This study identified cancer clinical trials activated at a National Cancer Institute (NCI)-designated cancer center from 1991 to 2014. The characteristics of trials activated or not at a satellite site within the county safety-net medical system were also identified. Over time, a decreasing proportion of trials were activated at the satellite site, which provides care to uninsured and underinsured patients. Trial complexity and costs accounted for much of this trend. Efforts to overcome these barriers will be key to equitable access to clinical trials, efficient accrual, and the generalizability of results.
Keywords: Cancer, Clinical trials, Underserved populations, Underrepresented minorities, Accrual
Abstract
Background.
Lack of access to available cancer clinical trials has been cited as a key factor limiting trial accrual, particularly among medically underserved populations. We examined the trends and factors in clinical trial availability within a major U.S. safety-net hospital system.
Materials and Methods.
We identified cancer clinical trials activated at the Harold C. Simmons Cancer from 1991 to 2014 and recorded the characteristics of the trials that were and were not activated at the Parkland Health and Hospital System satellite site. We used univariate and multivariate logistic regression to determine the association between trial characteristics and nonactivation status, and chi-square analysis to determine the association between the trial characteristics and the reasons for nonactivation.
Results.
A total of 773 trials were identified, of which 152 (20%) were not activated at Parkland. In multivariable analysis, nonactivation at Parkland was associated with trial year, sponsor, and phase. Compared with the 1991–2006 period, clinical trials in the 2007–2014 period were almost eightfold more likely not to be activated at Parkland. The most common reasons for nonactivation at Parkland were an inability to perform the study procedures (27%) and the startup costs (15%).
Conclusion.
Over time, in this single-center setting, a decreasing proportion of cancer clinical trials were available to underserved populations. Trial complexity and costs appeared to account for much of this trend. Efforts to overcome these barriers will be key to equitable access to clinical trials, efficient accrual, and the generalizability of the results.
Implications for Practice:
Despite numerous calls to increase and diversify cancer clinical trial accrual, the present study found that cancer clinical trial activation rates in a safety-net setting for medically underserved populations have decreased substantially in recent years. The principal reasons for study nonactivation were expenses and an inability to perform the study-related procedures, reflecting the increasing costs and complexity of cancer clinical trials. Future efforts need to focus on strategies to mitigate the increasing disparity in access to clinical research and cutting-edge therapies, which also threatens to hinder study accrual, completion rates, and generalizability.
Introduction
Accrual to cancer clinical trials remains a major challenge [1–3]. Currently, fewer than 5% of U.S. adult cancer patients enroll in clinical trials. Poor study accrual hinders study completion, wastes resources, and limits the generalizability of the results. Multiple factors limiting study enrollment have been identified. These include intrinsic patient characteristics, such as age [3–5], sex [3], race [3, 6–8], and socioeconomic status [6, 9]. Physician characteristics, attitudes toward patients [10], and communication skills [10–14] have also been associated with study accrual. Additionally, factors related to the consent process itself, such as its timing in relation to the cancer diagnosis and consenter experience, appear to influence patient interest in clinical research [15, 16]. Finally, increasingly numerous and stringent eligibility criteria have limited study enrollment [17–22].
Historically, cancer clinical trial accrual rates have been particularly low among underserved populations [3]. The reasons for these trends are multifactorial. Patient mistrust and a poor understanding of the protocols has been associated with decreased enrollment [23, 24]. A greater comorbidity burden could also limit eligibility of such patients disproportionately [25]. Finally, underserved populations might have less access to clinical trials [26]. Addressing these barriers to include these populations in clinical trials has emerged as a major focus for cancer physicians and researchers nationwide [27, 28].
Clinical trial availability represents an initial and essential step in the accrual process. Therefore, to determine the potential effect of cancer clinical trial availability in a medically underserved population, we examined the proportion of clinical trials within a National Cancer Institute (NCI)-designated cancer center that were activated in a major urban safety-net satellite site that provides care to low-income, uninsured, and vulnerable populations. Owing to the perceived increases in the cost and complexity of oncology clinical research over time—factors that can threaten trial activation and conduct in underserved settings—we focused, in particular, on the temporal trends.
Materials and Methods
Study Setting
The Harold C. Simmons Comprehensive Cancer Center is an NCI-designated cancer center within the University of Texas (UT) Southwestern Medical Center, located in Dallas, Texas. In 2013, Dallas County had a population of 2.48 million, of which 39% were Hispanic, 32% were non-Hispanic white, and 23% were African American [29]. The Parkland Health and Hospital System (Parkland) is the sole safety-net medical provider for all cancer treatment in Dallas County. As the Dallas County integrated safety-net health system, Parkland consists of a 900-bed tertiary care hospital and 12 community-oriented primary care clinics throughout Dallas County. Parkland is responsible for the medical care of all uninsured residents in Dallas County. The principal community oncology practice in Dallas County (Texas Oncology) does not accept Medicaid and does not have a charity plan. Thus, uninsured and underinsured individuals are referred to Parkland for cancer care at diagnosis. The inpatient facility and an outpatient primary care and specialty clinic building are located directly adjacent to the UT Southwestern campus. Annually, the Parkland system treats more than 1.5 million patients. Disease-specific oncology clinics are held 5 days per week in a designated oncology space, which includes a 24-chair infusion area.
The Parkland cancer program is accredited by the American College of Surgeons Commission on Cancer. Oncology clinic staff and administrators are full-time Parkland employees. As previously described [30], the oncology clinics are staffed entirely by UT Southwestern medical oncology faculty and fellows. Additionally, there are three full-time clinical research staff (one research manager and two research coordinators) in the Parkland oncology clinic, all of whom are Simmons Cancer Center Clinical Research Office employees.
A single scientific and safety review process for cancer clinical trials covers both UT Southwestern and Parkland. This includes approval and endorsement by a disease-oriented team, review and approval by the Simmons Cancer Center protocol review and monitoring committee, and review and approval by the UT Southwestern institutional review board (IRB). Additionally, a number of Parkland-specific steps are required, including submission of a study intake form that is distributed to all departments involved in the study (e.g., pathology, radiology, pharmacy). Once a study has IRB approval and all Parkland department approvals, it undergoes a final review and approval by the hospital research manager. At that point, it will be opened to accrual.
Data Sources and Variables
We identified clinical trials activated in the Simmons Cancer Center from the Velos database from 1991 (the first year for which data were available) through May 2014. Velos eResearch (Velos, Fremont, CA, http://www.velos.com) is a study management tool used to help investigators manage the set up and day-to-day activities of human research studies. We excluded the following study types from our analysis: pediatric clinical trials (because these are activated exclusively at the Children’s Medical Center), studies categorized as retrospective medical records reviews, single-patient investigational new drug (IND) applications, and trials opened exclusively at remote satellite sites (e.g., the Veterans Affairs North Texas Health Care System). We categorized trials according to the activation year, type (ancillary, correlative, prevention, screening, supportive, diagnostic, epidemiologic, observational, registry, interventional, therapeutic), phase (not applicable, pilot/feasibility, I, II, III), primary management group (breast, ear, nose and throat [ENT], gastrointestinal [GI], genitourinary [GU], gynecology, lung, malignant hematology, radiation oncology, other), investigator-initiated, rare disease (defined as tumor types with an incidence rate of ≤6 per 100,000, or fewer than 10 cases seen in a given year), and sponsor (external peer reviewed, industry, institutional, national cooperative group, not defined). Trials designated as having no phase were generally nontherapeutic studies. The “other” management group included the phase I program and developing programs (e.g., melanoma, sarcoma). The primary management categories were grouped according to clinical structure at Parkland: for example, lung-ENT are combined at a single clinic, just as are GI-GU. For these analyses, radiation oncology was selected as the reference group, because a single radiation oncology clinical operation provides care to both Parkland and Simmons Cancer Center patient populations. All the other groups have separate Parkland and Simmons clinical operations. We recorded whether the trial was activated at Parkland. We then grouped the trial characteristics into larger categories for statistical analysis. We grouped the activation year as 1991–2006 and 2007–2014, because 2007 marked a key point in restructuring and expanding the clinical research office at the Simmons Cancer Center. We selected this cutpoint a priori before reviewing the results of our analysis. For instances in which clinical trials spanned multiple phases (e.g., phase I/II trials), the categorization was assigned according to the earlier phase, because the earlier phase trials were more likely to have features affecting the activation process, such as complex study procedures.
We obtained the dominant reason for trial nonactivation at Parkland from the clinical research managers and coordinators and, in some instances, from the principal investigators. A senior clinical research manager (L.L.P.) conducted the interviews and recorded the reasons provided. This information was reviewed by an experienced clinical investigator (D.E.G.), who provided guidance on categorization and grouping. To limit the possibility of influencing and biasing the interviewed individuals, we did not provide the interviewees with a list of categories from which to choose. Instead, we recorded their feedback as raw data and subsequently assigned the categories. In some instances, the clinical research personnel had archived correspondence (typically electronic mail) that they referenced to provide the requested information. In cases in which individuals no longer held the clinical research position relevant to the trials in question but who still were employed at our medical center, they were contacted and interviewed. We did not contact former clinical research staff who had left our institution. Ultimately, the reasons for nonactivation at Parkland were categorized as follows: startup costs; startup timelines/approval process; intervention not available (e.g., hematopoietic stem cell transplants are not performed at Parkland; thus, clinical trials related to stem cell transplants are not feasible at Parkland); study population not seen (e.g., patients with graft-vs.-host disease are not routinely seen at Parkland owing to the lack of stem cell transplants); standard of care therapies not on formulary (which usually occurred with new treatments or combinations, such as nab-paclitaxel for lung cancer [although on the formulary for pancreatic cancer at Parkland]); the required oversight committee was not available (i.e., the lack of an institutional biosafety committee, precluding the activation of trials using agents considered potential biohazards, such as human and animal pathogens, recombinant DNA, viral vectors, pest insects); clinic scheduling; research staffing; clinician availability (faculty-level physician expertise for certain [typically rarer] malignancies might not be present at Parkland); and study procedures (e.g., frequent electrocardiograms [ECGs] or pharmacokinetics [PK] blood sampling). We grouped the reasons as site-related if it appeared unlikely that sponsor actions could have addressed them (e.g., study intervention not available) and as sponsor-related if it appeared likely that sponsor actions could have addressed them (e.g., study startup costs).
Statistical Analysis
Summary statistics, including the mean, median, and frequency, were recorded. We used univariate and multivariate logistic regression to determine the association between the trial characteristics and activation at Parkland. For the initial multivariate model, we entered all variables with p < .2 on univariate analysis. We then performed backward selection, removing the variables with the largest p value > .05 one by one to generate the final model. We used chi-square analysis to determine the association between the trial characteristics and the reasons for not activating the trial at Parkland. To compare trends in safety-net site activation by trial characteristics across time periods, we generated three-way tables analyzed by Cochran-Mantel-Haenszel p values. All statistical calculations were performed using SAS for Windows, version 9.3 (SAS Institute, Inc., Cary, NC, http://www.sas.com).
Results
Through the initial Velos report, we identified 1,175 clinical trials. The following trials were removed: 345 pediatric studies, 28 retrospective medical record reviews, 6 single-patient INDs, 2 duplicates, 19 that had only opened at satellite sites, and 2 that had never been intended for activation. Thus, 773 trials remained in the final study cohort. Of these, 77% were interventional/therapeutic trials, 36% were industry-sponsored, and 64% were phase II or phase III trials. Additional characteristics of these trials are listed in Table 1.
Table 1.
Baseline trial characteristics
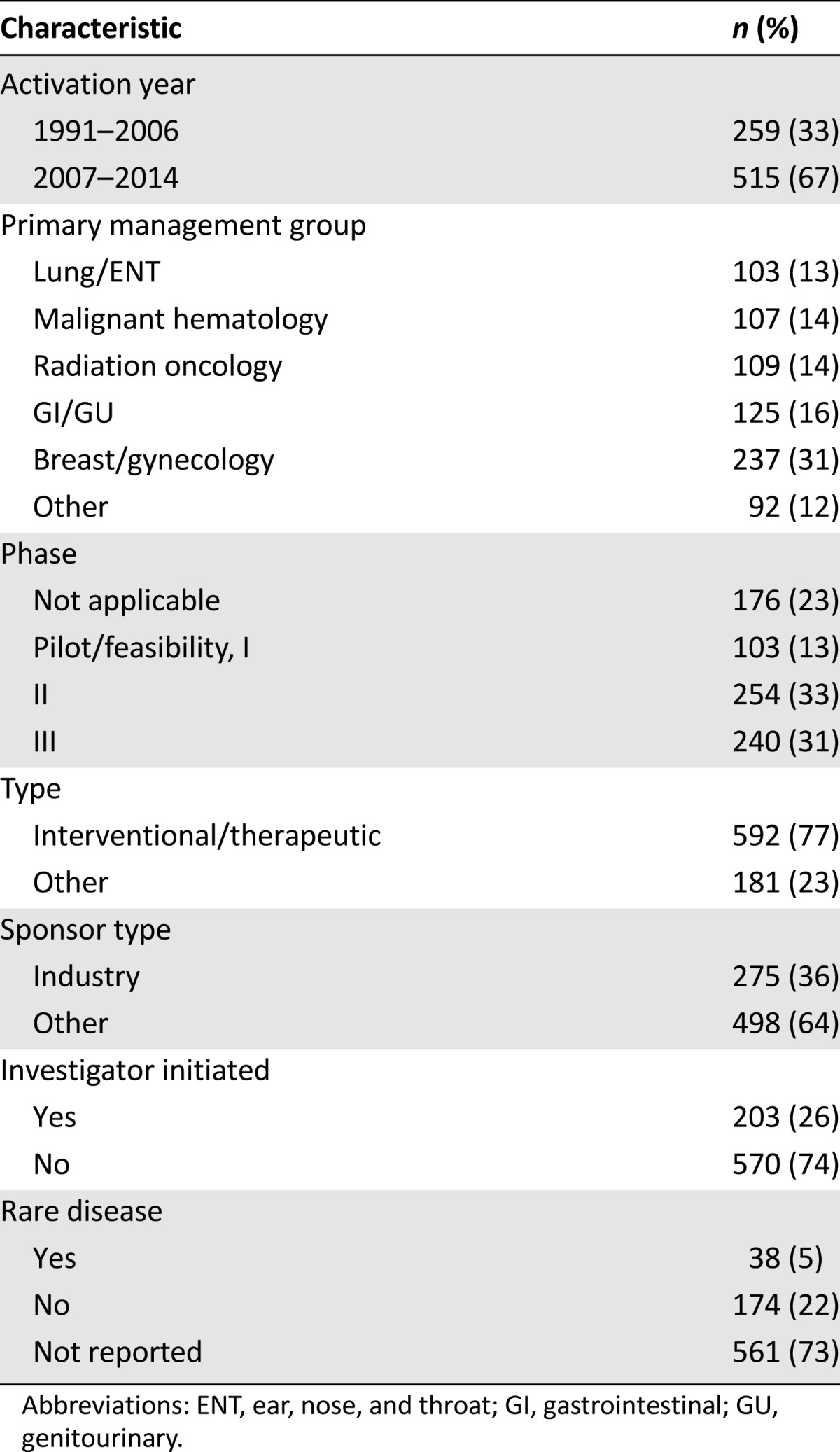
The overall Simmons clinical trial portfolio differed in a number of characteristics between the early (1991–2006) and late (2007–2014) time periods. First, although the late time period (7-year duration) represents less than one half the time represented in the early time period (15-year duration), two thirds of the trials in our study cohort were activated during the later time period. In general, trials activated during the late time period were more likely to be an earlier phase. For 1991–2006 and 2007–2014, respectively, the trial phases were as follows: not applicable (27% vs. 21%), pilot/feasibility/phase I (9% vs. 16%), phase II (32% vs. 33%), and phase III (32% vs. 30%). They were also slightly more likely to be interventional/therapeutic (72% vs. 79%) and to be industry-sponsored (28% vs. 40%).
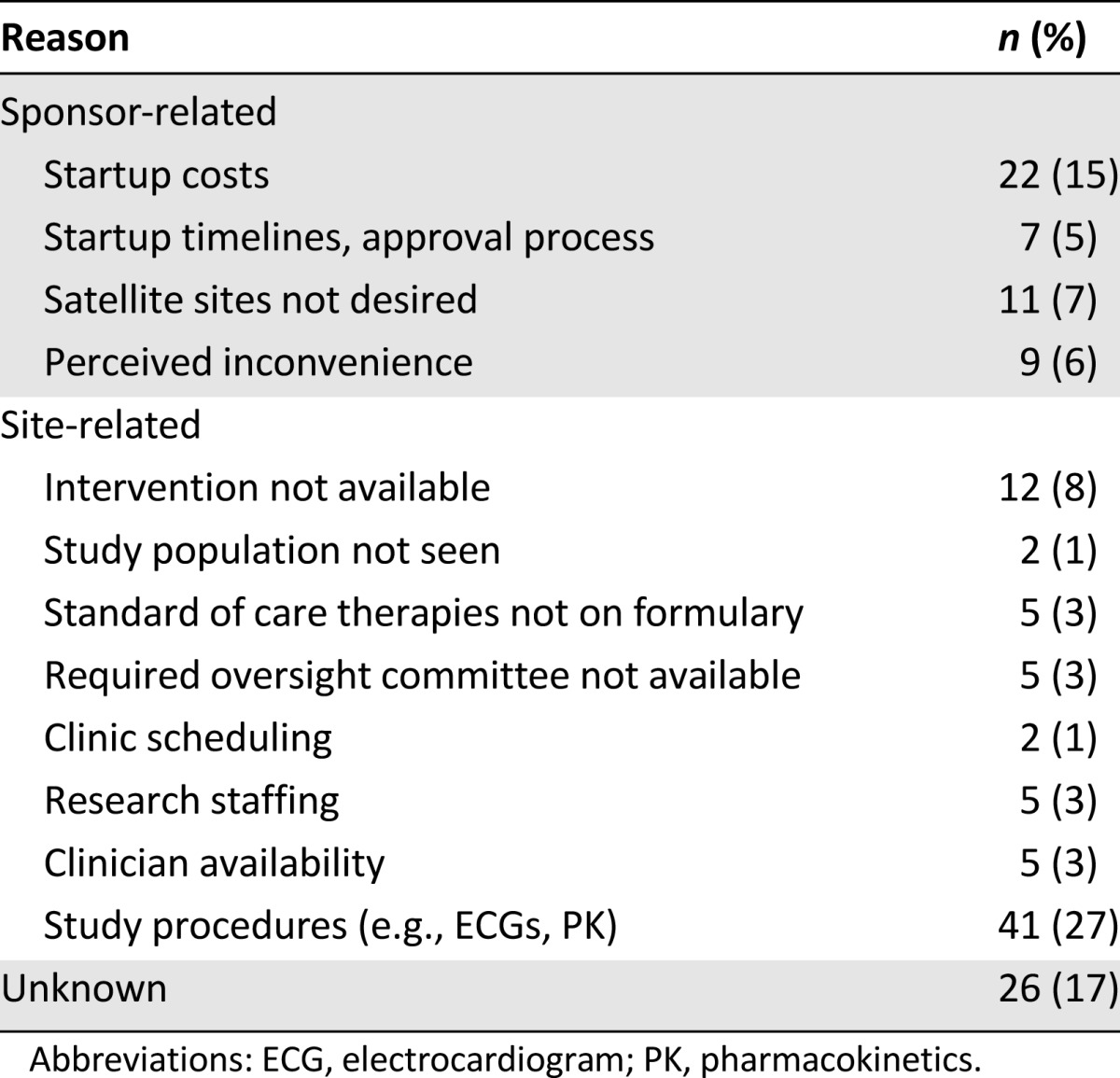
A total of 152 clinical trials (20%) were not activated at the safety-net site (Parkland). The principal reasons for nonactivation are listed in Table 2. The reason was considered sponsor-related in 34% of the cases, site-related in 49%, and unknown in 17%. Among the sponsor-related reasons, the startup costs were the most common. Among the site-related reasons, study procedures (e.g., ECGs and PK blood sampling) were the most common.
Table 2.
Reasons for trials not being activated at safety-net site
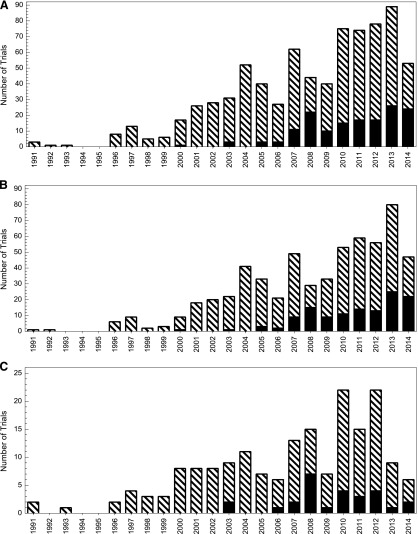
The association between the trial characteristics and nonactivation at the safety-net site is shown in Table 3 (univariate analysis) and Table 4 (multivariate analysis). We noted a clear increase in the likelihood of nonactivation over time (odds ratio, 7.94 for 2007–2014 compared with 1991–2006). Nonactivation rates by specific year are shown in Figure 1A for all trials, Figure 1B for interventional/therapeutic trials, and Figure 1C for noninterventional/therapeutic trials. Similar differences were observed when using other temporal cutpoints. For instance, the nonactivation rate in 1991–2003 was 3% versus 23% in 2004–2014 (p < .001). The nonactivation rate in 1991–2009 was 13% versus 27% in 2010–2014 (p < .001). Regarding the primary management group, the highest rates of safety-net nonactivation were among the hematologic malignancy and other (including phase I trials) categories. For the trial phase, the lowest rate of safety-net site activation occurred among the feasibility/pilot/phase I studies, with a progressive increase noted for each phase increase. Industry-sponsored trials were less likely to be activated at the safety-net site. Despite changes in the distribution of the trial phase and sponsor over time at our center, the trial activation year, management group, and phase remained strongly associated with activation status in a multivariable model incorporating each of these variables. Because interventional/therapeutic trials are often the principal focus of clinical trial accrual efforts, we analyzed the predictors of safety-net site activation for this subset (which represented 77% of the total trials in our study) in separate univariate and multivariate analyses (supplemental online Tables 1, 2), which yielded similar results.
Table 3.
Predictors of clinical trials not opening at safety-net site (all trials)—univariate analysis
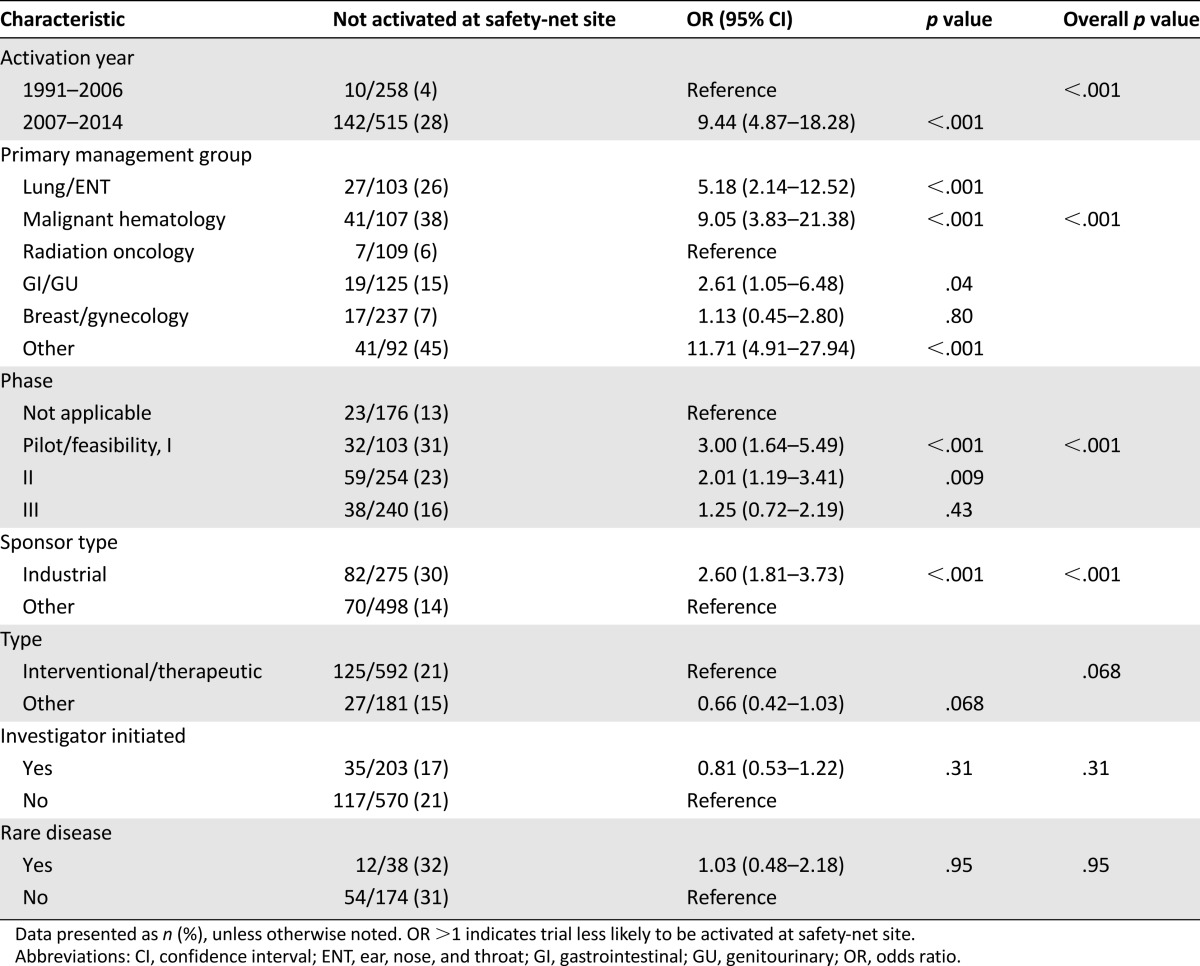
Table 4.
Predictors of clinical trials not opening at safety-net site (all trials)—multivariate analysis
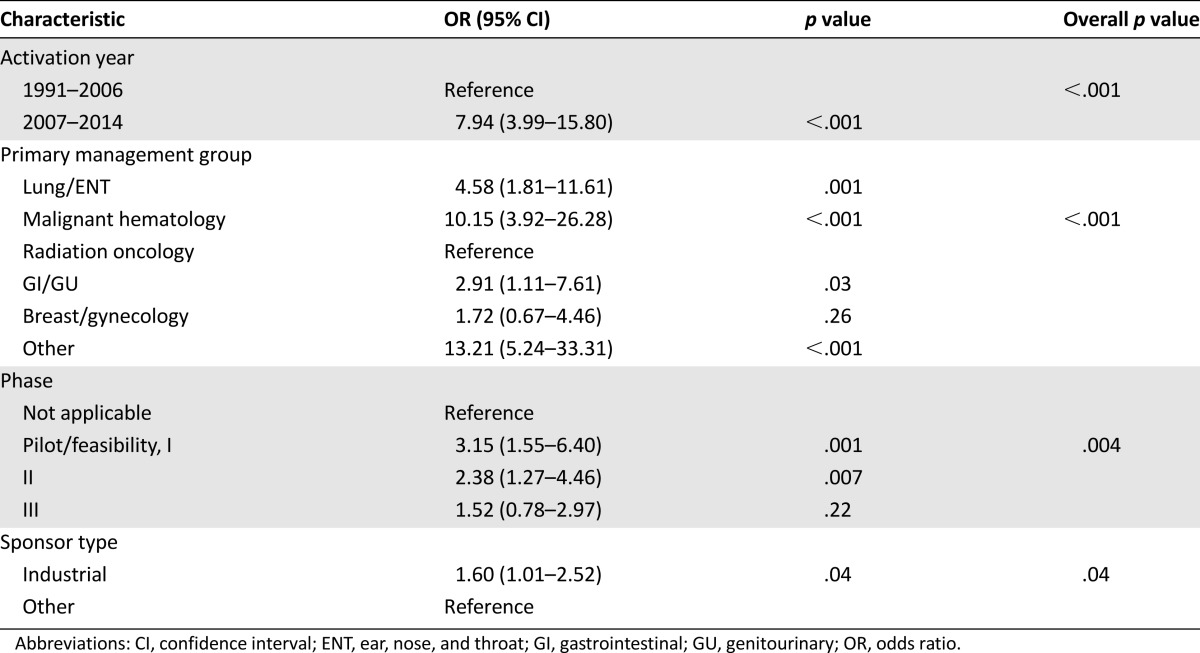
Figure 1.
Number and proportion of clinical trials not activated at safety-net site. (A): All trials. (B): Therapeutic/interventional trials. (C): Nontherapeutic/noninterventional trials. Black bars indicate not activated at safety-net site; hatched bars, activated at safety-net site. Data for 2014 represent January 1, 2014, through May 30, 2014.
We also examined the association between trial characteristics and the reasons for the trial not being activated at the safety-net site (Fig. 2). A nonsignificant association was found for trial activation year, with site-related reasons accounting for all nonactivations in 1991–2006 and 60% of the reasons for nonactivation in 2007–2014 (p = .16). Site-related reasons accounted for 78% of the nonactivations for phase I trials, 60% for phase II, and 55% for phase III (p = .10). Site-related reasons accounted for 65% of nonactivations for interventional/therapeutic trials compared with 40% of nonactivations for noninterventional/therapeutic trials (p = .04). Also, a significant association was found with management group (p = .03). For 17% of the nonactivations, the reasons were not available. In general, these trials were older (median year, 2008) than the trials for which reasons were available (median year, 2012).
Figure 2.
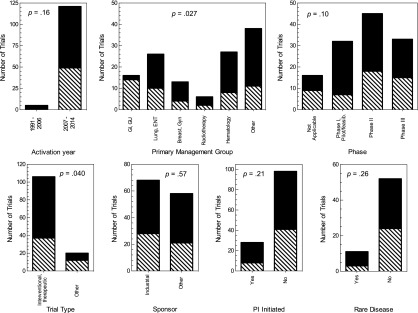
Reasons for safety-net nonactivation according to trial characteristics. Black bars indicate site-related; hatched bars, sponsor-related.
Abbreviations: feasib., feasibility; GI, gastrointestinal; GU, genitourinary; Gyn, gynecology; PI, primary investigator.
Finally, we found that the association between certain trial characteristics and nonactivation at the safety-net site differed over time. For instance, in 1991–2006, sponsor type was not associated with safety-net activation: only 4% of industry-sponsored and 4% of other sponsor trials were not activated at the safety-net site (p = 1.00). However, during the 2007–2014 time period, 39% of industry-sponsored trials were not activated at the safety-net site compared with 20% of other trials (p < .001; Cochran-Mantel-Haenszel for time trend p < .001). Similarly, the trial phase was associated with nonactivation at the safety-net site only during the more recent time period (Cochran-Mantel-Haenszel for time trend p = .005). In 1991–2006, the nonactivation rate was 4% for all study phases (p = 1.00). In 2007–2014, the nonactivation rates were 19% for no phase, 39% for pilot/feasibility/phase I, 33% for phase II, and 22% for phase III (p = .003).
Discussion
It has long been recognized that the lack of available trials is a major factor limiting accrual to adult cancer clinical trials [26, 31]. A number of potential underlying reasons exist for this lack of research opportunities. Recent years have seen trial eligibility criteria become increasingly numerous and stringent, resulting in the exclusion of a greater proportion of potential participants [17, 21, 32]. Transportation and scheduling issues could also limit study access [33]. Nowhere has this been a greater issue than for medically underserved populations and underrepresented minorities, which have particularly low clinical trial participation rates [3]. Among other cited reasons, a greater comorbidity burden and mistrust of the medical establishment could render these populations less eligible for, or interested in, clinical research opportunities [23–25]. Additionally, such individuals might have less access to available clinical trials [26].
To our knowledge, ours is the first study that systematically examines institutional availability of cancer clinical trials for these historically underrepresented populations. Specifically, we examined the trends and factors related to study activation in an urban safety-net healthcare system associated with a major academic medical center. To conduct the present analysis, we compared these study activation rates with those of the affiliated NCI-designated cancer center, which is staffed by the same academic medical oncology faculty and served by the same clinical research office. Most importantly, we found that, even when controlling for multiple clinical trial characteristics, a near eightfold increase has occurred in the proportion of cancer clinical trials that are not activated within the safety-net setting. Additionally, certain disease types (including lung cancer, head-and-neck cancer, and hematologic malignancies) and trial phases (specifically phase I) were associated with nonactivation. This distribution could exacerbate the access disparities because the burdens of smoking and smoking-related diseases are greatest in underserved populations [34, 35]. The variety of tumor types and multiple lines of previous therapy allowed on many phase I trials could also be particularly relevant to a safety-net setting.
We identified numerous reasons for trial nonactivation, including sponsor-related (most commonly, study cost) and site-related (most commonly, the inability to perform the study-related procedures such as frequent ECGs or PK blood sampling). How these can be addressed is a complex undertaking that will require input from all parties. With the goal of optimizing the clinical and biologic information gleaned from each research subject, clinical trials have become increasingly complicated and regularly feature requirements for archival tumor tissue, frequent phlebotomy for pharmacokinetic and pharmacodynamic assessments, frequent quality-of-life and patient-centered outcome determinations, and additional safety considerations such as regular cardiac monitoring [36]. Safety-net medical facilities, which might already be stretching resources to provide routine clinical care, will face disproportionate challenges to meeting these requirements. Issues related to trial startup costs could be multifactorial, potentially reflecting increasing charges from the study site or—in particular, since the recent economic downturn—less willingness to pay pre-existing charges on the part of the sponsor.
Some key reasons for the lack of trial activation at our safety-net clinical site are clearly defined, although not necessarily readily addressed. The lack of a hematopoietic stem cell transplant program at the site precludes activation of any clinical trial using that modality or studying a related medical condition (e.g., graft-vs.-host disease). Creation of an institutional biosafety committee or establishing an oversight agreement with the existing UT System institutional biosafety committee would permit consideration of perceived high-risk therapeutic agents, such as viral vectors and their byproducts. However, this limitation accounted for only 3% of the trials not activated at the safety-net site. The substantial increase in early-phase and industry-sponsored trials noted in our series might reflect our center’s maturation and growth as a regional cancer center. Separately, the trend could also be an indication of a general decline in the number of national cooperative trials because of financial constraints and restructuring. Regardless of the explanation, securing the interest and participation of industry partners is essential. This will require negotiation of study terms and conveyance of the potential benefits of safety-net site activation, such as increased and diversified accrual. Streamlining the startup processes and timelines could also increase sponsor interest in the site. Conducting phase I clinical trials at the safety-net site will require considerable effort and infrastructure development, unless a method exists to treat the patients within the existing phase I program at our university site. This is the clinical model used by our radiation oncology department (in which all patients are treated within a single clinical operation), which had the highest proportion of safety-net trial activation (94%) of any management group in our study. Even more broadly, perhaps the health plans newly available through the Affordable Care Act will expand the treatment options for underserved populations in Dallas and nationwide, providing patients with increased access to cancer clinical trials.
Additional areas that could be targeted to expand access to clinical trials among underserved populations include factors related to trial design and financing. The increased complexity of clinical trials in recent years reflects the heightened sophistication of scientific analyses in clinical research (e.g., predictive and pharmacodynamic biomarkers) and increased safety and regulatory requirements (e.g., extensive ECG analyses). These complexities affect not only the study costs and procedures but also the length of the protocol documents and number of eligibility criteria. Addressing these issues requires a careful balance between maximizing the yield of efficacy and biomarker data in a trial and providing real-world effectiveness readouts. Clinical trial financing requires consideration of multiple ethical principles. Coverage analyses determining which costs are assigned to study sponsors (i.e., research components) and which to the patient and/or third-party payor (i.e., standard of care components) are performed in a unified fashion, regardless of patients’ financial and insurance status. Any financial assistance provided to clinical trial participants must be provided equally to all participants and must not be considered potentially coercive to the potential study subjects.
The present study had a number of limitations. First, as suggested by the factors detailed in the present report, the present single-center experience might not be broadly generalizable. Nevertheless, our analysis had features that suggest our findings are unlikely to be unique. Although academic medical centers represent only 5% of all hospitals, they provide 37% of all charity care and 26% of all Medicaid hospitalizations [37]. Additionally, some observed trends seem unlikely to reflect site-specific considerations. For instance, although the capacity to perform clinical procedures at the safety-net site did not diminish during our study periods (and, indeed, likely increased), an increasing proportion of clinical trials were not activated there because of the inability to perform study-related procedures. This suggests that, over time, clinical trial protocols are becoming increasingly complex to implement and conduct [38]. Another limitation was that our analysis did not capture the possibility of clinical trials that were never activated at any site within our system because they could not be activated at the safety-net site. Unmeasured variables, such as changes in regulatory requirements, investigator mix, and clinical trial office personnel, could also affect trial activation. The possibility also exists of a misclassification of the reasons for nonactivation, which might have been lessened by having multiple clinical investigators assign categories and then measure concordance. Additionally, the reasons for trial nonactivation at the safety-net site were missing for 17% of cases, reflecting the inherent challenges of obtaining information on previous clinical trials that was not captured prospectively. That this proportion was not greater reflects the time distribution of the nonactivated trials: only 3% were from before 2004. In collecting this information, recall bias could also have been present among the personnel interviewed. We sought to minimize this effect by recording the interviewees’ responses as raw data and then assigning the categorization, rather than providing a list of reasons from which to choose.
Conclusion
In the present single-center setting, over time, an increasing proportion of cancer clinical trials were not accessible to the underrepresented minorities and medically underserved populations. This trend was not explained by changes in the types of clinical trials under consideration. Several sponsor- and site-related reasons underlie this phenomenon, most commonly concerns regarding startup costs and a limited ability to perform increasingly complex study procedures. These initial findings merit additional investigation. If confirmed, future discussion will need to focus on strategies to mitigate an increasing disparity in access to clinical research and cutting-edge therapies that also threatens to hinder study accrual, completion rates, and generalizability.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank Hannah Phan and Erin Williams from the Simmons Cancer Center Clinical Research Office for assistance running the Velos database reports to obtain the clinical trial protocol information, Helen Mayo, from the University of Texas Southwestern Medical Library, for assistance performing the literature searches, and the Simmons Cancer Center Disease Oriented Team leaders and managers for assistance collecting the reasons for clinical trial nonactivation. The present study was presented in abstract form to the 51st Annual Meeting of the American Society of Clinical Oncology, Chicago, Illinois, USA, May 29 to June 2, 2015, and presented at the 15th Annual Targeted Therapies of the Treatment of Lung Cancer Meeting, Santa Monica, California, USA, February 18–21, 2015. Biostatistical support was provided by the Biostatistics and Bioinformatics Shared Resource at the Harold C. Simmons Comprehensive Cancer Center, University of Texas Southwestern Medical Center, Dallas, Texas, USA, which is supported in part by National Cancer Institute Cancer Center Support Grant 1P30 CA 142543-03. A.M.L. is supported by Howard Hughes Medical Institute Med into Grad Grant 56006776 and NIH Training Grant 1T32GM10977601.
Footnotes
Editor's Note: See the related commentary,“Clinical Trials, Disparities, and Financial Burden: It's Time to Intervene,” on page 571 of this issue.
Author Contributions
Conception/Design: David Gerber, Ashley Lakoduk, Laurin Priddy
Collection and/or assembly of data: David Gerber, Ashley Lakoduk, Laurin Priddy
Data analysis and interpretation: David Gerber, Jingsheng Yan, Xian-Jin Xie
Manuscript writing: David Gerber, Ashley Lakoduk
Final approval of manuscript: David Gerber, Ashley Lakoduk, Laurin Priddy, Jingsheng Yan, Xian-Jin Xie
Disclosures
The authors indicated no financial relationships.
References
- 1.Viability of cancer clinical research: Patient accrual, coverage, and reimbursement. American Medical Association Council on Scientific Affairs. J Natl Cancer Inst. 1991;83:254–259. doi: 10.1093/jnci/83.4.254. [DOI] [PubMed] [Google Scholar]
- 2.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 3.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 6.Advani AS, Atkeson B, Brown CL, et al. Barriers to the participation of African-American patients with cancer in clinical trials: A pilot study. Cancer. 2003;97:1499–1506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- 7.Du W, Gadgeel SM, Simon MS. Predictors of enrollment in lung cancer clinical trials. Cancer. 2006;106:420–425. doi: 10.1002/cncr.21638. [DOI] [PubMed] [Google Scholar]
- 8.Shavers VL, Lynch CF, Burmeister LF. Factors that influence African-Americans’ willingness to participate in medical research studies. Cancer. 2001;91(suppl):233–236. doi: 10.1002/1097-0142(20010101)91:1+<233::aid-cncr10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg ML, Fremont A, Khan DC, et al. Lay patient navigator program implementation for equal access to cancer care and clinical trials: Essential steps and initial challenges. Cancer. 2006;107:2669–2677. doi: 10.1002/cncr.22319. [DOI] [PubMed] [Google Scholar]
- 10.Howerton MW, Gibbons MC, Baffi CR, et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109:465–476. doi: 10.1002/cncr.22436. [DOI] [PubMed] [Google Scholar]
- 11.Fallowfield L, Jenkins V, Farewell V, et al. Efficacy of a Cancer Research UK communication skills training model for oncologists: A randomised controlled trial. Lancet. 2002;359:650–656. doi: 10.1016/S0140-6736(02)07810-8. [DOI] [PubMed] [Google Scholar]
- 12.Hietanen PS, Aro AR, Holli KA, et al. A short communication course for physicians improves the quality of patient information in a clinical trial. Acta Oncol. 2007;46:42–48. doi: 10.1080/02841860600849067. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins V, Fallowfield L, Solis-Trapala I, et al. Discussing randomised clinical trials of cancer therapy: Evaluation of a Cancer Research UK training programme. BMJ. 2005;330:400. doi: 10.1136/bmj.38366.562685.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razavi D, Merckaert I, Marchal S, et al. How to optimize physicians’ communication skills in cancer care: Results of a randomized study assessing the usefulness of posttraining consolidation workshops. J Clin Oncol. 2003;21:3141–3149. doi: 10.1200/JCO.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Rasco DW, Xie Y, Yan J, et al. The impact of consenter characteristics and experience on patient interest in clinical research. The Oncologist. 2009;14:468–475. doi: 10.1634/theoncologist.2008-0268. [DOI] [PubMed] [Google Scholar]
- 16.Gerber DE, Rasco DW, Skinner CS, et al. Consent timing and experience: Modifiable factors that may influence interest in clinical research. J Oncol Pract. 2012;8:91–96. doi: 10.1200/JOP.2011.000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCusker J, Wax A, Bennett JM. Cancer patient accessions into clinical trials: A pilot investigation into some patient and physician determinants of entry. Am J Clin Oncol. 1982;5:227–236. doi: 10.1097/00000421-198204000-00072. [DOI] [PubMed] [Google Scholar]
- 18.Kotwall CA, Mahoney LJ, Myers RE, et al. Reasons for non-entry in randomized clinical trials for breast cancer: A single institutional study. J Surg Oncol. 1992;50:125–129. doi: 10.1002/jso.2930500215. [DOI] [PubMed] [Google Scholar]
- 19.Lee JY, Breaux SR. Accrual of radiotherapy patients to clinical trials. Cancer. 1983;52:1014–1016. doi: 10.1002/1097-0142(19830915)52:6<1014::aid-cncr2820520614>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 20.Simon MS, Du W, Flaherty L, et al. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol. 2004;22:2046–2052. doi: 10.1200/JCO.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Fuks A, Weijer C, Freedman B, et al. A study in contrasts: Eligibility criteria in a twenty-year sample of NSABP and POG clinical trials. National Surgical Adjuvant Breast and Bowel Program. Pediatric Oncology Group. J Clin Epidemiol. 1998;51:69–79. doi: 10.1016/s0895-4356(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 22.Gerber DE, Laccetti AL, Xuan L, et al. Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouton CP, Harris S, Rovi S, et al. Barriers to black women’s participation in cancer clinical trials. J Natl Med Assoc. 1997;89:721–727. [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis PM, Butow PN, Tattersall MH, et al. Randomized clinical trials in oncology: Understanding and attitudes predict willingness to participate. J Clin Oncol. 2001;19:3554–3561. doi: 10.1200/JCO.2001.19.15.3554. [DOI] [PubMed] [Google Scholar]
- 25.Adams-Campbell LL, Ahaghotu C, Gaskins M, et al. Enrollment of African Americans onto clinical treatment trials: Study design barriers. J Clin Oncol. 2004;22:730–734. doi: 10.1200/JCO.2004.03.160. [DOI] [PubMed] [Google Scholar]
- 26.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 27.Chen MS, Jr, Lara PN, Dang JH, et al. Twenty years post-NIH Revitalization Act: Enhancing minority participation in clinical trials (EMPaCT): Laying the groundwork for improving minority clinical trial accrual: Renewing the case for enhancing minority participation in cancer clinical trials. Cancer. 2014;120(suppl 7):1091–1096. doi: 10.1002/cncr.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawk ET, Habermann EB, Ford JG, et al. Five National Cancer Institute-designated cancer centers’ data collection on racial/ethnic minority participation in therapeutic trials: A current view and opportunities for improvement. Cancer. 2014;120(suppl 7):1113–1121. doi: 10.1002/cncr.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Census Bureau. State and county quickfacts. 2013. Available at http://quickfacts.census.gov/qfd/states/48/48113.html. Accessed November 26, 2014.
- 30.Yorio JT, Yan J, Xie Y, et al. Socioeconomic disparities in lung cancer treatment and outcomes persist within a single academic medical center. Clin Lung Cancer. 2012;13:448–457. doi: 10.1016/j.cllc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Javid SH, Unger JM, Gralow JR, et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316) The Oncologist. 2012;17:1180–1190. doi: 10.1634/theoncologist.2011-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Spall HG, Toren A, Kiss A, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: A systematic sampling review. JAMA. 2007;297:1233–1240. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- 33.Baggstrom MQ, Waqar SN, Sezhiyan AK, et al. Barriers to enrollment in non-small cell lung cancer therapeutic clinical trials. J Thorac Oncol. 2011;6:98–102. doi: 10.1097/JTO.0b013e3181fb50d8. [DOI] [PubMed] [Google Scholar]
- 34.Laaksonen M, Rahkonen O, Karvonen S, et al. Socioeconomic status and smoking: Analysing inequalities with multiple indicators. Eur J Public Health. 2005;15:262–269. doi: 10.1093/eurpub/cki115. [DOI] [PubMed] [Google Scholar]
- 35.Hardy D, Liu CC, Xia R, et al. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115:2199–2211. doi: 10.1002/cncr.24248. [DOI] [PubMed] [Google Scholar]
- 36.Ledford H. Translational research: 4 ways to fix the clinical trial. Nature. 2011;477:526–528. doi: 10.1038/477526a. [DOI] [PubMed] [Google Scholar]
- 37.Grover A, Slavin PL, Willson P. The economics of academic medical centers. N Engl J Med. 2014;370:2360–2362. doi: 10.1056/NEJMp1403609. [DOI] [PubMed] [Google Scholar]
- 38.Berger O, Grønberg BH, Sand K, et al. The length of consent documents in oncological trials is doubled in twenty years. Ann Oncol. 2009;20:379–385. doi: 10.1093/annonc/mdn623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.