This review explores the literature on HER2 expression in endometrial cancer, focusing on trastuzumab and other anti-HER2 therapy and resistance mechanisms characterized in breast cancer but germane to endometrial tumors. Potential opportunities to overcome inherent resistance to anti-HER2 therapy in endometrial cancer are discussed.
Keywords: Endometrial cancer, HER2, Uterine serous carcinoma, Resistance
Abstract
Endometrial cancer is the most common gynecologic cancer in the United States, diagnosed in more than 50,000 women annually. While the majority of women present with low-grade tumors that are cured with surgery and adjuvant radiotherapy, a significant subset of women experience recurrence and do not survive their disease. A disproportionate number of the more than 8,000 annual deaths attributed to endometrial cancer are due to high-grade uterine cancers, highlighting the need for new therapies that target molecular alterations specific to this subset of tumors. Numerous correlative scientific investigations have demonstrated that the HER2 (ERBB2) gene is amplified in 17%–33% of carcinosarcoma, uterine serous carcinoma, and a subset of high-grade endometrioid endometrial tumors. In breast cancer, this potent signature has directed women to anti-HER2-targeted therapies such as trastuzumab and lapatinib. In contrast to breast cancer, therapy with trastuzumab alone revealed no responses in women with recurrent HER2 overexpressing endometrial cancer, suggesting that these tumors may possess acquired or innate trastuzumab resistance mechanisms. This review explores the literature surrounding HER2 expression in endometrial cancer, focusing on trastuzumab and other anti-HER2 therapy and resistance mechanisms characterized in breast cancer but germane to endometrial tumors. Understanding resistance pathways will suggest combination therapies that target both HER2 and key oncogenic escape pathways in endometrial cancer.
Implications for Practice:
This review summarizes the role of HER2 in endometrial cancer, with a focus on uterine serous carcinoma. The limitations to date of anti-HER2 therapy in this disease site are examined, and mechanisms of drug resistance are outlined based on the experience in breast cancer. Potential opportunities to overcome inherent resistance to anti-HER2 therapy in endometrial cancer are detailed, offering opportunities for further clinical study with the goal to improve outcomes in this challenging disease.
Introduction
Endometrial cancer (EnCa) is the leading gynecologic malignancy in the United States, accounting for more than 50,000 new cases in 2014 and 8,590 deaths [1]. EnCa includes a broad range of histologic subtypes, the most common being endometrioid. Marked differences in clinical behavior have been observed in patients with EnCa, depending on histology, tumor grade, and extent of cancer spread. Investigators have suggested a classification system that separates endometrial tumors into type I and type II subsets to account for the striking divide in risk factors, clinical behavior, and approach to therapy [2].
Type I endometrial tumors account for 75%–85% of EnCa [3]. These tumors are usually of low-grade, endometrioid histology, and are most commonly diagnosed at stage I or II and confined to the uterus and cervix. The recurrence risk after surgery for these women is 2%–7%, portending an excellent prognosis [4, 5]. In contrast to type I cancers, type II endometrial tumors are high-grade (grades 2–3) with a spectrum of histologies, including uterine serous carcinoma (USC), carcinosarcoma, and clear cell carcinoma [3, 6, 7]. These cancers typically (40%–50%) present with disease outside the uterus (stage III or IV) and have a high propensity to recur after primary therapy. Common sites of metastasis include pelvic or para-aortic lymph nodes, the vagina, lungs, liver, and peritoneum, although spread to the brain, bones, and distant lymph nodes has been reported [3, 8, 9]. Unlike type I EnCa, upfront treatment of type II tumors frequently requires multimodality therapy, combining aggressive cytoreductive surgery to remove visible tumor, platinum-containing chemotherapy, and pelvic or abdominal radiation [10, 11]. While type II cancers account for only 15%–25% of all EnCa, these tumors are responsible for 75% of the mortality observed, likely related to their higher grade and stage at presentation [3]. Whether type I or type II, advanced, recurrent, or refractory EnCa portends a poor prognosis, as there are limited therapeutic modalities available, offering low response and, rarely, cure rates [12–14]. Mortality from EnCa is approximately 18%, similar to breast cancer. To improve outcomes, the development of therapeutic modalities that specifically address the molecular events that promote or contribute to the pathology of this subset of aggressive EnCa is imperative.
Genetic Features of Endometrial Cancer
Endometrial cancers harbor specific, and at times overlapping, molecular alterations in significant subsets of tumors, making this disease site fertile ground for targeted therapeutics [15]. While the clinical distinction of type I and II is controversial, most investigators agree that high-grade EnCa is a distinct entity from low-grade EnCa, with markedly different drivers of neoplasia that require alternate treatment strategies. Correlative scientific investigations have described molecular signatures specific to either type I or type II that may have therapeutic relevance [16]. Type I cancers frequently have molecular perturbation (gene mutation, gene amplification, or dysfunctional protein expression) of Ras (∼25%), β-catenin (20%–50%), and the phosphatase and tensin homolog (PTEN) tumor suppressor (30%–80%) [16, 17]. Oncogenic alterations in the phosphotidylinositol 3-kinase (PI3K) pathway appear to affect both type I and type II EnCa [16, 18, 19]. Importantly, EnCa has one of the highest rates of PI3K pathway aberrations of all cancers (30%–50%) [20–22], strongly suggesting that targeted therapeutics interacting with this crucial pathway may have heightened antitumor activity [22]. While alterations in the PI3K pathway are prevalent in type II EnCa, these tumors do not typically have Ras, β-catenin, or PTEN alterations. Two of the most commonly described genomic changes in type II tumors are amplification of the human epidermal growth factor receptor 2 (HER2, ERBB2) gene (17%–30%) [18, 23–26] and mutation of the TP53 gene (∼90%) [16, 27]. These data provide evidence to suggest that distinct subsets of patients with high-grade EnCa possess oncogenic alterations that may be susceptible to biologic strategies that target HER2 (ERBB2) and the PI3K pathway [15, 28].
HER2 as an Oncogenic Target
Amplification of the HER2 gene and overexpression of the HER2 protein have been described in many human malignancies, including breast, colon, gastric, esophageal, ovarian, and endometrial; for some of these cancers, anti-HER2 therapies have become a mainstay of treatment (Fig. 1) [29–34]. HER2, also known as HER2/neu, ERBB2, and CD340, is a 185-kDa member of the human epidermal growth factor receptor (HER) family of transmembrane tyrosine kinase receptors. Besides HER2, the HER family consists of three other tyrosine kinase receptors (HER1/EGFR, HER3, and HER4) that, when activated by ligand, can dimerize and induce signal transduction through the mitogen-activated protein kinase and PI3K signaling pathways [35]. This downstream activation leads to induction of genes that promote oncogenic transformation via cell survival, proliferation, angiogenesis, and metastasis [36, 37]. Unlike the other epidermal growth factor receptors, HER2 has no known ligand, highlighting the fact that it may be constitutively activated and could act independently to drive an invasive phenotype. Additionally, HER2, when overexpressed, preferentially forms heterodimers with other members of the HER family, leading to particularly potent downstream signaling [38]. HER2 activation has been used most successfully in breast cancer as a potent biomarker to select those women most likely to respond to anti-HER2 therapies such as trastuzumab, a monoclonal antibody; or lapatinib, a small molecule tyrosine kinase inhibitor (TKI). In breast cancer, 15%–30% of tumors have been found to harbor HER2 activation via gene amplification (HER2/CEP17 ratio >2.0) or protein overexpression (immunohistochemistry [IHC] score, 2+ or 3+) and are designated as HER2 “positive” [39]. While HER2 positivity was initially associated with the most guarded prognosis in breast cancer, the advent of a targeted anti-HER2 therapy has dramatically improved clinical outcomes [32, 39].
Figure 1.

The Cancer Genome Atlas analysis of HER2 mutation, deletion, and amplification across the 12 most common disease sites. Breast, esophageal, stomach, and uterine carcinomas all present with similar rates of gene amplification, while mutation appears to be most common in glioblastoma, cervical, and lung adenocarcinoma. Therapeutic response to anti-HER2 therapies has been most closely associated with HER2 gene amplification or protein overexpression, although understanding of HER2 mutations is expanding (http://www.cbioportal.org [33, 34]).
Abbreviations: adeno, adenocarcinoma; CNA, copy number alteration; CS, carcinosarcoma; GBM, glioblastoma; HER2, human epidermal growth factor receptor 2; squ, squamous cell; TCGA, The Cancer Genome Atlas.
As of April 2015, the U.S. Food and Drug Administration (FDA) has approved five therapies for HER2-positive breast, gastric, and non-small cell lung cancer that have improved prognosis for these patients. The first class of drugs includes monoclonal antibodies against the extracellular domain of the HER2 receptor, including trastuzumab, ado-trastuzumab emtansine (T-DM1), and pertuzumab [40–48]. In addition to these antibodies, there are two FDA-approved small-molecule TKIs, lapatinib and afatinib, that inhibit the intracellular kinase domain of the HER receptor to prevent signaling. Lapatinib inhibits both EGFR and HER2, and afatinib irreversibly inhibits EGFR, HER2, and HER4 [49–52]. Several other antibodies, antibody-drug conjugates, and small-molecule inhibitors of HER2 and other members of the HER family are under investigation, suggesting a vibrant pipeline of agents that could be used in multiple disease sites [53].
HER2 as a Biomarker
Controversy still exists as to whether HER2 protein expression or gene amplification should be used as the biomarker to identify those tumors most likely to respond to the various anti-HER2 therapies in endometrial cancer. In breast cancer, both have been used, and the current American Society of Clinical Oncology-College of American Pathologists HER2 test guideline recommendations are for either IHC or fluorescent in situ hybridization (FISH) to be performed upfront. When IHC reveals 3+ intensity in >10% of cells, no further testing is required. In the setting of 2+ IHC protein expression, experts commonly recommend FISH analysis to ensure that there is at least a HER2-to-centromere ratio of >2.0 to demonstrate increased HER2 dosage [54]. In the breast cancer literature, either of these outcomes define HER2-positive. In other disease sites, such as gastric carcinoma, the IHC criteria have been modified, as the cellular expression patterns differ, but the FISH ratios and guidelines remain identical to those of breast cancer [55–57]. In these alternate disease sites with subsets of tumors with HER2 overexpression, many investigators advocate for similar criteria to define HER2 positivity, although the data linking these criteria to therapeutic response are less robust compared with breast tumors [30, 31, 53, 58]. In endometrial cancer, approximately 60%–70% of high-grade carcinomas appear to overexpress HER2, with only a subset exhibiting HER2 gene amplification [59]. Because responses to HER2 therapy have yet to be demonstrated, no clear recommendations for testing have emerged. Most investigators recommend that both protein expression and gene amplification should be tested as biomarkers of HER2 response in future trials examining novel agents and combinations of therapies [60].
HER2 in Endometrial Cancer
Like breast cancer, high-grade EnCa has a 17%–30% rate of HER2 gene amplification, with up to 80% of tumors exhibiting HER2 protein overexpression [23, 59, 61–63]. The prevalence of HER2 overexpression appears to be disproportionately higher in tumors from black patients compared with white patients [24]. A review of specimens from the Gynecologic Oncology Group (GOG) protocol 177, which examined prospectively collected tumors from women with stage III-IV endometrial cancer, identified a 44% rate of HER2 overexpression by IHC 2-3+ staining and 12% HER2 amplification by FISH in the whole population [64]. There was increased positivity seen in the serous subtype, with 61% overexpressed and 21% amplified, consistent with broad genomic signatures described in The Cancer Genome Atlas (TCGA) experience [15, 64].
The clinical significance of HER2 overexpression or gene amplification remains controversial. Morrison et al. examined both HER2 expression and amplification in 483 women with endometrial cancer of a variety of histologies and found a correlation between HER2 expression and amplification and tumors of higher grade and stage, lymph node positivity, and survival outcomes [63]. Women with tumors positive for HER2 amplification and expression had a 5-year overall survival of 41%, compared with 83% in women with HER2-negative tumors. Even in women otherwise predicted to have the best prognosis with stage IA-IB endometrioid tumors, HER2 positivity was correlated with a worse survival outcome, with a 96% 5-year progression-free survival in women with HER2-negative tumors versus a 33% 4-year survival in women with tumors with elevated HER2 protein expression and gene amplification [65]. Similarly, a series of 68 USCs at the MD Anderson Cancer Center demonstrated 18% HER2 positivity by IHC, which was associated with a worse overall survival [23]. Not all studies have confirmed this association between HER2 and survival in endometrial cancer. In a series of type II cancers out of the Mayo Clinic, HER2 gene amplification by FISH did not correlate with overall survival (18 months vs. 29 months, p = .113) [62]. Regardless of a survival association, the signature is certainly prevalent in the subset of type II tumors known to be more clinically aggressive and chemotherapy refractory.
Although high-grade EnCa harbors this promising HER2 signature, the two published phase II trials of anti-HER2 therapy in recurrent EnCa manifested poor response rates. The phase II GOG229D trial included women with recurrent or persistent endometrial cancer of all histologies, without a requirement for tumor testing for HER2 expression. This study administered single-agent lapatinib to 30 evaluable patients [66, 67]. One woman in the trial had a partial response (3.3%). After enrollment, women who had available tumor tissue were evaluated by IHC for HER2 and EGFR expression, given the dual inhibition of these receptors by lapatinib. Of the 27 tumors analyzed, EGFR was expressed in 62% and HER2 in 8% in the initial hysterectomy specimens. Tumor DNA was also analyzed for EGFR mutations, which were found in three patients, one of whom was the woman with the clinical response. These mutations are of uncertain clinical significance, although all were in the tyrosine kinase domain, and similar mutations in this portion of EGFR have been predictive of response to TKIs in non-small cell lung cancer [66, 67]. The second trial, GOG181B, was a phase II trial of trastuzumab in women with HER2-positive (either amplification by FISH or overexpression by IHC 2+ or 3+) stage III-IV or recurrent endometrial cancer. Overall, 33 women (11.5% of those screened) were included in the study from 2000 to 2007, one-third of whom had USC. In this trial, there were no objective tumor responses noted in any of the patients. Although this trial was closed prematurely because of poor accrual, it achieved 86.5% power to confirm the null hypothesis [68].
Despite an extensive body of breast and gastric cancer literature suggesting HER2 amplification to be a biomarker for response to anti-HER2 therapy [69, 70], these targeted therapies failed to demonstrate any activity in EnCa, even in a preselected population enriched for HER2 positivity [69]. While there is disagreement regarding why lapatinib and trastuzumab failed to demonstrate any significant durable clinical benefit [60], these trials suggest that single-agent therapies directed against HER2 have limited effect in EnCa, possibly because of innate or drug-induced resistance pathways. Using the extensive breast cancer experience with trastuzumab resistance may allow investigators to identify and exploit mechanisms likely to be associated with HER2 therapy resistance in EnCa as well.
Using the extensive breast cancer experience with trastuzumab resistance may allow investigators to identify and exploit mechanisms likely to be associated with HER2 therapy resistance in EnCa as well.
Anti-HER2 Therapy Resistance Mechanisms
Resistance to anti-HER2 therapies can be intrinsic or acquired with treatment, and much research has elucidated molecular mechanisms potentially contributing to disease-specific resistance patterns. The breast cancer experience provides an instructive example of how resistance has been studied and overcome. Invasive breast cancers have a 15%–30% prevalence of HER2 overexpression, and while many women with HER2-positive tumors (gene amplified or protein overexpressed) respond to anti-HER2 therapies in the adjuvant and metastatic settings [40, 71], 20% of these patients never respond, and many more acquire resistance to these therapies after an initial response [36, 40, 45, 72–74]. Numerous trastuzumab resistance mechanisms in breast cancer have been reviewed by Pohlmann et al [36]. Those that appear most relevant to EnCa include (a) increased expression of a constitutively active p95HER2 truncated variant that signals but does not bind antibody [73, 75]; (b) upregulation of downstream activators such as via PIK3CA mutation [76], loss of PTEN function [77, 78], or increased AKT and S6K phosphorylation [76, 79, 80]; and (c) signaling through alternative pathways including other HER family members such as EGFR and HER3 [81–84] and unrelated pathways such as Notch [85, 86]. Significant preclinical and clinical efforts to examine and overcome anti-HER2 therapy resistance in breast cancer have focused on these fundamental areas, and such studies have led to improvements in clinical outcome [87, 88]. In EnCa, proposed strategies against anti-HER2 resistance mechanisms are largely untested, but mounting preclinical data suggest combination therapy targeting cancer cells simultaneously at multiple checkpoints in the HER2 signaling pathway may be a successful treatment approach in HER2-positive tumors.
HER2 Structural Alteration
Structural alterations in the HER2 target have been associated with trastuzumab resistance. The p95HER2 variant is a truncated form of the full HER2 receptor that lacks the N-terminal trastuzumab binding domain but retains the active kinase C-terminal domain. Breast tumors that express the truncated p95HER2 variant are resistant to trastuzumab therapy, as this protein lacks the extracellular domain (ECD) to which the antibody binds [89, 90]. The p95HER2 form has been shown to be generated following post-translational proteolysis that cleaves the HER2 ECD but may also be the result of alternative translation [91–93]. Elevated serum levels of the HER2 ECD in breast cancer patients have been correlated with HER2 gene amplification, metastasis, and poor survival [94–96]. Increased levels of the p95HER2 variant have been correlated with poor prognosis and trastuzumab resistance in HER2 positive breast cancer [73, 75, 97–99]. Preclinical in vitro and in vivo studies demonstrated that p95HER2 exhibited kinase activity that induced tumor proliferation that was resistant to trastuzumab therapy [71, 73, 75, 90]. In addition, investigators have hypothesized that p95HER2 is constitutively active and can signal without heterodimerization [100, 101]. This active form of HER2 can be targeted by anti-HER2 TKI agents such as lapatinib, which targets the ATP binding pocket of the HER2 intracellular kinase domain [102]. Lapatinib has been shown to induce an antitumor response in p95HER2-expressing tumors from breast cancer cell lines in preclinical investigations [75, 103, 104]. In one clinical study of women with breast cancer treated with lapatinib, p95HER2 expression was not correlated with response, indicating lapatinib was effective in some women with this variant [104]. Dual HER2 blockade with both trastuzumab and lapatinib has been associated with heightened cell death and tumor regression in mice bearing HER2-positive xenografts known to be refractory to trastuzumab therapy [105–107].
Given these preclinical results, several clinical studies are designed to determine if p95HER2 expression is a consistently reliable predictive biomarker for trastuzumab resistance. Some early results have validated specific cutoff levels of expression that can be assayed in a clinical trial setting [97, 108]. In contrast to reports showing resistance to trastuzumab as a single agent, early reports from the GeparQuattro and NeoAlto trials have suggested that women who overexpress the p95HER2 variant actually have improved clinical outcomes with trastuzumab-containing neoadjuvant regimens that incorporate cytotoxic chemotherapy compared with those who have decreased expression [109]. This intriguing observation was further explored in preclinical breast carcinoma models, with the finding that in p95HER2-overexpressing tumors cytotoxic chemotherapy stabilized full-length HER2 expression and, in turn, re-established trastuzumab sensitivity [109]. Expression of the p95HER2 variant is an important emerging biomarker for HER2 overexpressing breast cancer, as it may explain the antitumor synergy of dual HER2 therapy with lapatinib and trastuzumab and aid the future triage of combination HER2-directed therapies with conventional cytotoxic chemotherapies [87, 102, 110–115].
In EnCa, limited data suggest that the p95HER2 variant is expressed. As anti-HER2 therapies are not widely used in EnCa, p95HER2 expression has not yet been clinically linked to prognosis or treatment response. A preclinical in vitro model of USC recently confirmed that ECD shedding occurs most frequently in those tumors with HER2 gene amplification [116], confirming indirectly that p95HER2 is likely to be expressed. A study by our group confirmed that 46 of 86 (53%) tested high-grade EnCa samples expressed p95HER2 at levels above the threshold shown to confer trastuzumab resistance [117]. Additionally, we demonstrated that when the p95HER2 and full-length HER2 expression profiles in high-grade EnCa were compared with IHC-matched breast tumors, the p95HER2 levels in endometrial tumors were significantly higher [117]. These data suggest the HER2 landscape is fundamentally different in EnCa, possibly predisposing these tumors to innate trastuzumab resistance. In our preclinical experiments using USC xenografts, both from cell lines and derived from primary human tumor, we observed that USC xenograft tumors, regardless of HER2 amplification, were resistant to trastuzumab. When trastuzumab was combined with lapatinib, however, we observed potent synergistic antitumor activity in the two HER2 overexpressing xenograft tumors, although this effect was not seen in the nonamplified tumors [60]. The success of targeting both the intracellular and extracellular domains of HER2 is consistent with the theory of increased p95HER2 in EnCa.
Activation of Downstream PI3K Pathway Signaling
Activation of the PI3K pathway, primarily through inactivation of PTEN or gain-of-function mutation in the PIK3CA gene, has been implicated in resistance to HER2 therapies [77, 78, 118]. Compelling breast cancer literature has identified both PIK3CA gene mutation and PTEN status as highly associated with resistance to anti-HER2 therapies. In one study, the presence of a PIK3CA mutation or a lower level of expression of PTEN was associated with lower rates of pathologic response to trastuzumab in the neoadjuvant setting in HER2-positive breast cancer (29% vs. 67% and 33% vs. 72%, respectively) [119]. The large prospective, randomized CLEOPATRA trial demonstrated a worse progression-free survival in the setting of a PI3KCA mutation in both the trastuzumab and docetaxel with or without pertuzumab arms [120, 121]. While most data suggest downstream activation of the PI3K pathway uncouples response to HER2 blockade, the data are not consistent. In the analysis of the N9831 randomized trial of paclitaxel and trastuzumab, PTEN-negative tumors were associated with a longer disease-free survival compared with tumors with intact PTEN expression when trastuzumab was administered with paclitaxel [122].
Based on preclinical and clinical experiences in breast cancer, most investigators agree that downstream alterations are likely to modulate response to HER2-targeted therapies. This is of particular relevance to EnCa, which is known to harbor the highest rates of oncogenic alteration in the PI3K pathway of any solid tumor, suggesting this is a potential innate mechanism of resistance to anti-HER2 therapy. Notably, an analysis of the reported TCGA data in high-grade EnCa and breast cancer revealed that 85% of HER2-amplified tumors coexist with PIK3CA gain-of-function mutations compared with 32% of breast tumors (Fig. 2) [15, 123]. Unlike the majority of EnCa, HER2-amplified EnCa appears to lack mutation or homozygous deletion of PTEN, suggesting PTEN status may not impact response to anti-HER2 therapies. Preclinical investigation using non-immortalized, high-grade EnCa cell lines with and without HER2 gene amplification described that the presence of a PIK3CA gene mutation was a major determinant of response to anti-HER2 therapies in vitro and was associated with modulating response to specific mammalian target of rapamycin (mTOR) and PI3K inhibitors as single agents [124]. Dual inhibition of HER2 and specific, downstream PI3K pathway targets, such as PI3K, AKT, or mTOR, offers a rational option to counteract this potent resistance mechanism in EnCa. This approach is supported by preclinical and clinical studies of anti-HER2 therapy for breast cancer, including in the recently reported BOLERO-3 trial, in which the addition of an mTOR inhibitor led to synergistic antitumor activity, even in tumors previously refractory to single-agent anti-HER2 therapy [88, 125–127]. Alternatively, antibody-drug conjugates such as T-DM1 may circumvent the need for dual targeting, as the HER2 pathway is not specifically targeted but used only as a mechanism to introduce a nonspecific cytotoxic. In this setting, downstream activating mutations and changes in other HER family members would not be relevant to drug response, thus avoiding these resistance mechanisms, as evidenced by the success of T-DM1 in women with trastuzumab-resistant tumors [40].
Figure 2.

Cancer Genome Atlas analysis of PIK3CA and PTEN alterations in HER2-amplified high-grade EnCa and invasive breast carcinoma. Comparison of HER2 gene-amplified EnCa to invasive breast cancer reveals a significant difference in simultaneous gain-of-function PIK3CA mutation (Fisher’s exact test, p < .003). While HER2-positive breast carcinoma has a 32% rate of PIK3CA mutation, HER2-positive EnCa presents with a 73% mutation rate, suggesting downstream activation of the phosphotidylinositol 3-kinase pathway to be of greater impact in driving HER2-overexpressing EnCa compared with breast carcinoma. Notably, both HER2-overexpressing EnCa and breast carcinoma lack mutations or homozygous deletions in PTEN, although loss of this tumor suppressor may play a role in non-HER2-positive tumors (http://www.cbioportal.org [33, 34]). (A): High-grade EnCa (n = 60; ERBB2 amplified in 25%). (B): Invasive breast carcinoma (n = 482; ERBB2 amplified in 13%).
Abbreviations: EnCa, endometrial cancer; ERBB2, erb-b2 receptor tyrosine kinase 2 (also abbreviated HER2); HER2, human epidermal growth factor receptor 2; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha; PTEN, phosphatase and tensin homolog.
Signaling Through Alternate HER Family Proteins
Inhibition of the PI3K pathway via HER2, mTOR, AKT, or direct PI3K inhibition has been demonstrated to increase HER3 expression, a factor that has been linked to trastuzumab resistance in numerous disease sites, including breast, ovarian, gastric, and esophageal tumors [84, 128–130]. Since HER2 and HER3 proteins are known to dimerize with the highest affinity, numerous investigators have characterized HER3 expression in tumors and described how anti-HER2 therapy leads to a compensatory increase in HER3 expression and dimerization [84, 131]. Preclinical investigation has demonstrated that the HER2/HER3 dimer is essential to sustaining oncogenesis and promotes sustained HER2 activation of the PI3K pathway by preventing HER2 dephosphorylation [83]. Importantly, the HER2/HER3 dimer is impervious to trastuzumab therapy, as it prevents access to the trastuzumab binding site [82]. The baseline expressions of HER3 and HER2/HER3 dimers in EnCa are incompletely characterized, but given the emerging data in breast and other disease sites, HER3 likely contributes to a dynamic HER2 landscape that shifts when various HER2 and PI3K pathway blockades are applied [107]. In addition, HER3 has been described as a central node of oncogenic signaling that can dimerize with insulin growth factor receptor and c-met, promoting neoplastic growth through a variety of mechanisms that could contribute to drug resistance [84, 132]. Understanding the specific balance of HER2 and HER3 in EnCa will be vital to incorporating the new generation of HER3-specific inhibitors to abrogate resistance to anti-HER2 therapy. Given its ability to block HER2-HER3 dimerization, combination therapy with pertuzumab may be a successful strategy for endometrial cancer, as has been seen in breast cancer [46, 48].
Like HER3, EGFR (HER1, ERBB1) expression decreases sensitivity to trastuzumab [133]. EGFR overexpression by IHC has been linked to poor progression-free survival in patients with primary and metastatic breast cancer treated with trastuzumab, although not all studies show this relationship [134–136]. Because of lapatinib’s dual EGFR and HER2 inhibition, in contrast to the effects on trastuzumab therapy, increased copy number of EGFR has been linked to lapatinib sensitivity in breast cancer [137]. HER4 has also been demonstrated in vitro to be upregulated with acquisition of trastuzumab resistance, which can be reversed with anti-HER4 therapies [138]. Clinically, however, the results challenge these in vitro findings. Two studies demonstrated increased response to trastuzumab and improved clinical outcomes in patients with tumors high in HER4, and another study showed no relationship of HER4 to clinical response [139–141]. In summary, the role of the HER-family receptors in trastuzumab response or resistance is not fully elucidated and needs further evaluation in the setting of the new anti-HER2 therapies available. It is uncertain which alterations in these HER family members are important in EnCa, and understanding how these alterations affect sensitivity to anti-HER2 therapies will require in vitro and in vivo modeling so that clinical trials can be designed to circumvent these potential resistance mechanisms.
Signaling Through Alternate Pathways
Mounting evidence in the breast cancer literature implicates Notch pathway activation in HER2 blockade resistance [142]. The Notch signaling pathway plays an important role in multiple developmental and cellular processes, including regulation of cell proliferation, differentiation, apoptosis, and stem cell self-renewal [143]. Dysregulation of the Notch pathway has been demonstrated in a variety of malignancies, with Notch1 and Notch3 most commonly implicated in malignant transformation [144]. Both oncogenic and tumor-suppressive effects of the Notch pathway have been observed [145]. Given the role of Notch in human cancers, a variety of Notch pathway inhibitors have been developed [146], the most common of which are the γ-secretase inhibitors (GSIs) that block the cleavage of all of a subset of four Notch paralogs [147]. Preclinical investigations using breast carcinoma models highlighted that HER2-overexpressing cells manifested low Notch pathway activation, as evidenced by decreased Notch1 and target gene Hes and Hey expression compared with cell lines without HER2 overexpression [86]. When treated with trastuzumab, investigators noted an induction of Notch pathway signaling that conferred sensitivity to γ-secretase inhibition. Conversely, when trastuzumab-resistant breast tumor cells were treated with γ-secretase inhibitors, a significant elevation in HER2 expression was manifest that restored sensitivity to trastuzumab in vitro [86, 148, 149].
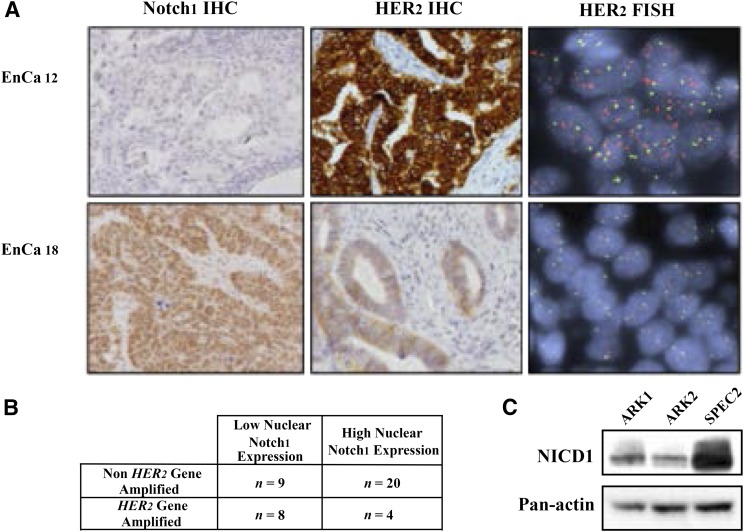
Notch expression has been explored in high-grade EnCa with and without HER2 gene amplification. In an in vivo study from our group, we demonstrated that GSI can induce single-agent antitumor effects via Notch inhibition as well as potentiate the activity of conventional cytotoxic chemotherapy (carboplatin and paclitaxel) in USC models that manifested elevated Notch1 protein expression and lacked HER2 overexpression [150]. It is not known whether this paradoxical cross-talk between HER2 and Notch exists in EnCa as it appears to do in breast carcinoma, but our unpublished data confirm the inverse relationship of HER2 and Notch expression at baseline (Fig. 3). The potential interplay between the Notch and HER2 pathways suggests investigators could use inhibition of one pathway, such as Notch, to induce tumor sensitivity to other classes of drugs, such as trastuzumab, where previously tumors were resistant. Given the dynamic nature of HER2 signaling that interacts with many other pathways, modulation of HER2 expression may be a promising avenue of investigation in HER2-negative tumors to induce responses to the emerging classes of anti-HER family agents.
Figure 3.
Relationship of activated Notch pathway and HER2 gene amplification in 41 high-grade EnCa samples and cell lines. (A): Representative panels of nuclear Notch1 protein expression, membranous HER2 protein expression, and nuclear HER2 gene amplification status. (B): In the retrospective cohort, nuclear Notch1 expression is inversely correlated with HER2 gene amplification (two-sided Fisher’s exact test, p = .045). (C): Elevated cleaved NICD1 noted in SPEC2, a cell line lacking HER2 gene amplification, compared with ARK1 and ARK2, both with HER2 overexpression. These data are consistent with the experience in breast cancer, suggesting markers of Notch activation assume an inverse relationship with HER2 expression. Adapted with permission from Groeneweg et al. [150].
Abbreviations: EnCa, endometrial cancer; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; NICD1, NOTCH1 intracellular domain.
The potential interplay between the Notch and HER2 pathways suggests investigators could use inhibition of one pathway, such as Notch, to induce tumor sensitivity to other classes of drugs, such as trastuzumab, where previously tumors were resistant.
Conclusion
Heightened activation of HER2 signaling is a hallmark of a significant proportion of high-grade endometrial tumors that are at the highest risk for progression, recurrence, and decreased patient survival. While therapeutic targeting of this pathway has become a platform for women with HER2-overexpressing breast cancer, clinical trials testing single-agent use of anti-HER2 therapies in EnCa have shown minimal clinical benefit due to innate and/or acquired resistance mechanisms that are poorly understood. Table 1 summarizes the two ongoing trials in EnCa that are using HER2 overexpression as a biomarker to select women for anti-HER2 therapies, both in concert with chemotherapy. One is a randomized phase II trial of trastuzumab in combination with paclitaxel and carboplatin for upfront therapy of USC (NCT01367002). The other is a phase I trial testing lapatinib with ixabepilone for recurrent uterine carcinoma and carcinosarcoma. Given the distinct range of responses to anti-HER2 therapies observed in the clinic among disease sites, disease-specific drivers of resistance need to be elucidated. Unlike some disease sites that exhibit oncogenic addiction to one signaling cascade, EnCa is a tumor marked by numerous gene and protein alterations that coexist and can circumvent targeted therapies. By understanding the factors associated with HER2-blockade sensitivity and resistance, we can optimize the use of HER2 blockade in the next generation of EnCa therapy and meaningfully alter patient outcomes.
Table 1.
Two ongoing trials in endometrial cancer that are using HER2 overexpression as a biomarker to select women for anti-HER2 therapies in concert with chemotherapy

Footnotes
For Further Reading: Nawar A. Latif, Ashley Haggerty, Stephanie Jean et al. Adjuvant Therapy in Early-Stage Endometrial Cancer: A Systematic Review of the Evidence, Guidelines, and Clinical Practice in the U.S. The Oncologist 2014;19:645–653.
Implications for Practice: Several phase III randomized controlled trials have shown that adjuvant radiation for high-intermediate risk early-stage endometrial cancer confers a benefit by reducing local regional recurrence with minimal added toxicity, but at increased cost and without significant overall survival benefit. This article reviews key trials regarding adjuvant radiation in early stage endometrial cancer and summarizes current clinical practice patterns and available guidelines. These facets must be considered and reviewed with each patient individually to determine the relative benefit to each patient.
Author Contributions
Conception/Design: Elisabeth J. Diver, Rosemary Foster, Bo R. Rueda, Whitfield B. Growdon
Provision of study material or patients: Elisabeth J. Diver
Collection and/or assembly of data: Elisabeth J. Diver, Rosemary Foster, Whitfield B. Growdon
Data analysis and interpretation: Elisabeth J. Diver, Whitfield B. Growdon
Manuscript writing: Elisabeth J. Diver, Rosemary Foster, Bo R. Rueda, Whitfield B. Growdon
Final approval of manuscript: Elisabeth J. Diver, Rosemary Foster, Bo R. Rueda, Whitfield B. Growdon
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94:642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Creutzberg CL, van Putten WL, Wárlám-Rodenhuis CC, et al. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: The Postoperative Radiation Therapy in Endometrial Carcinoma Trial. J Clin Oncol. 2004;22:1234–1241. doi: 10.1200/JCO.2004.08.159. [DOI] [PubMed] [Google Scholar]
- 6.Boruta DM, 2nd, Gehrig PA, Fader AN, et al. Management of women with uterine papillary serous cancer: A Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115:142–153. doi: 10.1016/j.ygyno.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 7.McCluggage WG. Malignant biphasic uterine tumours: Carcinosarcomas or metaplastic carcinomas? J Clin Pathol. 2002;55:321–325. doi: 10.1136/jcp.55.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauh-Hain JA, Winograd D, Growdon WB, et al. Prognostic determinants in patients with uterine and ovarian clear carcinoma. Gynecol Oncol. 2012;125:376–380. doi: 10.1016/j.ygyno.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8) suppl:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Rauh-Hain JA, Growdon WB, Schorge JO, et al. Prognostic determinants in patients with stage IIIC and IV uterine papillary serous carcinoma. Gynecol Oncol. 2010;119:299–304. doi: 10.1016/j.ygyno.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Homesley HD, Filiaci V, Markman M, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:526–531. doi: 10.1200/JCO.2006.06.4907. [DOI] [PubMed] [Google Scholar]
- 12.Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159–2166. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 13.Creutzberg CL. Adjuvant chemotherapy for endometrial cancer: Unproven. Int J Gynecol Cancer. 2010;20:1105–1108. doi: 10.1111/igc.0b013e3181f35473. [DOI] [PubMed] [Google Scholar]
- 14.Del Carmen MG, Boruta DM, 2nd, Schorge JO. Recurrent endometrial cancer. Clin Obstet Gynecol. 2011;54:266–277. doi: 10.1097/GRF.0b013e318218c6d1. [DOI] [PubMed] [Google Scholar]
- 15.Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dedes KJ, Wetterskog D, Ashworth A, et al. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011;8:261–271. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 17.Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;24:4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 18.Growdon WB, Roussel BN, Scialabba VL, et al. Tissue-specific signatures of activating PIK3CA and RAS mutations in carcinosarcomas of gynecologic origin. Gynecol Oncol. 2011;121:212–217. doi: 10.1016/j.ygyno.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Hayes MP, Wang H, Espinal-Witter R, et al. PIK3CA and PTEN mutations in uterine endometrioid carcinoma and complex atypical hyperplasia. Clin Cancer Res. 2006;12:5932–5935. doi: 10.1158/1078-0432.CCR-06-1375. [DOI] [PubMed] [Google Scholar]
- 20.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 21.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradford LS, Rauh-Hain A, Clark RM, et al. Assessing the efficacy of targeting the phosphatidylinositol 3-kinase/AKT/mTOR signaling pathway in endometrial cancer. Gynecol Oncol. 2014;133:346–352. doi: 10.1016/j.ygyno.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Slomovitz BM, Broaddus RR, Burke TW, et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J Clin Oncol. 2004;22:3126–3132. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- 24.Santin AD, Bellone S, Van Stedum S, et al. Amplification of c-erbB2 oncogene: A major prognostic indicator in uterine serous papillary carcinoma. Cancer. 2005;104:1391–1397. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- 25.Dedes KJ, Wetterskog D, Ashworth A, et al. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011;8:261–271. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 26.Salvesen HB, Carter SL, Mannelqvist M, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci USA. 2009;106:4834–4839. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lax SF, Kendall B, Tashiro H, et al. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: Evidence of distinct molecular genetic pathways. Cancer. 2000;88:814–824. [PubMed] [Google Scholar]
- 28.Slomovitz BM, Lu KH. Commenting on “HER2/neu overexpression: Has the Achilles’ heel of uterine serous papillary carcinoma been exposed? (88:263-5) by Santin AD”. Gynecol Oncol. 2004;92:386–387; author reply 387. doi: 10.1016/j.ygyno.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Khasraw M, Bell R. Primary systemic therapy in HER2-amplified breast cancer: A clinical review. Expert Rev Anticancer Ther. 2012;12:1005–1013. doi: 10.1586/era.12.62. [DOI] [PubMed] [Google Scholar]
- 30.Chan DS, Twine CP, Lewis WG. Systematic review and meta-analysis of the influence of HER2 expression and amplification in operable oesophageal cancer. J Gastrointest Surg . 2012;16:1821–1829. doi: 10.1007/s11605-012-1979-2. [DOI] [PubMed] [Google Scholar]
- 31.Hechtman JF, Polydorides AD. HER2/neu gene amplification and protein overexpression in gastric and gastroesophageal junction adenocarcinoma: A review of histopathology, diagnostic testing, and clinical implications. Arch Pathol Lab Med. 2012;136:691–697. doi: 10.5858/arpa.2011-0168-RS. [DOI] [PubMed] [Google Scholar]
- 32.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–5847. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 33.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 36.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res. 2009;15:7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muthuswamy SK, Li D, Lelievre S, et al. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 39.Baselga J. Treatment of HER2-overexpressing breast cancer. Ann Oncol. 2010;21(suppl 7):vii36–vii40. doi: 10.1093/annonc/mdq421. [DOI] [PubMed] [Google Scholar]
- 40.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 41.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 42.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 44.Burris HA, 3rd, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 45.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 46.Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 48.Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 49.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: A dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 50.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 51.Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343:342–350. doi: 10.1124/jpet.112.197756. [DOI] [PubMed] [Google Scholar]
- 52.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 53.Yan M, Parker BA, Schwab R, et al. HER2 aberrations in cancer: Implications for therapy. Cancer Treat Rev. 2014;40:770–780. doi: 10.1016/j.ctrv.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 56.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 57.Hicks DG, Whitney-Miller CL. The evolving role of HER2 evaluation for diagnosis and clinical decision making for breast and gastric adenocarcinoma. Biotechnic Histochem . 2013;88:121–131. doi: 10.3109/10520295.2012.751619. [DOI] [PubMed] [Google Scholar]
- 58.Hu Y, Bandla S, Godfrey TE, et al. HER2 amplification, overexpression and score criteria in esophageal adenocarcinoma. Mod Pathol. 2011;24:899–907. doi: 10.1038/modpathol.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groeneweg JW, Hernandez SF, Byron VF, et al. Dual HER2 targeting impedes growth of HER2 gene-amplified uterine serous carcinoma xenografts. Clin Cancer Res. 2014;20:6517–6528. doi: 10.1158/1078-0432.CCR-14-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santin AD. Letter to the Editor referring to the manuscript entitled: “Phase II trial of trastuzumab in women with advanced or recurrent HER-positive endometrial carcinoma: A Gynecologic Oncology Group study” recently reported by Fleming et al., (Gynecol Oncol., 116;15-20;2010) Gynecol Oncol. 2010;118:95–96; author reply 96–97. doi: 10.1016/j.ygyno.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 61.Santin AD, Bellone S, Gokden M, et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clin Cancer Res. 2002;8:1271–1279. [PubMed] [Google Scholar]
- 62.Konecny GE, Santos L, Winterhoff B, et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br J Cancer. 2009;100:89–95. doi: 10.1038/sj.bjc.6604814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.English DP, Roque DM, Carrara L, et al. HER2/neu gene amplification determines the sensitivity of uterine serous carcinoma cell lines to AZD8055, a novel dual mTORC1/2 inhibitor. Gynecol Oncol. 2013;131:753–758. doi: 10.1016/j.ygyno.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 64.Grushko TA, Filiaci VL, Mundt AJ, et al. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:3–9. doi: 10.1016/j.ygyno.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrison C, Zanagnolo V, Ramirez N, et al. HER-2 is an independent prognostic factor in endometrial cancer: Association with outcome in a large cohort of surgically staged patients. J Clin Oncol. 2006;24:2376–2385. doi: 10.1200/JCO.2005.03.4827. [DOI] [PubMed] [Google Scholar]
- 66.Galsky MD, Von Hoff DD, Neubauer M, et al. Target-specific, histology-independent, randomized discontinuation study of lapatinib in patients with HER2-amplified solid tumors. Invest New Drugs. 2012;30:695–701. doi: 10.1007/s10637-010-9541-0. [DOI] [PubMed] [Google Scholar]
- 67.Leslie KK, Sill MW, Lankes HA, et al. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol Oncol. 2012;127:345–350. doi: 10.1016/j.ygyno.2012.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fleming GF, Sill MW, Darcy KM, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121:3797–3803. doi: 10.1172/JCI57152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pazo Cid RA, Antón A. Advanced HER2-positive gastric cancer: Current and future targeted therapies. Crit Rev Oncol Hematol. 2013;85:350–362. doi: 10.1016/j.critrevonc.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 71.Anido J, Scaltriti M, Bech Serra JJ, et al. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J. 2006;25:3234–3244. doi: 10.1038/sj.emboj.7601191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilken JA, Maihle NJ. Primary trastuzumab resistance: New tricks for an old drug. Ann N Y Acad Sci. 2010;1210:53–65. doi: 10.1111/j.1749-6632.2010.05782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sperinde J, Jin X, Banerjee J, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res. 2010;16:4226–4235. doi: 10.1158/1078-0432.CCR-10-0410. [DOI] [PubMed] [Google Scholar]
- 74.Mohd Sharial MS, Crown J, Hennessy BT. Overcoming resistance and restoring sensitivity to HER2-targeted therapies in breast cancer. Ann Oncol. 2012;23:3007–3016. doi: 10.1093/annonc/mds200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scaltriti M, Rojo F, Ocaña A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 76.Kataoka Y, Mukohara T, Shimada H, et al. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010;21:255–262. doi: 10.1093/annonc/mdp304. [DOI] [PubMed] [Google Scholar]
- 77.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 78.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 79.Chan CT, Metz MZ, Kane SE. Differential sensitivities of trastuzumab (Herceptin)-resistant human breast cancer cells to phosphoinositide-3 kinase (PI-3K) and epidermal growth factor receptor (EGFR) kinase inhibitors. Breast Cancer Res Treat. 2005;91:187–201. doi: 10.1007/s10549-004-7715-1. [DOI] [PubMed] [Google Scholar]
- 80.Serra V, Scaltriti M, Prudkin L, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–2557. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Narayan M, Wilken JA, Harris LN, et al. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res. 2009;69:2191–2194. doi: 10.1158/0008-5472.CAN-08-1056. [DOI] [PubMed] [Google Scholar]
- 82.Wehrman TS, Raab WJ, Casipit CL, et al. A system for quantifying dynamic protein interactions defines a role for Herceptin in modulating ErbB2 interactions. Proc Natl Acad Sci USA. 2006;103:19063–19068. doi: 10.1073/pnas.0605218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sergina NV, Rausch M, Wang D, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garrett JT, Olivares MG, Rinehart C, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci USA. 2011;108:5021–5026. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pandya K, Meeke K, Clementz AG, et al. Targeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrence. Br J Cancer. 2011;105:796–806. doi: 10.1038/bjc.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Osipo C, Patel P, Rizzo P, et al. ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a gamma-secretase inhibitor. Oncogene. 2008;27:5019–5032. doi: 10.1038/onc.2008.149. [DOI] [PubMed] [Google Scholar]
- 87.Kümler I, Tuxen MK, Nielsen DL. A systematic review of dual targeting in HER2-positive breast cancer. Cancer Treat Rev. 2014;40:259–270. doi: 10.1016/j.ctrv.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 88.André F, O’Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580–591. doi: 10.1016/S1470-2045(14)70138-X. [DOI] [PubMed] [Google Scholar]
- 89.Molina MA, Sáez R, Ramsey EE, et al. NH(2)-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin Cancer Res. 2002;8:347–353. [PubMed] [Google Scholar]
- 90.Arribas J, Baselga J, Pedersen K, et al. p95HER2 and breast cancer. Cancer Res. 2011;71:1515–1519. doi: 10.1158/0008-5472.CAN-10-3795. [DOI] [PubMed] [Google Scholar]
- 91.Pupa SM, Ménard S, Morelli D, et al. The extracellular domain of the c-erbB-2 oncoprotein is released from tumor cells by proteolytic cleavage. Oncogene. 1993;8:2917–2923. [PubMed] [Google Scholar]
- 92.Scott GK, Robles R, Park JW, et al. A truncated intracellular HER2/neu receptor produced by alternative RNA processing affects growth of human carcinoma cells. Mol Cell Biol. 1993;13:2247–2257. doi: 10.1128/mcb.13.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Christianson TA, Doherty JK, Lin YJ, et al. NH2-terminally truncated HER-2/neu protein: Relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998;58:5123–5129. [PubMed] [Google Scholar]
- 94.Kandl H, Seymour L, Bezwoda WR. Soluble c-erbB-2 fragment in serum correlates with disease stage and predicts for shortened survival in patients with early-stage and advanced breast cancer. Br J Cancer. 1994;70:739–742. doi: 10.1038/bjc.1994.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fehm T, Maimonis P, Katalinic A, et al. The prognostic significance of c-erbB-2 serum protein in metastatic breast cancer. Oncology. 1998;55:33–38. doi: 10.1159/000011832. [DOI] [PubMed] [Google Scholar]
- 96.Mansour OA, Zekri AR, Harvey J, et al. Tissue and serum c-erbB-2 and tissue EGFR in breast carcinoma: Three years follow-up. Anticancer Res. 1997;17(4B):3101–3106. [PubMed] [Google Scholar]
- 97.Duchnowska R, Sperinde J, Chenna A, et al. Quantitative measurements of tumoral p95HER2 protein expression in metastatic breast cancer patients treated with trastuzumab: Independent validation of the p95HER2 clinical cutoff. Clin Cancer Res. 2014;20:2805–2813. doi: 10.1158/1078-0432.CCR-13-2782. [DOI] [PubMed] [Google Scholar]
- 98.Scaltriti M, Nuciforo P, Bradbury I, et al. High HER2 expression correlates with response to the combination of lapatinib and trastuzumab. Clin Cancer Res. 2015;21:569–576. doi: 10.1158/1078-0432.CCR-14-1824. [DOI] [PubMed] [Google Scholar]
- 99.Montemurro F, Prat A, Rossi V, et al. Potential biomarkers of long-term benefit from single-agent trastuzumab or lapatinib in HER2-positive metastatic breast cancer. Mol Oncol. 2014;8:20–26. doi: 10.1016/j.molonc.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Segatto O, King CR, Pierce JH, et al. Different structural alterations upregulate in vitro tyrosine kinase activity and transforming potency of the erbB-2 gene. Mol Cell Biol. 1988;8:5570–5574. doi: 10.1128/mcb.8.12.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Angelini PD, Zacarias Fluck MF, Pedersen K, et al. Constitutive HER2 signaling promotes breast cancer metastasis through cellular senescence. Cancer Res. 2013;73:450–458. doi: 10.1158/0008-5472.CAN-12-2301. [DOI] [PubMed] [Google Scholar]
- 102.Burris HA, 3rd, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 103.Scaltriti M, Chandarlapaty S, Prudkin L, et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res. 2010;16:2688–2695. doi: 10.1158/1078-0432.CCR-09-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han SW, Cha Y, Paquet A, et al. Correlation of HER2, p95HER2 and HER3 expression and treatment outcome of lapatinib plus capecitabine in HER2-positive metastatic breast cancer. PLoS ONE. 2012;7:e39943. doi: 10.1371/journal.pone.0039943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rimawi MF, Wiechmann LS, Wang YC, et al. Reduced dose and intermittent treatment with lapatinib and trastuzumab for potent blockade of the HER pathway in HER2/neu-overexpressing breast tumor xenografts. Clin Cancer Res. 2011;17:1351–1361. doi: 10.1158/1078-0432.CCR-10-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scaltriti M, Verma C, Guzman M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 107.Garrett JT, Sutton CR, Kuba MG, et al. Dual blockade of HER2 in HER2-overexpressing tumor cells does not completely eliminate HER3 function. Clin Cancer Res. 2013;19:610–619. doi: 10.1158/1078-0432.CCR-12-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De P, Hasmann M, Leyland-Jones B. Molecular determinants of trastuzumab efficacy: What is their clinical relevance? Cancer Treat Rev. 2013;39:925–934. doi: 10.1016/j.ctrv.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 109.Parra-Palau JL, Morancho B, Peg V, et al. Effect of p95HER2/611CTF on the response to trastuzumab and chemotherapy. J Natl Cancer Inst. 2014;106:pii: dju291. doi: 10.1093/jnci/dju291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: Results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30:1989–1995. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- 111.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 112.Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: Final results from the EGF104900 Study. J Clin Oncol. 2012;30:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 113.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rimawi MF, Mayer IA, Forero A, et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J Clin Oncol. 2013;31:1726–1731. doi: 10.1200/JCO.2012.44.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Prat A, Baselga J. Dual human epidermal growth factor receptor 2 (HER2) blockade and hormonal therapy for the treatment of primary HER2-positive breast cancer: One more step toward chemotherapy-free therapy. J Clin Oncol. 2013;31:1703–1706. doi: 10.1200/JCO.2012.48.4998. [DOI] [PubMed] [Google Scholar]
- 116.Todeschini P, Cocco E, Bellone S, et al. Her2/neu extracellular domain shedding in uterine serous carcinoma: Implications for immunotherapy with trastuzumab. Br J Cancer. 2011;105:1176–1182. doi: 10.1038/bjc.2011.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Growdon WB, Groeneweg J, Byron V, et al. HER2 over-expressing high grade endometrial cancer expresses high levels of p95HER2 variant. Gynecol Oncol. 2015;137:160–166. doi: 10.1016/j.ygyno.2015.01.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hutchinson L. Targeted therapies: Activated PI3K/AKT confers resistance to trastuzumab but not lapatinib. Nat Rev Clin Oncol. 2010;7:424. doi: 10.1038/nrclinonc.2010.113. [DOI] [PubMed] [Google Scholar]
- 119.Sueta A, Yamamoto Y, Yamamoto-Ibusuki M, et al. An integrative analysis of PIK3CA mutation, PTEN, and INPP4B expression in terms of trastuzumab efficacy in HER2-positive breast cancer. PLoS ONE. 2014;9:e116054. doi: 10.1371/journal.pone.0116054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baselga J, Swain SM. CLEOPATRA: A phase III evaluation of pertuzumab and trastuzumab for HER2-positive metastatic breast cancer. Clin Breast Cancer. 2010;10:489–491. doi: 10.3816/CBC.2010.n.065. [DOI] [PubMed] [Google Scholar]
- 121.Baselga J, Cortés J, Im SA, et al. Biomarker analyses in CLEOPATRA: A phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32:3753–3761. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 122.Perez EA, Dueck AC, McCullough AE, et al. Impact of PTEN protein expression on benefit from adjuvant trastuzumab in early-stage human epidermal growth factor receptor 2-positive breast cancer in the North Central Cancer Treatment Group N9831 trial. J Clin Oncol. 2013;31:2115–2122. doi: 10.1200/JCO.2012.42.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.English DP, Bellone S, Cocco E, et al. Oncogenic PIK3CA gene mutations and HER2/neu gene amplifications determine the sensitivity of uterine serous carcinoma cell lines to GDC-0980, a selective inhibitor of Class I PI3 kinase and mTOR kinase (TORC1/2) Am J Obstet Gynecol. 2013;209:465.e1–e9. doi: 10.1016/j.ajog.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 125.García-García C, Ibrahim YH, Serra V, et al. Dual mTORC1/2 and HER2 blockade results in antitumor activity in preclinical models of breast cancer resistant to anti-HER2 therapy. Clin Cancer Res. 2012;18:2603–2612. doi: 10.1158/1078-0432.CCR-11-2750. [DOI] [PubMed] [Google Scholar]
- 126.Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jerusalem G, Fasolo A, Dieras V, et al. Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2011;125:447–455. doi: 10.1007/s10549-010-1260-x. [DOI] [PubMed] [Google Scholar]
- 128.Berghoff AS, Bartsch R, Preusser M, et al. Co-overexpression of HER2/HER3 is a predictor of impaired survival in breast cancer patients. Breast. 2014;23:637–643. doi: 10.1016/j.breast.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 129.Gala K, Chandarlapaty S. Molecular pathways: HER3 targeted therapy. Clin Cancer Res. 2014;20:1410–1416. doi: 10.1158/1078-0432.CCR-13-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Green AR, Barros FF, Abdel-Fatah TM, et al. HER2/HER3 heterodimers and p21 expression are capable of predicting adjuvant trastuzumab response in HER2+ breast cancer. Breast Cancer Res Treat. 2014;145:33–44. doi: 10.1007/s10549-014-2925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tzahar E, Waterman H, Chen X, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mujoo K, Choi BK, Huang Z, et al. Regulation of ERBB3/HER3 signaling in cancer. Oncotarget. 2014;5:10222–10236. doi: 10.18632/oncotarget.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Diermeier S, Horváth G, Knuechel-Clarke R, et al. Epidermal growth factor receptor coexpression modulates susceptibility to Herceptin in HER2/neu overexpressing breast cancer cells via specific erbB-receptor interaction and activation. Exp Cell Res. 2005;304:604–619. doi: 10.1016/j.yexcr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 134.Gallardo A, Lerma E, Escuin D, et al. Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer. 2012;106:1367–1373. doi: 10.1038/bjc.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee HJ, Seo AN, Kim EJ, et al. Prognostic and predictive values of EGFR overexpression and EGFR copy number alteration in HER2-positive breast cancer. Br J Cancer. 2015;112:103–111. doi: 10.1038/bjc.2014.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smith BL, Chin D, Maltzman W, et al. The efficacy of Herceptin therapies is influenced by the expression of other erbB receptors, their ligands and the activation of downstream signalling proteins. Br J Cancer. 2004;91:1190–1194. doi: 10.1038/sj.bjc.6602090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fabi A, Merola R, Ferretti G, et al. Epidermal growth factor receptor gene copy number may predict lapatinib sensitivity in HER2-positive metastatic breast cancer. Expert Opin Pharmacother. 2013;14:699–706. doi: 10.1517/14656566.2013.779672. [DOI] [PubMed] [Google Scholar]
- 138.Mohd Nafi SN, Generali D, Kramer-Marek G, et al. Nuclear HER4 mediates acquired resistance to trastuzumab and is associated with poor outcome in HER2 positive breast cancer. Oncotarget. 2014;5:5934–5949. doi: 10.18632/oncotarget.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sassen A, Diermeier-Daucher S, Sieben M, et al. Presence of HER4 associates with increased sensitivity to Herceptin in patients with metastatic breast cancer. Breast Cancer Res. 2009;11:R50. doi: 10.1186/bcr2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Portier BP, Minca EC, Wang Z, et al. HER4 expression status correlates with improved outcome in both neoadjuvant and adjuvant Trastuzumab treated invasive breast carcinoma. Oncotarget. 2013;4:1662–1672. doi: 10.18632/oncotarget.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yonemori K, Tsuta K, Shimizu C, et al. Immunohistochemical expression of HER1, HER3, and HER4 in HER2-positive breast cancer patients treated with trastuzumab-containing neoadjuvant chemotherapy. J Surg Oncol. 2010;101:222–227. doi: 10.1002/jso.21486. [DOI] [PubMed] [Google Scholar]
- 142.Yamaguchi H, Chang SS, Hsu JL, et al. Signaling cross-talk in the resistance to HER family receptor targeted therapy. Oncogene. 2014;33:1073–1081. doi: 10.1038/onc.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 144.McAuliffe SM, Morgan SL, Wyant GA, et al. Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc Natl Acad Sci USA. 2012;109:E2939–E2948. doi: 10.1073/pnas.1206400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Avila JL, Kissil JL. Notch signaling in pancreatic cancer: Oncogene or tumor suppressor? Trends Mol Med. 2013;19:320–327. doi: 10.1016/j.molmed.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: Clinical development advances challenges. Pharmacol Ther. 2014;141:140–149. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rizzo P, Osipo C, Foreman K, et al. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 148.Ju JH, Yang W, Oh S, et al. HER2 stabilizes survivin while concomitantly down-regulating survivin gene transcription by suppressing Notch cleavage. Biochem J. 2013;451:123–134. doi: 10.1042/BJ20121716. [DOI] [PubMed] [Google Scholar]
- 149.Han M, Deng HY, Jiang R. Effect of trastuzumab on notch-1 signaling pathway in breast cancer sk-br3 cells. Chin J Cancer Res. 2012;24:213–219. doi: 10.1007/s11670-012-0213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Groeneweg JW, Hall TR, Zhang L, et al. Inhibition of gamma-secretase activity impedes uterine serous carcinoma growth in a human xenograft model. Gynecol Oncol. 2014;133:607–615. doi: 10.1016/j.ygyno.2014.03.560. [DOI] [PubMed] [Google Scholar]