Incidence rates for breast cancer continue to rise in the People’s Republic of China. The purpose of this study was to analyze differences in characteristics of breast malignancies between China and the U.S. Chinese women were diagnosed at younger ages with higher stage and larger tumors and underwent more aggressive surgical treatment. Prospective trials should be conducted to address screening, surgical, and tumor discrepancies between China and the U.S.
Keywords: Breast cancer, China, Disparities
Abstract
Background and Objective.
Incidence of and mortality rates for breast cancer continue to rise in the People’s Republic of China. The purpose of this study was to analyze differences in characteristics of breast malignancies between China and the U.S.
Methods.
Data from 384,262 breast cancer patients registered in the U.S. Surveillance, Epidemiology, and End Results (SEER) program from 2000 to 2010 were compared with 4,211 Chinese breast cancer patients registered in a Chinese database from 1999 to 2008. Outcomes included age, race, histology, tumor and node staging, laterality, surgical treatment method, and reconstruction. The Pearson chi-square and Fisher’s exact tests were used to compare rates.
Results.
Infiltrating ductal carcinoma was the most common type of malignancy in the U.S. and China. The mean number of positive lymph nodes was higher in China (2.59 vs. 1.31, p < .001). Stage at diagnosis was higher in China (stage IIA vs. I, p < .001). Mean size of tumor at diagnosis was higher in China (32.63 vs. 21.57 mm). Mean age at diagnosis was lower in China (48.28 vs. 61.29 years, p < .001). Moreover, 2.0% of U.S. women underwent radical mastectomy compared with 12.5% in China, and 0.02% in China underwent reconstructive surgery.
Conclusion.
Chinese women were diagnosed at younger ages with higher stage and larger tumors and underwent more aggressive surgical treatment. Prospective trials should be conducted to address screening, surgical, and tumor discrepancies between China and the U.S.
Implications for Practice:
Breast cancer patients in China are diagnosed at later stages than those in America, which might contribute to different clinical management and lower 5-year survival rate. This phenomenon suggests that an earlier detection and treatment program should be widely implemented in China. By comparing the characteristics of Chinese and Chinese-American patients, we found significant differences in tumor size, lymph nodes metastasis, and age at diagnosis. These consequences indicated that patients with similar genetic backgrounds may have different prognoses due to the influence of environment and social economic determinates.
Abstract
摘要
背景与目的. 中华人民共和国的乳腺癌发病率和死亡率正在持续升高。本研究旨在分析中国与美国乳腺癌特征的差异。
方法. 对2000 – 2010年期间美国监测、流行病学与最终结果(SEER)项目中登记的384 262例乳腺癌患者数据与1999 – 2008年间中国数据库中登记的4 211例中国乳腺癌患者数据进行比较。结果包括年龄、种族、组织学、肿瘤与淋巴结分期、发病侧、手术方案,以及乳房再造术。使用Pearson卡方检验和Fisher精确检验对比率进行比较。
结果. 浸润性导管癌是美国和中国乳腺癌最常见的类型。中国患者平均阳性淋巴结数目较多(2.59 vs. 1.31,P < 0.001),诊断时分期较高(IIA vs. I期,P < 0.001)、平均肿瘤体积较大(32.63 vs. 21.57 mm)且平均年龄较低(48.28 vs. 61.29岁,P < 0.001)。此外,美国女性患者中有2.0%接受乳腺癌根治术治疗,而中国的比例为12.5%,中国有0.02%的患者接受了乳房再造术。
结论. 中国女性诊断时较为年轻、分期较高、肿瘤体积较大,且更多接受较激进的手术治疗。应开展前瞻性临床研究来阐明中国和美国之间筛查、手术和肿瘤差异的问题。The Oncologist 2015;20:1044–1050
对临床实践的提示:中国乳腺癌患者诊断时分期晚于美国患者,这可能导致临床管理的差异和5年生存率较低。这一现象提示中国应广泛开展早期诊断和治疗方案。通过对中国患者和美籍华人患者的特征进行比较,我们发现肿瘤体积、淋巴结转移和诊断年龄存在显著差异。这些结果提示由于环境和社会经济学决定因素的影响,遗传背景相似的患者可能具有不同的预后。
Introduction
Breast cancer maintains one of the highest incidences per year among common cancers, and physicians worldwide struggle with determining the optimal course of screening, diagnosis, and treatment methods for patients. With heavy international focus on breast cancer research, progress continues to be made to alleviate mortality and morbidity rates from breast cancer. Because of increased education and screening, deaths per 100,000 breast cancer patients in the U.S. have decreased from 33.2 in 1989 to 21.9 in 2009 [1]. Diagnosis is often made during annual screening visits and less often by patient self-examination. With earlier diagnoses and subsequent lower stage tumors, treatment can entail lumpectomy and/or radiation and chemotherapy rather than the more aggressive modified radical mastectomy for later, higher stage diagnoses.
In contrast with the decreasing rates of breast cancer-related mortality in the U.S., multiple recent studies have illustrated stable or increasing rates of mortality over the past few years in the People’s Republic of China. Although 5-year survival rates for breast cancer patients in the U.S. have increased from 84.3% in 1989 to 90.5% in 2005, those in China have remained around 76%–82% [1–3]. This difference could be related to the U.S. maintaining well-supported national screening protocols, easy access to breast surgeons, and a lack of a negative social stigma surrounding screening and treatment. Given the population of China, mortality rates due to breast cancer constitute a significant portion of the international burden of breast cancer. Many of these deaths are preventable with earlier identification and treatment. Many studies have described specific characteristics of breast malignancies in China; however, no descriptive study has been conducted comparing breast cancer characteristics in China and the U.S.
The aims of this paper are twofold. First, through a systematic comparison of data from the U.S. Surveillance, Epidemiology, and End Results (SEER) program from 2000 to 2010 [1] and a similar database created at the Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CICAMS) in Beijing, China, from 1999 to 2008, we aimed to analyze differences between the two nations pertaining to demographics and tumor characteristics on breast cancer diagnosis. These differences included the following outcomes at diagnosis: age, race, histology, tumor and node staging, and laterality. Second, we aimed to analyze differences pertaining to surgical treatment between patients in the U.S. and China, using the same databases. These differences included modified radical mastectomy versus radical mastectomy and reconstruction.
Materials and Methods
Retrospective Study
Patient Selection in the U.S.
All patients with a diagnosis of breast cancer included in the U.S. data were included in this study. Patients with a diagnosis before 2000 and after 2010 were excluded from this study. Within the years 2000–2010, there were no specific exclusion criteria. Methods of patient selection for the SEER database can be found on the SEER website (http://seer.cancer.gov/csr/1975_2010/).
Patient Selection in China
Data collection for patients with breast cancer in China was conducted through the use of a multicenter retrospective hospital-based database that focused on a cohort of Chinese women with malignant primary breast cancer. In order to acquire an extensive and representative cohort of patients for this study that would be comparable to data acquired through the U.S. SEER program, the nation of China was stratified into seven geographic areas (north, northeast, central, south, east, northwest, and southwest). The purpose of this approach was to construct a large group of study patients that was representative of both urban and rural areas and including women belonging to both high and low socioeconomic classes of diverse geographic regions. Within each of the seven geographic regions, purposive sampling was used to choose one tertiary public cancer hospital with certain inclusion characteristics. These characteristics were as follows: chosen hospitals were the public cancer hospitals and regional referral centers providing pathology diagnosis, surgery, radiotherapy, medical oncologic treatment, and follow-up care for breast cancer patients. Chosen public cancer hospitals were the larger university hospitals specific to each region. Furthermore, patient sources at each hospital had to contain patients from the entire designated geographic study region. Finally, each hospital chosen was located in a major city, based on the premise that Chinese women with breast cancer tend to seek treatment in bigger cities rather than less equipped rural hospitals. With this hospital inclusion criteria, the list of hospitals through which study patients were acquired included CICAMS (north China); Liaoning Cancer Hospital (northeast China); Second Xiangya Hospital, Central South University (central China); Sun Yat-Sen University Cancer Center (south China); Zhejiang Cancer Hospital (east China); First Affiliated Hospital of Medical College, Xi’an Jiaotong University (northwest China); and Sichuan Cancer Hospital (southwest China). Further methodology detailing how this enrollment process allowed for a cohort to be designed that was representative of all geographic regions without selection bias can be found in a previously published manuscript using this database [4].
Each selected hospital in China provided the research team with medical records of patients diagnosed with primary breast cancer between 1999 and 2008. Patients diagnosed with primary breast cancer and included in this study were required to meet the following criteria: pathology-confirmed primary breast cancer, inpatient admission data within the selected month of the study hospital, and receipt (current or completed) of treatment (surgical, medical, or radiotherapeutic) for the diagnosed malignancy through the specific hospital site. Patients with benign tumors or tumors suspicious for malignancy without confirmation were excluded from this study [4].
From 1999 to 2008, 45,200 patients with breast cancer were treated in the 7 hospitals. We identified the study group by random sampling of this population. One month was randomly selected within each year (1999–2008), and all inpatient primary breast cancer cases for that month were reviewed. Inpatient records from January and February were excluded from revision because of potential confounding effects of Chinese New Year vacation time on patient care. Each hospital collected data for a minimum of 50 cases for each randomly chosen month during 1999 to 2008, thus each hospital collected a minimum of 500 cases within these 10 years. We previously reported the detailed methods used and the basic characteristics of the patients included in this study [4].
Clinical Variable Measures
Each Chinese hospital record was reviewed by local clerks within each hospital according to our designated protocol that was taught to clerks at CICAMS in Beijing. Standard case report forms (CRFs) designed by the CICAMS research team were used to acquire specific information from the medical record of each included patient. The reliability and validity of the CRFs were deemed appropriate through a prior pilot study in which details of hospitals and patient enrollment are further outlined [4]. Information gathered through CRFs included patient demographics, risk factors, diagnostic imaging test modalities and results, tumor size and pathology, and surgical and chemotherapeutic treatments. Histology information was collected through updated World Health Organization histological classification criteria. After data collection, variables acquired were coded and categorized.
All Chinese data collected were stripped of any personal identifiers according to approved study procedures and aggregated into a secure master database to which only the primary research team was granted access. Acquired SEER data were received in an already deidentified manner. Patient consent for this study was not required, given the lack of risks and retrospective deidentified aspects. Both the Cancer Foundation of China and Vanderbilt University institutional review boards granted approval of the aforementioned methods.
Statistical Analysis
Descriptive statistical tests were used to illustrate and compare information regarding the following characteristics of both American and Chinese populations: age, histology, the number of examined and positive lymph nodes, laterality, stage and tumor size on diagnosis, and radiation and surgical treatment. Chemotherapy data were not analyzed, given the lack of specific chemotherapeutic data in the SEER database.
Means and standard deviations were calculated for quantitative variables from the Chinese and American databases. Differences of rates were analyzed with Pearson chi-square and/or Fisher’s exact tests. Given that the Chinese database did not contain overall population data, the latest recorded incidence of breast cancer was acquired through the National Cancer Registry for 2009.
Results
Patient Population
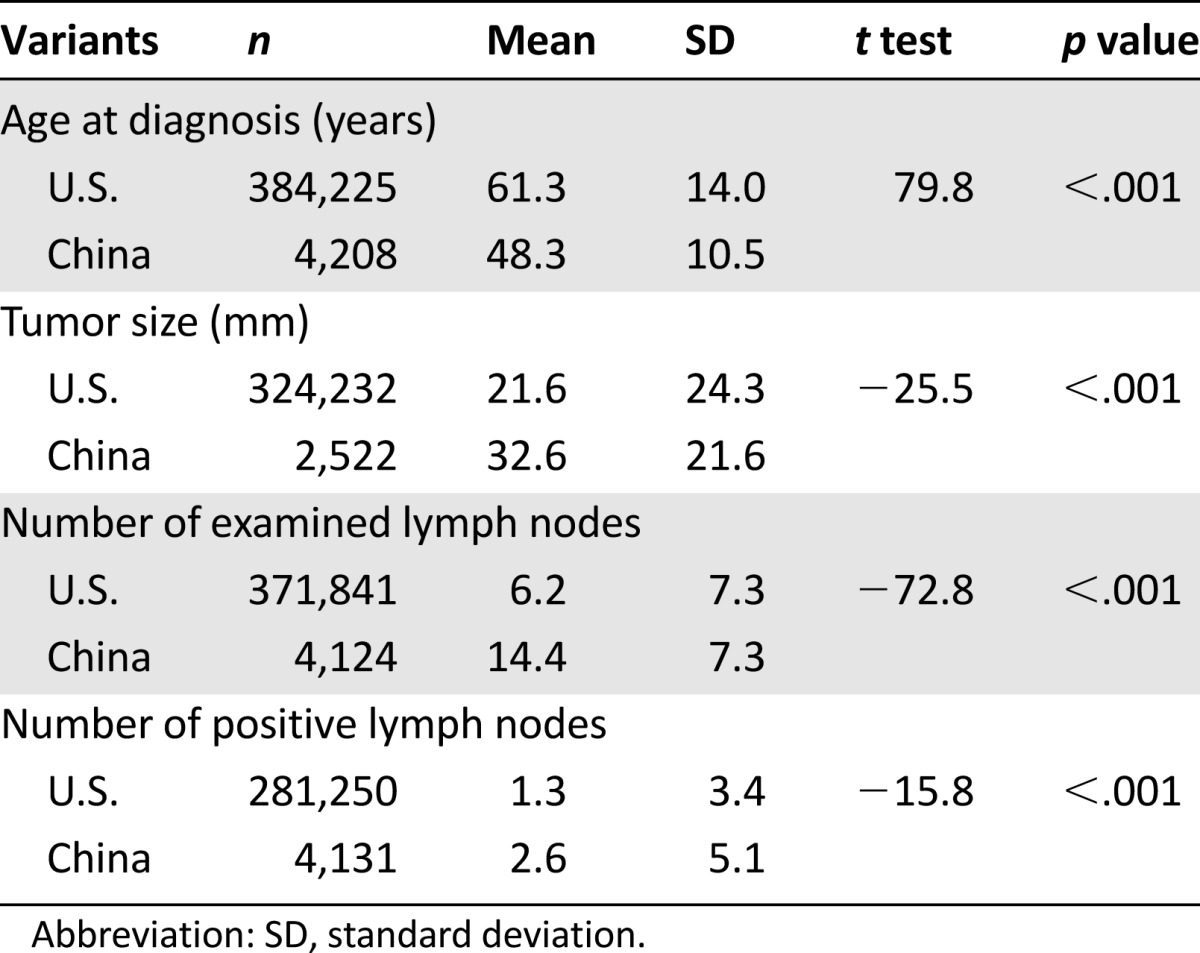
From 2000 to 2010, 384,262 patients were registered in the breast cancer SEER database in the U.S., with 37 cases having unlisted ages at diagnosis. From 1999 to 2008, 4,211 patients were registered in the breast cancer CICAMS database in China, with 3 cases having unlisted ages at diagnosis. The mean age at diagnosis in the U.S. was 61.29 years, with an age range of 10 to 114 years. The mean age at diagnosis in China was 48.28 years, with an age range of 21 to 85 years. The t tests showed that the age at diagnosis in the U.S. was significantly higher than that of China (t value 79.83, p < .001) (Table 1).
Table 1.
Characteristics of breast cancer in the U.S. and China
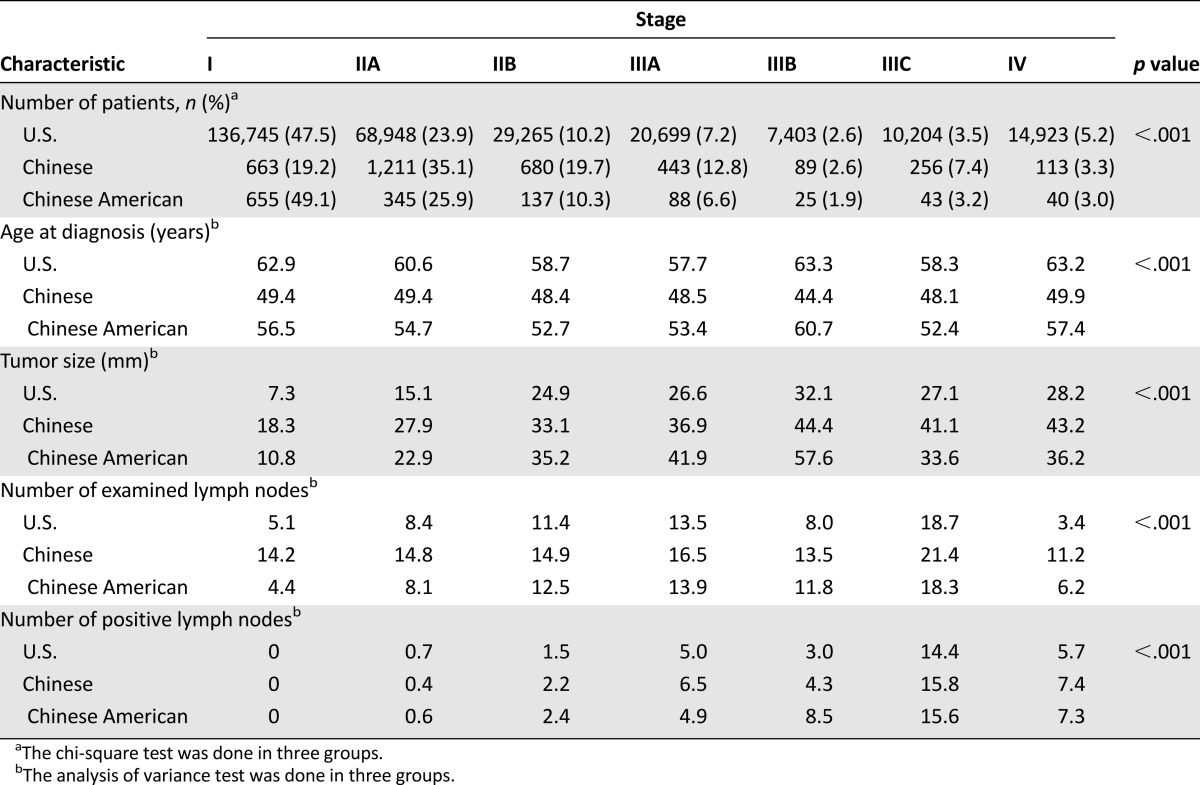
U.S. women included in the study population consisted of the following races (n = 384,262): 325,573 white (84.7%), 39,656 black (10.3%), 15,340 Asian or Pacific Islander (4.0%), 926 American Indian/Alaska Native (0.2%), and 2,767 unknown (0.7%). All women in China included in the study population were of Chinese origin (n = 4,211). Among the U.S. Asian or Pacific Islander group, 1,928 were of Chinese origin and will be referred to as Chinese Americans. The average age on diagnosis of Chinese Americans was 55.31 years, significantly higher than that of Chinese women in China (p < .001) (Table 2).
Table 2.
Characteristics of breast cancer by stage in U.S., Chinese, and Chinese-American women
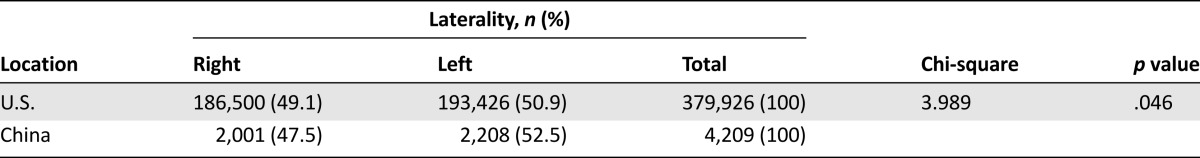
The histological subtype with the highest frequency in both the U.S. and China was infiltrating ductal carcinoma (63.3% and 81.8%, respectively). A significantly higher proportion of tumors originated in the right breast in patients in the U.S. compared with those in China (Pearson chi-square value 3.989, p = .046) (Table 3). A t test value of −25.546 indicated that primary tumors in China were significantly larger on diagnosis than those in the U.S. (32.63 vs. 21.57 mm, p < .001) (Table 1).
Table 3.
Laterality of breast cancer in the U.S. and China
Tumor and Node Stages at Diagnosis
Overall, 288,187 U.S. patients, 3,455 Chinese patients, and 1,333 Chinese-American patients were included in the stage analyses with stages I–IV (Table 2). The stage at diagnosis occurring with the highest frequency in the U.S. was stage I (47.45%), and the stage at diagnosis occurring with the highest frequency in Chinese-American patients was also stage I (49.14%). The stage at diagnosis occurring with the highest frequency in China was stage IIA (35.05%). A Pearson chi-square value of 1372.53 demonstrated that patients in China were diagnosed at a significantly higher stage than those in the U.S., including Chinese Americans (p < .001).
In patients with stage I malignancies, the average age at diagnosis was 62.87 years in U.S. women, 56.47 years among Chinese Americans, and 49.35 years in Chinese women. In patients with stage IIA malignancies, the average ages at diagnosis were 60.56, 54.7, and 49.44 years in U.S., Chinese-American, and Chinese women, respectively (Table 2).
Stage I patients in the U.S. were diagnosed with an average tumor size of 7.31 mm compared with 10.83 mm in Chinese-American women and 18.34 mm in women in China (p < .05) (Table 2).
Positive Lymph Nodes
In the U.S. and China, the number of positive lymph nodes with the highest frequency was zero (49.9% and 53.8%, respectively). A Pearson chi-square test value of 618.5 demonstrated that the rate of having positive lymph nodes on diagnosis in the U.S. was significantly lower than that of China (32% vs. 49%, p < .001). A t test value of −15.84 indicated that breast cancer patients in China were found to have a significantly higher mean number of positive nodes in comparison with those in the U.S. (2.59 vs. 1.31, p < .001) (Table 1).
Surgical Method and Reconstruction
In order to create a standardized comparison, we included data pertaining only to radical mastectomy and modified radical mastectomy in this study with 90,547 U.S. patients and 3,739 Chinese patients. In the U.S., 98.3% of women at stage I underwent a modified radical mastectomy compared with 1.7% undergoing a radical mastectomy. In China, 93.9% of women at stage I underwent a modified radical mastectomy and 6.1% underwent a radical mastectomy. Although 96.5% of U.S. women at stage IV underwent modified radical mastectomy, 77.3% of women in China underwent modified radical mastectomy and 22.7% of women in China underwent radical mastectomy. A Pearson chi-square value of 1,706.7 demonstrated that significantly more U.S. patients chose modified radical mastectomy and more Chinese patients chose radical mastectomy (p < .001) (Table 4).
Table 4.
Surgery methods in the U.S. and China
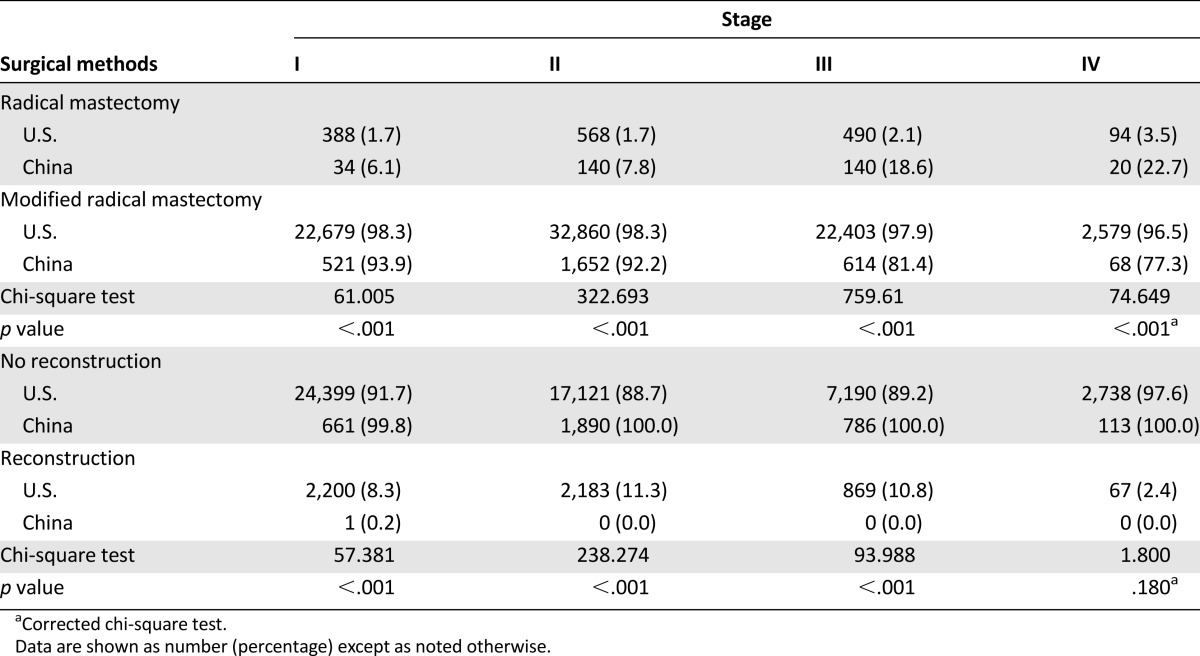
In the U.S., 9.9% of 74,502 of patients underwent immediate reconstruction, whereas 0.02% of 4,185 patients in China underwent immediate reconstruction. A Pearson chi-square value of 455.1 demonstrated that a significantly higher number of women in the U.S. underwent immediate reconstruction, independent of stage, compared with China (p < .001) (Table 4).
Discussion
Discrepancies among diagnosis, characteristics, and surgical treatment of breast malignancies between developing nations and the U.S. are vast. There has not been a comparison to date between diagnosis and treatment patterns in China and those of a developed nation [5]. In a multicenter systematic retrospective comparison, we found significant differences between characteristics and treatment of breast malignancies of patients in the U.S. and China, with the exception of tumor histology. The following significant differences in breast malignancies between patients in the U.S. and China on diagnosis were validated: mean patient age is 13 years lower in China, mean numbers of both examined and positive lymph nodes in China are approximately twice those of the U.S., and the U.S. exhibits a higher number of right-sided breast malignancies [6]. Furthermore, stage at diagnosis is lower in the U.S., mean size of the primary tumor is around 11 mm smaller in the U.S., a greater proportion of women in China receive radical versus modified radical mastectomies, and fewer women in China undergo immediate breast reconstruction. The most common histology found in both the U.S. and China was infiltrating ductal carcinoma (63.3% and 81.8%, respectively).
Given that there is not a standardized screening protocol in China as there is in the U.S., many Chinese women who are diagnosed with invasive malignancies at younger ages often see a physician when they notice concerning changes in the breasts [7]. In this retrospective study, we found that mean age at diagnosis is significantly lower in China (48.28 years) than in the U.S. (61.29 years). In addition, women in China exhibited a significantly advanced average stage on diagnosis (stage IIA compared with stage I) based on primary tumor size and a greater number of positive lymph nodes [8]. This study showed more examined lymph nodes in Chinese patients than that in American patients (14.4 vs. 6.2, p < .001), probably due to less use of sentinel lymph node biopsy (SLNB) in China. It was shown that more than 95% cases with stages I–III in the Chinese database did not have SLNB, indicating the underutilization of this method in China (data not shown). Because there is no record of SLNB in the SEER database, we could not compare this difference between groups of patients. Although there is a current drive in China to establish a national screening protocol similar to those in developed nations, a noteworthy comparison is the significantly lower average age of stage I patients in China (49.35 vs. 62.87 years) and the larger tumor size of stage I patients in China compared with stage I patients in the U.S. (18.34 vs. 7.31 mm). Perhaps the issue is not a lack of screening but rather more aggressive environmental characteristics that cause larger, earlier malignancies and fundamental differences between these tumors despite the similar infiltrating ductal carcinoma demonstrated in this study. To investigate this, we analyzed variables based on stage for Chinese-American women through SEER data [1] and found that Chinese-American women were diagnosed most frequently as stage I (49.14%) at a mean age higher than Chinese women (56.47 vs. 49.35 years) and with a tumor size significantly smaller than that of Chinese women (10.83 vs. 18.34 mm). These results encourage the theory that environmental factors and lack of screening in China significantly contribute to the more advanced malignancies diagnosed.
Because of the later stages at diagnosis, a higher proportion of women in China do not undergo surgery, or they undergo radical mastectomy rather than modified radical mastectomy, according to our data. We believe that this difference is partly due to conservative patient choice of surgical method in China, after the surgeon explains both the Halsted radical mastectomy and modified radical mastectomy. We believe the difference is also partly due to more advanced tumors in China, necessitating more aggressive surgical excision. Furthermore, a significantly higher number of women undergo immediate reconstruction in the U.S. (9.9%) compared with women in China (0.02%). Reconstruction beyond the immediate setting was not analyzed because of the lack of these data in our Chinese database. This trend of decreased surgical and reconstructive treatment and operations that are more aggressive than those in the U.S. could be multifactorial. One reason could be the higher percentage of metastatic advanced stage tumors on diagnosis in China compared with the U.S. Given the advanced stages of these tumors, surgical and/or radiation treatment would not be curative. Regarding reconstructive treatment, this aspect of care is not covered by insurance in China, and that could contribute to the extremely low rate of treatment demonstrated in this study.
A notable consideration is the conservative nature of Chinese patients, particularly those of lower socioeconomic status in rural areas [9, 10]. Social stigmas regarding breast cancer, surgery, and reconstruction likely play a role in more women opting out of surgical treatment. This could be compounded by a lack of patient education regarding the efficacy of these surgical treatments [11, 12]. Radical mastectomies may be performed at a higher rate in China because of the advanced stages at which Chinese women are diagnosed due to a lack of health care access, patient education, and adequate screening, among other factors [13, 14]. Although we did not analyze differences between rural and urban groups regarding choice of radical mastectomy versus modified radical mastectomy, a prior study using this database established that breast-conserving surgery increased by 15% in areas of higher socioeconomic status compared with only 6% in areas of lower socioeconomic status [15]. Further studies regarding the specific reasons for these surgical trends must be conducted to increase the number of women who undergo less aggressive surgical treatment, perhaps with earlier diagnosis. Future studies should also address possible reasons for minimal reconstruction in China, aside from insurance coverage, to address the needs of patients regarding self-image. Finally, given the overall advancement of chemotherapy, endocrine therapy, and targeted therapy, further studies comparing such treatment in China and the U.S. must be conducted to assess how to optimize both surgical and medical aspects of care in China.
Our values of tumor characteristics and surgical treatment reported over a similar 10-year period demonstrate significant discrepancies between diagnosis and treatment of breast malignancies in China compared with the U.S. These discrepancies have a direct influence on the increasing rates of incidence in China in recent years [16]. It is likely that several of these differences pertaining to the advanced nature of malignancies on diagnosis in China are related to the lack of patient knowledge and a standardized screening protocol [17], especially when observing our results comparing Chinese-American patients to those in China. Differences pertaining to surgical and reconstructive treatments could be related to the conservative nature of Chinese women or to the lack of knowledge regarding surgical options and efficacy. These differences can also reflect the advanced nature of Chinese malignancies. A limitation of this study is the slight difference in time periods analyzed between the two nations. A strength of this study is the use of extensive study cohorts from both nations, enabling high statistical power of outcomes. A necessary follow-up of this study includes acquiring information regarding patient knowledge and preferences in China to address screening and surgical discrepancies. Additional studies must explore the aforementioned differences in stage I tumor characteristics between China and the U.S., specifically including Chinese-American patients.
Conclusion
Although the current literature suggests inadequate screening of breast malignancies and subsequently higher rates of mortality in developing nations such as China, a large-scale study comparing specific diagnostic-related differences between China and a developed nation with lower mortality rates has not been conducted. This study demonstrated that women in China are diagnosed at significantly younger ages, with larger, higher stage tumors and more positive lymph nodes. A smaller proportion of women in China undergo surgical and immediate reconstructive treatment compared with women in the U.S., and a higher proportion of Chinese women undergo radical versus modified radical mastectomies. Follow-up studies are needed to understand why Chinese women acquire breast cancers at an average of 13 years younger than their female U.S. counterparts. Follow-up studies are also needed to further characterize the reasons for these global health care discrepancies and could dramatically affect comorbidity and mortality rates for breast cancer in China, if addressed. This study provides descriptive knowledge that health care providers and policy makers can use to address specific differences in treatment and ultimately reduce the international disease burden of breast cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
We thank the Cancer Institute of the Chinese Academy of Medical Sciences (CICAMS) for providing their expertise in the development of this study. We also thank the local investigators from Beijing, Liaoning (Shenyang), Hunan (Changsha), Guangdong (Guangzhou), Zhejiang (Hangzhou), Shannxi (Xian), and Sichuan (Chengdu) for data collection and assisting us with completing this project successfully. This work was in part supported by the NIH Fogarty International Center through the Fogarty Global Health Fellows Program Consortium, composed of the University of North Carolina, Johns Hopkins University, Morehouse School of Medicine, and Tulane University (5R25TW009340-02).
Author Contributions
Conception/Design: You-Lin Qiao
Provision of study material or patients: Bin Zhang, Zhong-Hua Tang, Guo-Ji Chen, Xiao-Ming Xie, Xiao-Zhou Xu, Hong-Jian Yang, Jian-Jun He, Hui Li, Jia-Yuan Li
Collection and/or assembly of data: Bin Zhang, Zhong-Hua Tang, Xiao-Ming Xie, Hong-Jian Yang, Jian-Jun He, Hui Li, Jia-Yuan Li, Jin-Hu Fan
Data analysis and interpretation: Priya G. Sivasubramaniam, Bai-Lin Zhang, Qian Zhang, Jennifer S. Smith
Manuscript writing: Priya G. Sivasubramaniam, Bai-Lin Zhang, Qian Zhang
Final approval of manuscript: Priya G. Sivasubramaniam, Bai-Lin Zhang, Qian Zhang, Jennifer S. Smith, Bin Zhang, Zhong-Hua Tang, Guo-Ji Chen, Xiao-Ming Xie, Xiao-Zhou Xu, Hong-Jian Yang, Jian-Jun He, Hui Li, Jia-Yuan Li, Jin-Hu Fan, You-Lin Qiao
Disclosures
The authors indicated no financial relationships.
References
- 1.Surveillance, Epidemiology, and End Results program. Available at http://www.seer.cancer.gov.
- 2.Chor JS-Y. Cancer survivors: What we know, what we need to know - Asian perspective. Available at http://www.wcrf.org/sites/default/files/WCRFConference_JChor.pdf.
- 3.Sankaranarayanan R, Swaminathan R, Brenner H, et al. Cancer survival in Africa, Asia, and Central America: A population-based study. Lancet Oncol. 2010;11:165–173. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Zhang BN, Fan JH, et al. A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer. 2011;11:364. doi: 10.1186/1471-2407-11-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhoo-Pathy N, Yip CH, Hartman M, et al. Breast cancer research in Asia: Adopt or adapt Western knowledge? Eur J Cancer. 2013;49:703–709. doi: 10.1016/j.ejca.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Weiss HA, Devesa SS, Brinton LA. Laterality of breast cancer in the United States. Cancer Causes Control. 1996;7:539–543. doi: 10.1007/BF00051887. [DOI] [PubMed] [Google Scholar]
- 7.Taib NA, Yip CH, Low WY. Recognising symptoms of breast cancer as a reason for delayed presentation in Asian women--the psycho-socio-cultural model for breast symptom appraisal: Opportunities for intervention. Asian Pac J Cancer Prev. 2011;12:1601–1608. [PubMed] [Google Scholar]
- 8.Yang HJ, Yu XF, He XM, et al. Age interactions in breast cancer: An analysis of a 10-year multicenter study in China. J Int Med Res. 2012;40:1130–1140. doi: 10.1177/147323001204000333. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Li J, Zheng S, et al. Breast cancer stage at diagnosis and area-based socioeconomic status: A multicenter 10-year retrospective clinical epidemiological study in China. BMC Cancer. 2012;12:122. doi: 10.1186/1471-2407-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Love RR, Ginsburg OM, Coleman CN. Public health oncology: A framework for progress in low- and middle-income countries. Ann Oncol. 2012;23:3040–3045. doi: 10.1093/annonc/mds473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu TY, Liu YL, Chung S. Improving breast cancer outcomes among women in China: Practices, knowledge, and attitudes related to breast cancer screening. Int J Breast Cancer. 2012;2012:921607. doi: 10.1155/2012/921607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar Y, Mishra G, Gupta S, et al. Cancer screening for women living in urban slums--acceptance and satisfaction. Asian Pac J Cancer Prev. 2011;12:1681–1685. [PubMed] [Google Scholar]
- 13.Huang Y, Zhou K, Li H, et al. Knowledge, attitudes, and behaviour regarding breast cancer screening among women from different socio-economic regions in southwest China: A cross-sectional study. Asian Pac J Cancer Prev. 2011;12:203–209. [PubMed] [Google Scholar]
- 14.Yu Z, Jia CX, Geng CZ, et al. Risk factors related to female breast cancer in regions of northeast China: A 1:3 matched case-control population-based study. China Med J (Engl) 2012;125:733–740. [PubMed] [Google Scholar]
- 15.Zhang B, Song Q, Zhang B, et al. A 10-year (1999 ~ 2008) retrospective multi-center study of breast cancer surgical management in various geographic areas of China. Breast. 2013;22:676–681. doi: 10.1016/j.breast.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Chen WQ, Zheng RS, Zeng HM, et al. Trend analysis and projection of cancer incidence in China between 1989 and 2008 [in Chinese]. Zhonghua Zhong Liu Za Zhi. 2012;34:517–524. doi: 10.3760/cma.j.issn.0253-3766.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Zhao FH, Chen JF, Gao XH, et al. Effectiveness and health economic analysis of strategies on cervical cancer screening and early diagnosis and treatment [in Chinese]. Zhonghua Zhong Liu Za Zhi. 2012;34:632–636. doi: 10.3760/cma.j.issn.0253-3766.2012.08.017. [DOI] [PubMed] [Google Scholar]


