A retrospective study of 40 patients with Waldenström macroglobulinemia treated with the mammalian target of rapamycin inhibitor everolimus was performed to investigate the imaging characteristics and radiographic patterns of pneumonitis. Drug-related pneumonitis was noted in 23 patients (58%). The most common findings were bilateral ground glass and reticular opacities, demonstrating cryptogenic organizing pneumonia and nonspecific interstitial pneumonia patterns.
Keywords: Mammalian target of rapamycin inhibitor, mTOR, Pneumonitis, Drug toxicity, Computed tomography, Waldenström macroglobulinemia
Abstract
Background.
This study determined the frequency of drug-related pneumonitis during mammalian target of rapamycin (mTOR) inhibitor therapy in Waldenström macroglobulinemia patients and investigated the imaging characteristics and radiographic patterns of pneumonitis.
Materials and Methods.
A total of 40 patients (23 men, 17 women; 43–84 years old) with Waldenström macroglobulinemia treated in 2 trials of the mTOR inhibitor everolimus were retrospectively studied. Chest computed tomography (CT) scans during therapy were reviewed for abnormalities suspicious for drug-related pneumonitis by the consensus of three radiologists, evaluating the extent, distributions, and specific findings. The radiographic patterns of pneumonitis were classified using the American Thoracic Society/European Respiratory Society classification of interstitial pneumonia.
Results.
Drug-related pneumonitis was noted in 23 patients (58%). The median time from the initiation of therapy to the onset of pneumonitis was 5.7 months. Lower lungs were involved in all 23 patients, with a higher extent than in the other zones (p < .001). The distribution was peripheral and lower in 11 patients (48%) and mixed and multifocal in 10 (44%). The findings were bilateral in 20 patients (87%). Ground glass opacities (GGOs) and reticular opacities were present in all 23 patients, with consolidation in 12, traction bronchiectasis in 2, and centrilobular nodularity in 1. The pattern of pneumonitis was classified as cryptogenic organizing pneumonia (COP) in 16 (70%) and nonspecific interstitial pneumonia (NSIP) in 7 (30%), with overlapping features of COP and NSIP in 7 patients.
Conclusion.
Drug-related pneumonitis was noted on CT in 58% of Waldenström macroglobulinemia patients treated with mTOR inhibitor therapy. Most common findings were bilateral GGOs and reticular opacities, with or without consolidation, in peripheral and lower lungs, demonstrating COP and NSIP patterns.
Implications for Practice:
The present study has demonstrated that drug-related pneumonitis during mammalian target of rapamycin (mTOR) inhibitor therapy is highly frequent, occurring in 58% of patients with Waldenström macroglobulinemia. The radiographic patterns of pneumonitis demonstrated cryptogenic organizing pneumonia and nonspecific interstitial pneumonia patterns, with overlapping features in 30% of the patients. The present study describes an initial attempt of a radiographic pattern-based approach to drug-related pneumonitis in the era of molecular targeting therapy, with a cohort of patients with Waldenström macroglobulinemia receiving mTOR inhibitor therapy as a paradigm, which might contribute to further understanding and in-depth interpretation of lung toxicity during novel cancer therapy.
Introduction
Drug-related pneumonitis represents one of the major categories of drug toxicity in cancer patients receiving systemic therapy and can be caused by direct cytotoxic, oxidative, or immune-mediated injuries [1]. Although a spectrum of radiographic manifestations are noted in drug-related pneumonitis, the lung’s response to injury is limited and commonly demonstrates several types of histopathologic manifestations with corresponding radiographic patterns [1]. These can be described according to the classification of interstitial pneumonias and related lung diseases. Recent advances in the genome-based approach to cancer have resulted in a dramatic increase in new molecular targeting agents that are approved or being tested in the clinical setting. Thus, drug-related pneumonitis as a manifestation of lung toxicity is frequently noted among cancer patients treated with different types of novel therapeutic agents. Because imaging plays a major role in detecting and monitoring drug-related pneumonitis during therapy, a systematic approach based on the radiographic patterns of pneumonitis is needed to fully characterize and accurately document pneumonitis due to lung toxicity from various therapeutic agents.
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase and is involved in the critical junctures of the PI3K/Akt/mTOR pathway, an established oncogenic driver in humans [2]. Everolimus (also known as RAD001) is a rapamycin analog and exerts its antitumor activity by selectively inhibiting mTOR [2]. Everolimus has been approved for treatment of renal cell carcinomas (RCCs), subependymal giant cell astrocytoma in tuberous sclerosis, advanced pancreatic neuroendocrine tumors, and advanced hormone receptor-positive, human epidermal growth receptor (HER)2-negative breast cancer [2–4]. Everolimus is under investigation as a therapeutic agent for other solid and hematologic malignancies, including Waldenström macroglobulinemia, which is a B-cell lymphoproliferative disorder characterized by bone marrow infiltration of lymphoplasmacytic cells and is associated with IgM monoclonal gammopathy [2, 5, 6].
Drug-related pneumonitis is a well-recognized class-effect toxicity of mTOR inhibitors [7]. Several previous reports have studied drug-related pneumonitis during mTOR inhibitor therapy in patients with advanced RCC [8–11]. In a previous study of 178 patients with advanced RCC treated with the mTOR inhibitor, temsirolimus, by Maroto et al., 52 patients (29%) developed radiographically identified drug-related pneumonitis. Of the 52 patients with pneumonitis, 16 (31%) had respiratory symptoms, highlighting the high incidence of radiographically detected pneumonitis during mTOR inhibitor therapy that might not be recognized clinically by treating physicians [9]. Another study of 46 metastatic RCC patients treated with mTOR inhibitors, either temsirolimus or everolimus, noted the computed tomography (CT) evidence of pneumonitis in 14 patients (30%). Those 14 patients more frequently achieved stable disease as a tumor response outcome than did those without pneumonitis, indicating the possibility of pneumonitis as a marker for therapeutic benefit [10]. Soria et al. studied 64 patients with advanced non-small-cell lung cancer (NSCLC) treated with everolimus, and reported that 24 patients (25%) had newly occurring or worsening radiographic changes suggestive of drug-related pneumonitis [12]. Although these studies have described the CT findings of pneumonitis to some extent and reported the presence of ground glass opacities (GGOs) and consolidations, a detailed radiographic characterization of the distribution of the findings and classification of patterns according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) classifications of interstitial pneumonias has not been performed [13, 14]. Given the high incidence of radiographically noted pneumonitis during mTOR inhibitor therapy that might not accompany respiratory symptoms at the time of radiographic presentation, the detailed description of the radiographic patterns of pneumonitis during mTOR inhibitor therapy according to the ATS/ERS classifications of interstitial pneumonias will help assist in the accurate diagnosis, further enhance the awareness of the entity, and contribute to patient management.
The purpose of the present study was to determine the frequency of drug-related pneumonitis on chest CT scans during mTOR inhibitor therapy in patients with Waldenström macroglobulinemia and investigate the CT characteristics and radiographic patterns of pneumonitis according to the ATS/ERS classifications of interstitial pneumonias.
Materials and Methods
Patients
The study population included 40 patients with Waldenström macroglobulinemia treated in two trials of the mTOR inhibitor everolimus at the Dana-Farber Cancer Institute. These patients had a baseline and at least one follow-up chest CT scan during therapy available for review. Six patients were treated in a phase II trial of single-agent everolimus in patients with relapsed/refractory Waldenström macroglobulinemia and received everolimus 10 mg orally daily [2]. Thirty-four patients were treated in a phase I trial of everolimus combined with rituximab with or without bortezomib; 6 patients were treated with everolimus and rituximab (everolimus dose, 5 mg in 3 patients and 10 mg in 3 patients) and 28 patients with everolimus, rituximab, and bortezomib (everolimus dose, 5 mg in 4 patients and 10 mg in 24 patients). The protocols were approved by the institutional review board, and all patients provided written informed consent. Patients without a baseline and at least one follow-up chest CT scan available for review were excluded from the present study, which focused on the radiographic assessment (n = 25).
Chest CT Examinations
Baseline chest CT scans were performed before the initiation of everolimus therapy (median time from the baseline CT scan to therapy initiation, 1.7 weeks). All the chest CT scans after the initiation of therapy, until the termination of therapy or the last follow-up examination for those still receiving therapy, were included as follow-up CT scans. According to the clinical trial protocols, in patients treated in the phase II trial, a CT scan of the chest, abdomen, and pelvis was performed at 8 and 24 weeks of therapy and every 12 weeks thereafter [2]. In patients treated in the phase I trial, a CT scan was performed at 24 weeks of therapy.
The standard clinical chest CT protocol at the Dana-Farber Cancer Institute uses a 64-row multiple detector CT scanner (Aquilion 64; Toshiba America Medical Systems, Tustin, CA, http://www.toshiba.com). Iodinated intravenous contrast agent was used if it was not medically contraindicated. Patients were scanned in the supine position from the cranial to caudal direction from the clavicles to the adrenal glands at end-inspiration. Axial images with 5-mm thickness were reconstructed using standard and lung algorithms. Axial images reconstructed with lung algorithms were reviewed on picture archiving communication systems workstations (Centricity; GE Healthcare, Princeton, NJ, http://www.gehealthcare.com) with a window level of −700 HU and a window width of 1,500 HU.
Radiologic Review of Chest CT During mTOR Inhibitor Therapy
A retrospective imaging review was performed on the baseline chest CT and follow-up chest CT scans performed during mTOR inhibitor therapy. All chest CT scans were reviewed for abnormalities suspicious for drug-related pneumonitis by consensus of three radiologists (M.N., H.H., N.R.). The radiologists were aware that the patients had Waldenström macroglobulinemia and were being treated with mTOR inhibitor therapy using everolimus. However, they were not aware of the detailed clinical data, including adverse events and tumor progression. Each set of baseline and follow-up scans that belonged to a patient were reviewed sequentially in one review session, and the radiologists were aware of the scan dates.
The chest CT images were evaluated for the presence of parenchymal and interstitial lung abnormalities suspicious for drug-related pneumonitis. The radiologists were instructed to disregard the findings indicative of tumor involvement of the lung [15, 16]. The abnormalities suspicious for drug-related pneumonitis were evaluated for (a) extent in terms of the upper, middle, and lower lung zones using a 5-point scale for each zone (0, no involvement; 1, <5%; 2, 5%–25%; 3, 25%–50%; 4, >50%); (b) distribution in terms of peripheral, diffuse, central, or mixed; (c) distribution in terms of upper predominant, lower predominant, diffuse, multifocal, or focal; and (d) lobar involvement (right upper lobe, right middle lobe, right lower lobe, left upper lobe excluding lingula, lingula, and left lower lobe). The presence or absence of other observations, including traction bronchiectasis, consolidation, reticular opacities, ground glass opacities, centrilobular nodularity, and honeycombing, was recorded. For cases indicative of pneumonitis, the radiographic patterns were classified, referring to the ATS/ERS international multidisciplinary classification of interstitial pneumonias and related conditions [13, 14], as the (a) usual interstitial pneumonia pattern, (b) nonspecific interstitial pneumonia (NSIP) pattern, (c) cryptogenic organizing pneumonia (COP) pattern, (d) acute interstitial pneumonia/acute respiratory distress syndrome pattern, (e) hypersensitivity pneumonitis pattern, and (f) not applicable.
From the examination of sequential CT images, the radiologists recorded whether the observed pulmonary abnormalities were likely to have been caused by drug-related pneumonitis, using the following scale: 1, definitively not; 2, probably not; 3, equivocal; 4, probably; and 5, definitively. Patients with definitively (score 5) and probably (score 4) were considered to have radiographically detected pneumonitis. For the patients with definitive and probable drug-related pneumonitis, the medical records were reviewed for the respiratory symptoms at the initial presentation of radiographic pneumonitis.
Statistical Analysis
Differences in the demographic data and clinical characteristics were tested using the chi-square test for categorical data and the Wilcoxon test for continuous data between patients with and without drug-related pneumonitis based on the CT review. The median time to the development of drug-related pneumonitis on CT scans was calculated using the Kaplan-Meier method. A Wilcoxon sign rank test was used to compare the time from the initiation of therapy to the last follow-up chest CT scan for patients with and without drug-related pneumonitis. All p values were based on a two-sided hypothesis; p < .05 was considered significant.
Results
The clinical and baseline disease characteristics and therapeutic regimen for the 40 patients are summarized in Table 1. Drug-related pneumonitis was noted in 23 patients (23 of 40; 58%), which was scored as definitive for pneumonitis in 19 and probable in 4 patients. The median time between the initiation of mTOR therapy and the onset of pneumonitis was 5.7 months (95% confidence interval [CI] for the median, 5.5–6.0). The demographic data and baseline disease characteristics of the patients with and without pneumonitis showed no statistically significant imbalances (Table 1). No significant differences were found between the 2 groups in terms of therapeutic regimen (p = .29 across 3 different regimens; p = .96 for everolimus monotherapy vs. combination therapy), the therapeutic dosage of everolimus (5 mg vs. 10 mg; p = .69), or the number of previous therapies for Waldenström macroglobulinemia (p = .94). Respiratory symptoms at the onset of radiographic pneumonitis were present in 8 of the 23 patients (35%). The most common symptom was cough, noted in 5 patients, and dyspnea, noted in 3 patients. No difference was seen in the frequency of respiratory symptoms between the COP and NSIP groups (p = .59).
Table 1.
Patient demographics and clinical characteristics
The details of the imaging characteristics of the 23 patients with drug-related pneumonitis are summarized in Table 2. Lower lung zones were involved in all 23 patients, with higher extent of involvement compared with the upper and middle lung zones (median extent of involvement in each zone; upper, <5%; middle, 5%–25%; lower, 25%–50%; p < .001). Two types of distributions of the lung abnormalities were assessed (Table 2). When these 2 types of distributions were combined, the distribution was most commonly peripheral and lower in 11 (48%) and mixed and multifocal in 10 patients (44%); peripheral and multifocal distribution (n = 1) and a diffuse distribution by both sets of assessments (n = 1) were also noted. The lung abnormalities on CT were bilateral in 20 (87%), only left-sided in 2 (9%), and only right-sided in 1 (4%) patient. All lobes were involved in 12 patients (52%). Bilateral lower lobes were involved in 20 patients (87%), including 12 with all-lobe involvement.
Table 2.
Imaging characteristics of pneumonitis in 23 patients
In terms of the specific CT findings, GGOs and reticular opacities were present in all 23 patients, with coexistent consolidations in 12 patients, traction bronchiectasis in 2 patients, and centrilobular nodularity in 1 patient. None of the patients had honeycombing.
The CT findings of pneumonitis were classified as a COP pattern in 16 (70%) and an NSIP pattern in 7 of the 23 patients (30%; Figs. 1, 2, respectively). Overlapping features of the COP and NSIP patterns were noted in 7 patients, including 5 in the COP group and 2 in the NSIP group (Fig. 3). No difference was noted between the COP and NSIP groups in terms of the clinical and treatment characteristics and time between therapy initiation and the development of pneumonitis on CT (p > .14). None of the patients underwent bronchoscopy, bronchoalveolar lavage, or lung biopsy.
Figure 1.
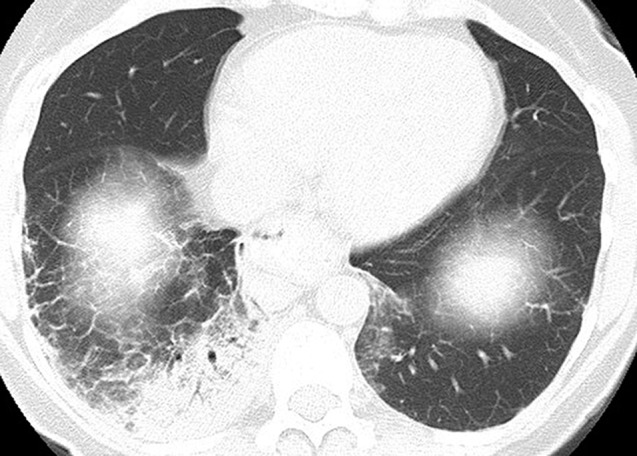
A 66-year-old woman with Waldenström macroglobulinemia. Chest computed tomography (CT) scan at 6 months of therapy demonstrated consolidation and ground glass and reticular opacities, demonstrating a cryptogenic organizing pneumonia pattern on the CT scan.
Figure 2.
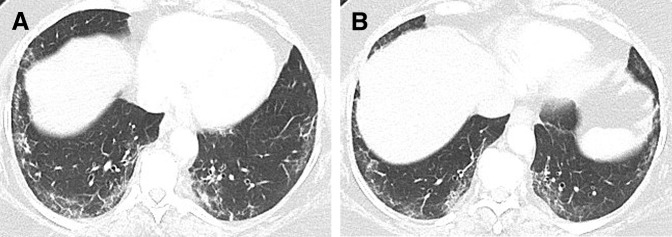
A 66-year-old woman with Waldenström macroglobulinemia. Chest computed tomography scan at 6 months of therapy showed consolidation and ground glass and reticular opacities in a lower and peripheral distribution, demonstrating a nonspecific interstitial pneumonia pattern.
Figure 3.
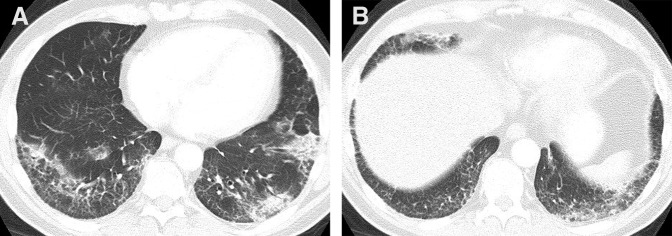
A 59-year-old man with Waldenström macroglobulinemia. Chest computed tomography scan at 6 months of therapy demonstrated ground glass and reticular opacities with areas of consolidations, indicative of a nonspecific interstitial pneumonia pattern, with secondary features of a cryptogenic organizing pneumonia pattern.
In 10 patients, everolimus was withheld at the time of pneumonitis and was restarted after a temporary hold (median, 2.5 weeks; range, 1–5 weeks) with a lower dose (5 mg) in 8 patients. Everolimus was continued with a lower dose (5 mg) in 3 patients and was discontinued in 2. In 8 patients, the therapy was continued without lowering the dose and without any additional treatment for pneumonitis. A steroid was given in 10 patients (n = 7 in the temporary hold group; n = 2 in the discontinued group; and n = 1 in the continued with lower dose group). Additional follow-up chest CT scans after the onset of pneumonitis were available for 11 patients, and a decrease in pulmonary findings were noted 8 patients (n = 6 in the temporary hold group; n = 1 in the discontinued group; and n = 1 in the no treatment change group).
Discussion
Drug-related pneumonitis diagnosed from CT findings was highly frequent among patients with Waldenström macroglobulinemia receiving mTOR inhibitor therapy, occurring in 58% of the patients. The median onset was 5.7 months since the initiation of therapy. The radiographic patterns of pneumonitis demonstrated COP and/or NSIP patterns, with overlapping features in 30% of the patients. Although the high frequency of drug-related pneumonitis has been recognized in previous clinical studies, a detailed description of the radiographic patterns of pneumonitis according to the ATS/ERS international multidisciplinary classification of interstitial pneumonias has not been previously reported. The present study described an initial exercise of a radiographic pattern-based approach to drug-related pneumonitis in the era of molecular targeting therapy, with a cohort of Waldenström macroglobulinemia receiving mTOR inhibitor therapy as a paradigm.
The frequency of radiographically noted pneumonitis was 58% (23 of 40) in the present cohort of Waldenström macroglobulinemia patients, higher than the previously reported frequency of 36% in metastatic RCC patients treated with everolimus or temsirolimus [10], 29% of advanced RCC patients treated with temsirolimus [9], and 25% of the patients with advanced NSCLC treated with everolimus [12]. Although the exact reason for the greater frequency in the present cohort is unknown, several factors could have contributed to the difference. It is possible that the frequency of the development of pneumonitis could differ among different tumor types, presumably in association with the different demographic data and clinical characteristics of the patients. The frequency of lung parenchymal metastasis from the tumors might, at least in part, affect the results. It can sometimes be difficult to make a radiologic diagnosis of pneumonitis in the presence of a significant tumor burden with lung metastasis or lymphangitic spread, which is common in patients with advanced NSCLC and RCC. However, it is rare in patients with Waldenström macroglobulinemia, with a propensity to involve the bone marrow and lymph nodes. One of the reasons for focusing on the cohort of Waldenström macroglobulinemia as a paradigm to demonstrate the pattern-based approach for pneumonitis was the low likelihood of significant lung parenchymal involvement of the tumor, which allowed a detailed assessment and sensitive detection of pneumonitis on imaging studies.
In the present study, 70% of the patients received rituximab with or without bortezomib, which might have contributed to the higher frequency because both agents have been reported to be associated with pneumonitis in some previous reports [7, 17–22]. Also, rituximab has recently been used to treat interstitial lung diseases [23, 24]. In the present study, however, 4 of the 6 patients (67%) treated with everolimus alone developed pneumonitis, and different therapeutic regimens had no significant association with the development of pneumonitis (p = .29).
The median time to the onset of pneumonitis from the initiation of therapy was 5.7 months (95% CI for the median, 5.5–6.0 months), longer than the 56 days (range, 31–214) among RCC patients treated with everolimus or temsirolimus [10]. In a previous study by Maroto et al. of advanced RCC patients treated with temsirolimus, 60% of the pneumonitis cases developed within 8 weeks of therapy [9]. The timing of the development of radiographically detected pneumonitis is certainly influenced by the timing of the oncology follow-up scans, which are subject to specific predetermined intervals according to tumor type, stage, and therapeutic regimen. In the study of RCC by Maroto et al., the CT scan was performed every 8 weeks to assess the tumor response to therapy [9]. In a study by Soria et al., a chest CT scan was performed every 4 weeks for the first 16 weeks and every 8 weeks thereafter [12]. The frequent CT scanning in these reports of solid malignancy was mainly because imaging is essential to assess the response to therapy in these tumors. In contrast, the follow-up imaging intervals of the cohort of Waldenström macroglobulinemia patients was much longer, because serum M protein and bone marrow evaluations play a major role in the response evaluation for these patients. According to the trial protocols, most of the patients in the present study underwent their follow-up CT scan at 24 weeks of therapy, with earlier scans performed in some patients. It should also be noted that 4 patients had pneumonitis noted on the CT scan at 8 weeks or earlier.
Respiratory symptoms were present only in 8 of the 23 patients (35%) at the development of pneumonitis, consistent with previous observations. Respiratory symptoms were present in 16 of 52 RCC patients (31%) in a study by Maroto et al. [9] and in 50% of the patients treated with temsirolimus in a study by Duran et al. [25], further emphasizing the importance of the radiological diagnosis and awareness of this common toxicity. The most common symptom was cough followed by dyspnea, also consistent with the previous report [25]. The low frequency of respiratory symptoms at the time of radiographic detection of pneumonitis also raises a possibility that the real onset of pneumonitis might be sooner than 6 months in our cohort, which were not detected until the 6-month imaging study for asymptomatic patients without earlier imaging studies.
The detailed assessment of the CT characteristics of pneumonitis has provided a comprehensive view of the imaging manifestation of this entity. The lung abnormalities involved lower lung zones with higher extent and most commonly had a peripheral and lower distribution, noted in 48% (11 of 23) of the cases. This follows the features seen in several representative entities of interstitial lung diseases, such as COP, NSIP, and idiopathic pulmonary fibrosis [14, 26]. Another common distribution was mixed and multifocal, noted in 44% of the cases (10 of 23). This distribution is somewhat nonspecific; however, it is seen in cases of COP. The findings were bilateral in 87% and involved all lobes in 52% of the cases, indicating the extensive nature of the lung abnormalities. Because the distribution is a key to interpreting interstitial lung diseases, including drug-related pneumonitis, the awareness of the common two types of distribution is important to assess the chest CT findings of patients undergoing mTOR inhibitor therapy.
For the specific CT findings, GGOs and reticular opacities were present in all cases, along with consolidation in 52% (12 of 23), consistent with the previous study by Maroto et al. reporting that GGOs and consolidation, either alone or combined, were the most commonly observed abnormalities on chest CT scans in patients with pneumonitis [9]. Other previous studies have also reported similar findings, consisting of predominantly GGOs and reticular opacities, sometimes with consolidation [10, 25]. The distribution of these abnormalities was not reported in detail in these previous studies. Notably, none of the patients had honeycombing, in contrast to the findings in pneumonitis associated with conventional chemotherapeutic agents such as bleomycin [27]. Although the mechanism of pneumonitis as a class-effect toxicity of mTOR inhibitors remains unclear, the different imaging manifestation is indicative of pathophysiology that is distinct from the toxicities of conventional agents.
The radiographic pattern of pneumonitis was classified as the COP pattern in 70% of the cases and as the NSIP pattern in 30%. Overlapping features of COP and NSIP patterns were noted in 30%, which is reasonable given the well-known overlap of CT findings of these entities in idiopathic cases [28]. Although none of the cases had histological confirmation of the radiologic patterns, it is worthwhile to understand the constellation of the overall imaging characteristics of pneumonitis according to the ATS/ERS classification. Although drug-related pneumonitis can manifest with diverse imaging findings, a systematic investigation focusing on a specific agent and a specific tumor will often identify several common features across individual cases and can help to contribute to improve diagnostic accuracy. Because almost all interstitial lung diseases can present with “GGOs” and “reticular opacities” to some extent, further in-depth characterization with distribution analysis and pattern classification are needed to help radiologic interpretation in clinical practice. To that end, the present study has described an initial exercise of the pattern-based approach to drug-related pneumonitis during mTOR inhibitor therapy, which could contribute to optimize the assessment and documentation of pneumonitis during molecular targeting therapy. The approach is also applicable to other types of tumors treated with other anticancer agents, including novel agents for which the toxicity profiles will be of great interest and could affect the therapeutic course.
The limitations of the present study included the retrospective design with a small number of patients treated at a single institution. Patients without chest CT scans available for review were excluded from the present study. The diagnosis of drug-related pneumonitis was predominantly based on the CT findings, which also added a limitation to the study. However, a similar approach has been used in previous publications by others addressing drug-related pneumonitis during mTOR inhibitor therapy [9, 25]. Histologic confirmation was not available for the present cohort, which is often the case with patients with known active malignancy receiving ongoing treatment. It should also be noted that, although the histologic evaluation helps to determine the morphologic pattern of lung injury, histologic examination alone might not fully identify the etiology of lung injury. Rather, etiologies such as drug-related toxicity often require a multidisciplinary approach, including clinical and radiological correlation. Most patients received other agents in addition to the mTOR inhibitor, raising the possibility of effects from these additional agents on the development of pneumonitis. However, the incidence of pneumonitis was similar between the monotherapy group and combination therapy group. A larger study with a prospective cohort treated with single-agent mTOR inhibitor therapy is needed to further address the issue.
Conclusion
Drug-related pneumonitis was noted on CT scans in 58% of Waldenström macroglobulinemia patients treated with mTOR inhibitor therapy. The most common findings were bilateral GGOs and reticular opacities, with or without consolidation, in the peripheral and lower lungs. The radiographic pattern of pneumonitis most commonly demonstrated a COP pattern, followed by the NSIP pattern, with overlapping features in one third of the patients. The radiographic pattern-based approach to drug-related pneumonitis described in the present study could contribute to further understanding and in-depth interpretation of lung toxicity during molecular targeting therapy for cancer.
Acknowledgment
M.N. was supported by National Cancer Institute Grant 1K23CA157631.
Author Contributions
Conception/Design: Mizuki Nishino, Erica N. Boswell, Hiroto Hatabu, Irene M. Ghobrial, Nikhil H. Ramaiya
Provision of study material or patients: Irene M. Ghobrial
Collection and/or assembly of data: Mizuki Nishino, Erica N. Boswell, Hiroto Hatabu, Irene M. Ghobrial, Nikhil H. Ramaiya
Data analysis and interpretation: Mizuki Nishino, Hiroto Hatabu, Irene M. Ghobrial, Nikhil H. Ramaiya
Manuscript writing: Mizuki Nishino, Erica N. Boswell, Hiroto Hatabu, Irene M. Ghobrial, Nikhil H. Ramaiya
Final approval of manuscript: Mizuki Nishino, Erica N. Boswell, Hiroto Hatabu, Irene M. Ghobrial, Nikhil H. Ramaiya
Disclosures
Mizuki Nishino: Bristol-Myers Squibb (C/A); Hiroto Hatabu: Canon USA, Inc., AZE Inc. (RF); Irene M. Ghobrial: Novartis (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Erasmus JJ, McAdams HP, Rossi SE. High-resolution CT of drug-induced lung disease. Radiol Clin North Am. 2002;40:61–72. doi: 10.1016/s0033-8389(03)00109-x. [DOI] [PubMed] [Google Scholar]
- 2.Ghobrial IM, Gertz M, Laplant B, et al. Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory Waldenstrom macroglobulinemia. J Clin Oncol. 2010;28:1408–1414. doi: 10.1200/JCO.2009.24.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 4.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 5.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 6.Ansell SM, Tang H, Kurtin PJ, et al. Temsirolimus and rituximab in patients with relapsed or refractory mantle cell lymphoma: A phase 2 study. Lancet Oncol. 2011;12:361–368. doi: 10.1016/S1470-2045(11)70062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouabdallah K, Ribrag V, Terriou L, et al. Temsirolimus in the treatment of mantle cell lymphoma: Frequency and management of adverse effects. Curr Opin Oncol. 2013;25(suppl 2):S1–S12. doi: 10.1097/CCO.0b013e32835de8ee. [DOI] [PubMed] [Google Scholar]
- 8.Willemsen AE, van Herpen CM. mTOR inhibitor-related pulmonary toxicity; Incidence even higher. Acta Oncol. 2013;52:1234. doi: 10.3109/0284186X.2013.770166. [DOI] [PubMed] [Google Scholar]
- 9.Maroto JP, Hudes G, Dutcher JP, et al. Drug-related pneumonitis in patients with advanced renal cell carcinoma treated with temsirolimus. J Clin Oncol. 2011;29:1750–1756. doi: 10.1200/JCO.2010.29.2235. [DOI] [PubMed] [Google Scholar]
- 10.Dabydeen DA, Jagannathan JP, Ramaiya N, et al. Pneumonitis associated with mTOR inhibitors therapy in patients with metastatic renal cell carcinoma: Incidence, radiographic findings and correlation with clinical outcome. Eur J Cancer. 2012;48:1519–1524. doi: 10.1016/j.ejca.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson BJ, Cauley DH, Ng C, et al. mTOR inhibitor associated noninfectious pneumonitis in patients with renal cell cancer: Management, predictors, and outcomes. BJU Int. 2014;113:376–382. doi: 10.1111/bju.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soria JC, Shepherd FA, Douillard JY, et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol. 2009;20:1674–1681. doi: 10.1093/annonc/mdp060. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society. European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 14.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishino M, Cardarella S, Dahlberg SE, et al. Interstitial lung abnormalities in treatment-naïve advanced NSCLC patients (pts): Prevalence and impact on survival. J Clin Oncol. 2014;32(suppl):e19030a. [Google Scholar]
- 16.Nishino M, Cardarella S, Araki T et al. Radiographic interstitial lung abnormalities in advanced NSCLC patients during platinum-based chemotherapy: A systematic study in a cohort with wild-type EGFR, ALK, BRAF, and KRAS. Paper presented at: Annual meeting of the Radiological Society of North America; December 4, 2013; Chicago, IL. [Google Scholar]
- 17.Yamaguchi T, Sasaki M, Itoh K. Bortezomib-induced pneumonitis during bortezomib retreatment in multiple myeloma. Jpn J Clin Oncol. 2012;42:637–639. doi: 10.1093/jjco/hys074. [DOI] [PubMed] [Google Scholar]
- 18.Kang W, Kim JS, Cho SH, et al. Nonspecific interstitial pneumonitis after bortezomib and thalidomide treatment in a multiple myeloma patient. Yonsei Med J. 2010;51:448–450. doi: 10.3349/ymj.2010.51.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao CW, Liao WC, Tu CY, et al. Rituximab-induced pneumonitis mimicking miliary tuberculosis. Eur Respir Rev. 2013;22:587–588. doi: 10.1183/09059180.00003813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ergin AB, Fong N, Daw HA. Rituximab-induced bronchiolitis obliterans organizing pneumonia. Case Rep Med. 2012;2012:680431. doi: 10.1155/2012/680431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber NA, Ganti AK. Pulmonary toxicities from targeted therapies: A review. Target Oncol. 2011;6:235–243. doi: 10.1007/s11523-011-0199-0. [DOI] [PubMed] [Google Scholar]
- 22.Arulkumaran N, Suleman R, Cecconi M, et al. Rituximab associated pneumonitis in antineutrophil cytoplasmic antibody-associated vasculitis. J Clin Rheumatol. 2012;18:39–41. doi: 10.1097/RHU.0b013e31823ee5bf. [DOI] [PubMed] [Google Scholar]
- 23.Lota HK, Keir GJ, Hansell DM, et al. Novel use of rituximab in hypersensitivity pneumonitis refractory to conventional treatment. Thorax. 2013;68:780–781. doi: 10.1136/thoraxjnl-2013-203265. [DOI] [PubMed] [Google Scholar]
- 24.Keir GJ, Maher TM, Ming D, et al. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology. 2014;19:353–359. doi: 10.1111/resp.12214. [DOI] [PubMed] [Google Scholar]
- 25.Duran I, Siu LL, Oza AM, et al. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur J Cancer. 2006;42:1875–1880. doi: 10.1016/j.ejca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Nishino M, Itoh H, Hatabu H. A practical approach to high-resolution CT of diffuse lung disease. Eur J Radiol. 2014;83:6–19. doi: 10.1016/j.ejrad.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis SJ, Cleverley JR, Müller NL. Drug-induced lung disease: High-resolution CT findings. AJR Am J Roentgenol. 2000;175:1019–1024. doi: 10.2214/ajr.175.4.1751019. [DOI] [PubMed] [Google Scholar]
- 28.Lynch DA, Travis WD, Müller NL, et al. Idiopathic interstitial pneumonias: CT features. Radiology. 2005;236:10–21. doi: 10.1148/radiol.2361031674. [DOI] [PubMed] [Google Scholar]


