;23:6083–6096. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 37.Taylor S, Harley C, Ziegler L, et al. Interventions for sexual problems following treatment for breast cancer: A systematic review. Breast Cancer Res Treat. 2011;130:711–724. doi: 10.1007/s10549-011-1722-9. [DOI] [PubMed] [Google Scholar]
- 38.Alfano CM, Smith AW, Irwin ML, et al. Physical activity, long-term symptoms, and physical health-related quality of life among breast cancer survivors: A prospective analysis. J Cancer Surviv. 2007;1:116–128. doi: 10.1007/s11764-007-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2008;107:167–180. doi: 10.1007/s10549-007-9548-1. [DOI] [PubMed] [Google Scholar]
- 40.Monnier A. Clinical management of adverse events in adjuvant therapy for hormone-responsive early breast cancer. Ann Oncol. 2007;18(suppl 8):viii36–viii44. doi: 10.1093/annonc/mdm264. [DOI] [PubMed] [Google Scholar]
- 41.Carter J, Goldfrank D, Schover LR. Simple strategies for vaginal health promotion in cancer survivors. J Sex Med. 2011;8:549–559. doi: 10.1111/j.1743-6109.2010.01988.x. [DOI] [PubMed] [Google Scholar]