Abstract
Background:
Vanderbilt University affiliate Friends in Global Health was funded in 2008 to support comprehensive HIV/AIDS services in north-central Nigeria. We summarise programme characteristics and trends in enrolment and quality of data collection in this rural, resource-limited environment.
Methods:
We used routinely collected programme data in supported sites from June 1 2009 to September 30, 2013.Baseline characteristics were defined as those collected closest to a 90-day window period before and after enrolment. Summary characteristics were compared by site and enrolment year.
Results:
We enrolled 3,960 HIV-infected patients into care (68% women), median age of 32 years [interquartile range (IQR): 27–40]. Most clients were married (79%) and unemployed (60%). At enrolment, median CD4+ cell count was 230 cells/μL (IQR: 114–390) and haemoglobin was 10.7 g/dL (IQR: 9.3–11.9). Advanced clinical disease [World Health Organization (WHO) clinical stage III/IV] at enrolment was documented in 29% of clients. Cumulative enrolment increased from 377 patients in 2009 to 3,960 patients by 2013.With each successive year, more clients were enrolled at earlier stages of disease; in 2009, 37% of patients were identified as WHO clinical stage I, while in 2013, 55% of patients were so classified. While documentation of clinical staging remained stable, the completeness of CD4+ cell count and haemoglobin data declined with time.
Conclusion:
Expanded testing in a comprehensive HIV programme in rural Nigeria brought persons to care at earlier stages of illness. Yet, as clinical services expanded, data collection quality declined. The paradox of successful scaling up HIV services but deteriorating quality of data underscores the importance of data management training and quality improvement efforts.
Keywords: HIV/AIDS, PEPFAR, trends, enrolment, data quality, rural Nigeria
Introduction
Sub-Saharan Africa is home to 23.5 million (69%) of the 34 million persons living with HIV in the world in 2012, with Nigerians representing the second largest nationality affected by the HIV/AIDS epidemic.1,2 In order to address this issue, the U.S. President's Emergency Plan for AIDS Relief (PEPFAR) implemented a 5-year partnership framework in 2010 with the Nigerian government. The primary objectives included: prevention of new HIV infections; ensuring that at least 50% of HIV-infected persons have access to quality care; increasing access to antiretroviral treatment (ART) from 32 to 80%; and increasing the level of HIV programme financing by the Government of Nigeria (GON) from 7 to 50%.3 Though significant progress has been made, challenges to providing access to HIV treatment services in Nigeria persist, especially in rural settings.4 Compared with urban settings, rural areas have disproportionately fewer healthcare workers, poor public works and clinical infrastructure, and less access to health services.4 In addition, patient populations in rural areas tend to be less educated, have higher rates of unemployment, and lower incomes than those living in urban settings.4 As a result, these resource-constrained areas are severely impacted by the HIV/AIDS epidemic.
Vanderbilt University (VU) and its non-governmental affiliate Friends in Global Health, LLC (FGH) received PEPFAR funding in 2008 to support the provision of comprehensive HIV/AIDS services in the rural areas of Niger and Kwara states and the Federal Capital, Abuja. These states are located in north-central Nigeria, which has the highest HIV prevalence rate in the country (2010 adult HIV prevalence of 7.5% as compared to the national HIV prevalence of 3.6%).5 The HIV epidemic in Nigeria is driven by heterosexual transmission, and the two states are traversed by a major expressway linking the northern and southern parts of the country, with several HIV transmission ‘hot spots’. VU/FGH supported activities were organised around direct technical assistance to facilities to implement HIV clinical services and human capacity development.6 Supported services included: HIV care and treatment for adults and children (including the provision of antiretroviral medications and medications to treat opportunistic infections); prevention of mother–child HIV transmission (PMTCT); HIV testing and counseling (HTC); TB testing and treatment; support for orphans and vulnerable children; and the strengthening of strategic information and laboratory services.6 Investigating patient characteristics and temporal changes in essential clinical indices (e.g., CD4+ cell count, haemoglobin, WHO clinical staging) is a useful approach to evaluating gaps in HIV service delivery and identifying opportunities for programme improvement.7 The aim of this study is to examine programme characteristics and trends in client enrolment and data collection in order to provide insight into the challenges faced in the implementation of a comprehensive HIV programme in a resource-limited rural setting.
MATERIALS AND METHODS
Setting
This observational cohort study was conducted in five VU/FGH hospitals (first and last enrolment dates in cleaned databases): Gawu Babangida Rural Hospital (19 May 2009–20 August 2013), Kuta Rural Hospital (8 November 2010–5 July 2012), and Umaru Yar'Adua Hospital Sabon Wuse (1 July 2010–23 November 2012) in Niger State; Sobi Specialist Hospital (9 June 2009–3 November 2013) and Lafiagi General Hospital (9 June 2009–27 August 2013) in Kwara State. A “hub and spoke” operating model links these five facilities to nine satellite ‘feeder’ clinics that provide HTC and PMTCT services.8,9
Eligibility criteria
All HIV-positive male and female patients aged ≥ 15 years initiating treatment in VU/FGH supported sites from June 1, 2009 and September 30, 2013 were included in the study. Patients < 15 years old and those enrolled into care outside the study period were excluded from this analysis.
Care and treatment procedures
Patients were enrolled into the HIV care and treatment programme if they tested HIV-positive in a VU/FGH-supported clinic (these included PMTCT, HTC, and TB settings) or if they were referred from other clinics. HIV testing was performed using the national serial rapid HIV-testing protocol. At enrolment, all patients received an initial evaluation, including CD4+ cell count, haematology, chemistry and World Health Organization (WHO) clinical staging. Subsequently, a physician conducted a comprehensive physical exam with additional screening for TB and other opportunistic infections.
As a result of changes in Nigerian guidelines for ART initiation in June 2010, there were two eligibility criteria. Prior to June 2010, patients eligible for ART initiation included those with WHO clinical stage I/II disease with CD4+ count < 200 cells/μL, or with WHO clinical stage III disease and CD4+ count 200–350 cells/μL, or WHO clinical stage IV disease irrespective of CD4+ cell count. After June 2010, initiation guidelines included any patient with a CD4+ cell count ≤ 350 cells/μL; or with WHO clinical stage III or IV disease regardless of CD4+ cell count. First line adult ART regimen included a combination of zidovudine (ZDV) plus lamivudine (3TC) plus nevirapine (NVP) or efavirenz (EFV). Some patients received tenofovir (TDF)/emtricitabine (FTC) instead of ZDV/3TC. Second-line therapy included two new nucleoside reverse transcriptase inhibitors plus lopinavir/ritonavir. All pregnant women with WHO clinical stage III or IV disease and/or having a CD4+ count < 350 cells/μL were eligible for ART initiation over the entire study period. Patients were followed up every 3 months, in accordance with national guidelines and deemed lost to follow-up (LTFU) after no effective contact within 3 months of the last scheduled follow-up. Patients who re-engage in care during the period of observation are not counted as LTFU. Periodic (monthly) lists of patients LTFU were generated to prioritise active case finding by community health workers and volunteers.
Data management
Friends in Global Health, LLC had strategic information monitoring systems in both Niger and Kwara states. All data collection and documentation activities were performed by medical records and data entry personnel at the sites, supervised by FGH monitoring and evaluation (M&E) officers. During the project period covered by this analysis, there were two M&E senior staff at the Abuja FGH office and one to two data entry/medical records personnel in each of the study facilities. Information on services provided were collected using national data collection tools, including patient monitoring and management (PMM) forms and facility-based registers. Data from the forms were then uploaded into an electronic health records system (“CAREWare™”) at FGH sites. Medical records and data entry personnel received periodic training and written instructions were placed at each site for reference purposes. Processes for data validation included periodic data quality assessments, which involved running data discrepancy reports and working with the site staff to obtain resolutions to missing, incomplete, illogical, or inconsistent data, where feasible. Baseline clinical characteristics were defined as those collected closest to a 90-day window period before or after enrolment. Categories of data collected included: demographics (sex, age, marital status, educational level and service entry into the programme), clinical information (weight, height, WHO clinical stage, WHO performance/functional status, and TB status), and select laboratory tests (CD4+ cell count, Hepatitis B, pregnancy status, syphilis/VDRL, haemoglobin, liver enzymes (ALT/SGPT), and serum creatinine). Weight, height, functional status, CD4+ cell count, haemoglobin, creatinine, and WHO clinical stage were collected at enrolment. Out-of-range and missing data were queried. Each site addressed data queries and clean data were extracted for final analyses. Out-of-range data that could not be confirmed were recorded as missing.
Statistical methods
Participant demographic and clinical characteristics were tabulated over total enrolment by clinic site. To compare the distribution of characteristics by site, we employed chi-square tests for categorical variables and Kruskal–Wallis rank sum test for continuous variables. Continuous variables were reported as medians [interquartile range (IQR)]. Trends in cumulative enrolment and data collection for select laboratory variables (haemoglobin and CD4+ cell count) and WHO clinical stage were analysed by year across the study period. To compare the distribution of characteristics by study year, we employed Kruskal–Wallis tests for categorical variables and Spearman rank correlation test for continuous variables. We use Kaplan–Meier estimates to summarise overall 1 year retention. All hypothesis testing was two-sided with a level of significance set at 5%. We employed R-software 3.0.2 (www.r-project.org.) for all data analyses. Analysis scripts are available at http://biostat.mc.vanderbilt.edu/ArchivedAnalyses
Ethical considerations
This study was classified as ‘exempt’ from ethical approval by the Vanderbilt University Institutional Review Board. Personal identifiers were removed prior to data extraction. Programme personnel were trained on confidentiality and secure data handling.
Results
Between June 1, 2009 and September 30, 2013, the five clinics cumulatively enrolled 3,960 HIV-infected patients into care. Umaru Yar' Adua Hospital had the highest number of enrolled patients (n = 1551), followed by Sobi Specialist Hospital (n = 910). Of the 3,960 enrolled clients, 68% were women (Table 1). The median age was 32 years (IQR: 27–40). Of the 2,528 patients with documented educational level, 73% were literate. Majority of the enrollees were married (79%) and unemployed (60%).
Table 1. Summary of patient demographics by clinic, FGH Nigeria Cohort, 2009–2013.
| GBRH | Kuta | LGH | SBSH | UMYMH | Combined | P-value | |
| (n = 867) | (n = 125) | (n = 507) | (n = 910) | (n = 1551) | (n = 3960) | ||
| Age (years), median (IQR)a | 32 (27–40) | 35 (30–41) | 30 (25–39) | 34 (28–41) | 31 (26–39) | 32 (27–40) | < 0.001 |
| Female, n (%)b | 562 (65%) | 80 (64%) | 381 (75%) | 643 (71%) | 1036 (67%) | 2702 (68%) | < 0.001 |
| Education, n (%) | < 0.001 | ||||||
| Missing | 533 (61%) | 36 (29%) | 82 (16%) | 187 (21%) | 594 (38%) | 1432 (36%) | |
| None | 120 (36%) | 40 (45%) | 247 (58%) | 174 (24%) | 113 (12%) | 694 (27%) | |
| Started primary | 18 (5%) | 1 (1%) | 22 (5%) | 32 (4%) | 50 (5%) | 123 (5%) | |
| Completed primary | 62 (19%) | 13 (15%) | 49 (12%) | 174 (24%) | 281 (29%) | 579 (23%) | |
| Secondary | 99 (30%) | 22 (25%) | 48 (11%) | 171 (24%) | 340 (36%) | 680 (27%) | |
| Post secondary | 19 (6%) | 9 (10%) | 24 (6%) | 111 (15%) | 44 (5%) | 207 (8%) | |
| Qur'anic | 16 (5%) | 4 (4%) | 35 (8%) | 61 (8%) | 129 (13%) | 245 (10%) | |
| Marital status, n (%) | < 0.001 | ||||||
| Missing | 444 (51%) | 11 (9%) | 66 (13%) | 147 (16%) | 528 (34%) | 1196 (30%) | |
| Divorced | 10 (2%) | 2 (2%) | 3 (1%) | 13 (2%) | 69 (7%) | 97 (4%) | |
| Married | 343 (81%) | 94 (82%) | 404 (92%) | 606 (79%) | 746 (73%) | 2193 (79%) | |
| Separated | 17 (4%) | 4 (4%) | 1 ( < 1%) | 19 (2%) | 18 (2%) | 59 (2%) | |
| Single | 22 (5%) | 10 (9%) | 27 (6%) | 84 (11%) | 103 (10%) | 246 (9%) | |
| Widowed | 31 (7%) | 4 (4%) | 6 (1%) | 41 (5%) | 87 (9%) | 169 (6%) | |
| Occupation, n (%) | < 0.001 | ||||||
| Missing | 457 (53%) | 14 (11%) | 98 (19%) | 165 (18%) | 531 (34%) | 1265 (32%) | |
| Employed | 46 (11%) | 19 (17%) | 32 (8%) | 302 (41%) | 291 (29%) | 690 (26%) | |
| Other | 20 (5%) | 0 (0%) | 113 (28%) | 121 (16%) | 62 (6%) | 316 (12%) | |
| Retired | 4 (1%) | 0 (0%) | 1 ( < 1%) | 3 ( < 1%) | 4 ( < 1%) | 12 ( < 1%) | |
| Student | 7 (2%) | 3 (3%) | 10 (2%) | 15 (2%) | 14 (1%) | 49 (2%) | |
| Unemployed | 333 (81%) | 89 (80%) | 253 (62%) | 304 (41%) | 649 (64%) | 1628 (60%) |
aAge is reported as median (interquartile range, IQR).
bTo compare the distribution of study characteristics for participants by sex, we employ chi-square tests. Similarly, we use a Kruskal–Wallis test for continuous variables by clinic.
The median BMI for all enrolled clients was 20.8 kg/m2 (Table 2). Median CD4+ cell count and haemoglobin at enrolment were 230 cells/μL (IQR: 114–390) and 10.7 g/dL (IQR: 9.3–11.9), respectively. Only 2% of clients were bedridden. Of the four study sites, Gawu Babangida Hospital had the largest proportion of patients in WHO clinical stage III/IV disease (41%, Table 2). Clients in Sobi Specialist Hospital had the lowest median CD4+ cell count values (184, IQR: 87–332), but were most likely to be initiated on ART within 90 days of enrolment (proportion of patients on ART within 90 days of enrolment = 61%).
Table 2. Summary of patient clinical characteristics by clinic, FGH Nigeria Cohort, 2009–2013.
| GBRH | Kuta | LGH | SBSH | UMYMH | Combined | P-valueb | |
| (n = 867) | (n = 125) | (n = 507) | (n = 910) | (n = 1551) | (n = 3960) | ||
| Height (cm), median (IQR)a,c | 162 (156–167) | 161 (152–167) | 160 (155–165) | 162 (157–169) | 163 (157–169) | 162 (156–168) | < 0.001 |
| Missing, n (%) | 281 (32%) | 69 (55%) | 221 (44%) | 205 (23%) | 689 (44%) | 1465 (37%) | |
| Weight (kg), median (IQR)a,c | 54 (47–62) | 53 (50–60) | 50 (45–58) | 55 (47–63) | 57 (50–65) | 55 (48–63) | < 0.001 |
| Missing, n (%) | 43 (5%) | 24 (19%) | 37 (7%) | 35 (4%) | 116 (7%) | 255 (6%) | |
| BMI (kg/m2), median (IQR)a | 20.7 (18.6–22.9) | 21.6 (18.7–23.5) | 19.6 (17.7–21.7) | 20.6 (18.1–23.4) | 21.6 (19.3–24.3) | 20.8 (18.5–23.4) | < 0.001 |
| Missing, n (%) | 283 (33%) | 72 (58%) | 225 (44%) | 213 (23%) | 719 (46%) | 1512 (38%) | |
| Functional status, n (%)c | < 0.001 | ||||||
| Missing | 23 (3%) | 1 (1%) | 32 (6%) | 56 (6%) | 94 (6%) | 206 (5%) | |
| Bedridden | 14 (2%) | 0 (0%) | 1 ( < 1%) | 10 (1%) | 37 (3%) | 62 (2%) | |
| Ambulatory | 68 (8%) | 3 (2%) | 19 (4%) | 50 (6%) | 139 (10%) | 279 (7%) | |
| Working | 762 (90%) | 121 (98%) | 455 (96%) | 794 (93%) | 1281 (88%) | 3413 (91%) | |
| CD4 count (cells/μL), median (IQR)a,c | 240 (116–402) | 247 (138––448) | 261 (137–433) | 184 (87–332) | 242 (124–403) | 230 (114–390) | < 0.001 |
| Missing, n (%) | 110 (13%) | 44 (35%) | 94 (19%) | 104 (11%) | 336 (22%) | 688 (17%) | |
| CD4 count category, n (%)c | |||||||
| < 50 | 72 (10%) | 8 (10%) | 24 (6%) | 100 (12%) | 105 (9%) | 309 (9%) | |
| 51–200 | 254 (34%) | 23 (28%) | 129 (31%) | 325 (40%) | 403 (33%) | 1134 (35%) | |
| 201–350 | 198 (26%) | 20 (25%) | 113 (27%) | 200 (25%) | 316 (26%) | 847 (26%) | |
| >350 | 233 (31%) | 30 (37%) | 147 (36%) | 181 (22%) | 391 (32%) | 982 (30%) | |
| Haemoglobin (g/dL), median (IQR)a.c | 10.8 (9.5–12.1) | 11.3 (10–12.8) | 10.1 (8.9–11.1) | – | 10.7 (9.3–11.9) | 10.7 (9.3–11.9) | < 0.001 |
| Missing, n (%) | 263 (30%) | 68 (54%) | 201 (40%) | 910 (100%) | 500 (32%) | 1942 (49%) | |
| Creatininec | – | 0.6 (0.6–0.8) | 0.7 (0.6–1) | – | 0.7 (0.5–0.8) | 0.7 (0.6–0.9) | < 0.001 |
| Missing, n (%) | 867 (100%) | 85 (68%) | 139 (27%) | 910 (100%) | 910 (59%) | 2911 (74%) | |
| WHO clinical stage, n (%)c | < 0.001 | ||||||
| Missing | 19 (2%) | 2 (2%) | 55 (11%) | 73 (8%) | 142 (9%) | 291 (7%) | |
| I | 360 (42%) | 80 (65%) | 209 (46%) | 335 (40%) | 868 (62%) | 1852 (50%) | |
| II | 144 (17%) | 18 (15%) | 115 (25%) | 231 (28%) | 271 (19%) | 779 (21%) | |
| III | 311 (37%) | 25 (20%) | 114 (25%) | 248 (30%) | 242 (17%) | 940 (26%) | |
| IV | 33 (4%) | 0 (0%) | 14 (3%) | 23 (3%) | 28 (2%) | 98 (3%) | |
| HAART status, n (%) | < 0.001 | ||||||
| HAART >90 days from enrolment | 65 (7%) | 36 (29%) | 75 (15%) | 90 (10%) | 79 (5%) | 345 (9%) | |
| HAART in 90 days from enrolment | 420 (48%) | 45 (36%) | 276 (54%) | 558 (61%) | 825 (53%) | 2124 (54%) | |
| No HAART | 287 (33%) | 23 (18%) | 154 (30%) | 254 (28%) | 626 (40%) | 1344 (34%) | |
| Not ART-naive | 95 (11%) | 21 (17%) | 2 ( < 1%) | 8 (1%) | 21 (1%) | 147 (4%) |
aContinuous variables are reported as medians (interquartile range).
bTo compare the distribution of study characteristics for participants by site, we employ chi-square tests. Similarly, we use a Kruskal–Wallis test for continuous variables by clinic.
cWeight, height, functional status, CD4, haemoglobin, creatinine, WHO clinical stage are collected at enrolment. Enrolment data are collected in a window of ± 90 days from date of enrolment.
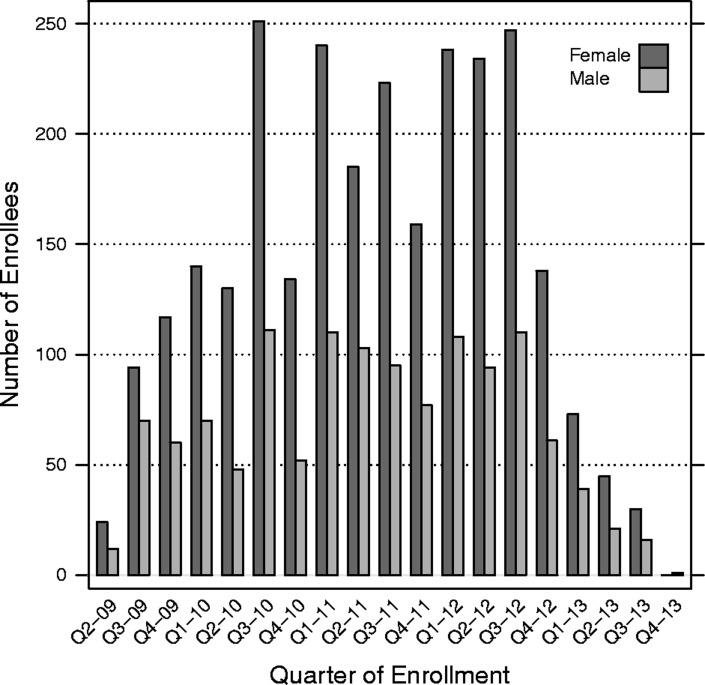
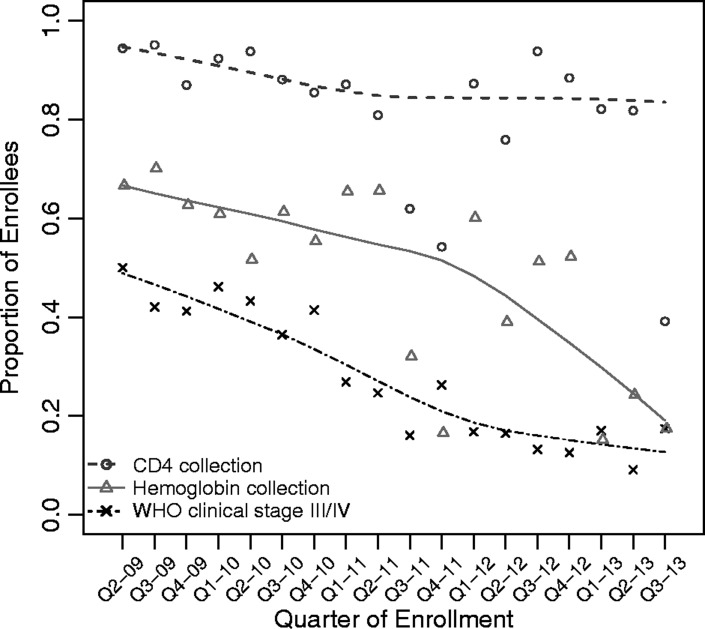
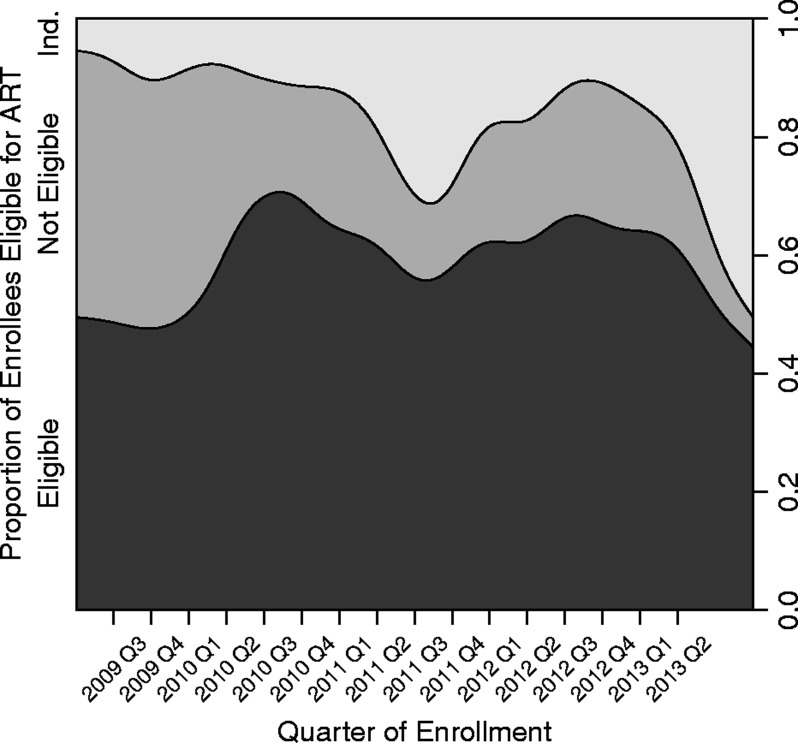
Cumulative patient enrolment rose from 377 patients in 2009 to 3,960 patients by 2013 (Table 3). Kuta and Umaru Yar Adua clinics enrolled patients only beginning in 2010. The largest relative percentage increase in enrolment by calendar year occurred between 2009 and 2010 (250% increase), but the majority of clinics enrolled the largest volume of patients during the third and fourth years of scale up (P < 0.001; Table 3, Fig. 1, see supplementary material online for this article (www.maneyonline.com/doi/suppl/10.1179/2047773215Y.0000000007)). With each successive year of the programme, more patients were enrolled at earlier stages of HIV disease (P < 0.001). In 2009, 37% of patients were identified as WHO clinical stage I, while in 2013, 55% of patients were classified as WHO clinical stage I. Whereas documentation of WHO clinical staging fluctuated above 90%, the completeness of CD4+ cell count and haemoglobin data declined with time (P < 0.001; Fig. 2). The completeness of data collection for CD4+ cell count declined from 91% in 2009 to 73% in 2013 and for haemoglobin from 66% in 2009 to 18% in 2013, respectively (Table 3, Fig. 2). During scale up, the proportion of patients who were eligible for ART increased from 48% in 2009 to 60% in 2013 (P < 0.001; Table 3). The proportion of patients who were unable to be assessed for ART eligibility peaked in 2013 at 24% (Table 3, Fig. 3). Overall, patient retention among enrollees at 1 year was 70% (95% CI: 68–71%). Overall, patient retention among patients on treatment at 1 year following antiretroviral initiation was 62% (95% CI: 60–64%).
Table 3. Trends in enrolment, sex, WHO clinical stage, CD4+ cell count, haemoglobin and data collection, FGH Nigeria Cohort, 2009–2013.
| 2009 | 2010 | 2011 | 2012 | 2013 | Combined | ||
| Cumulative enrollment, n | (n = 377) | (n = 936) | (n = 1192) | (n = 1230) | (n = 225) | (n = 3960) | P-valued |
| Clinic enrolment, n (%) | 377 | 1313 | 2505 | 3735 | 3960 | < 0.001 | |
| GBRH | 208 (55%) | 447 (48%) | 19 (2%) | 91 (7%) | 102 (45%) | 867 (22%) | |
| Kuta | 0 (0%) | 41 (4%) | 72 (6%) | 12 (1%) | 0 (0%) | 125 (3%) | |
| LGH | 92 (24%) | 157 (17%) | 117 (10%) | 85 (7%) | 56 (25%) | 507 (13%) | |
| SBSH | 77 (20%) | 265 (28%) | 245 (21%) | 256 (21%) | 67 (30%) | 910 (23%) | |
| UMYMH | 0 (0%) | 26 (3%) | 739 (62%) | 786 (64%) | 0 (0%) | 1551 (39%) | |
| Age (years), median (IQR) | 34 (27–40) | 32 (27–40) | 31 (26–39) | 32 (27–40) | 34 (29–40) | 32 (27–40) | 0.68 |
| Female, n (%) | 235 (62%) | 655 (70%) | 807 (68%) | 857 (70%) | 148 (66%) | 2702 (68%) | 0.33 |
| WHO clinical stage, n (%) | < 0.001 | ||||||
| I | 135 (37%) | 310 (35%) | 610 (55%) | 685 (62%) | 112 (55%) | 1852 (50%) | |
| II | 67 (19%) | 195 (22%) | 216 (20%) | 244 (22%) | 57 (28%) | 779 (21%) | |
| III | 140 (39%) | 354 (40%) | 257 (23%) | 160 (14%) | 29 (14%) | 940 (26%) | |
| IV | 20 (6%) | 29 (3%) | 21 (2%) | 24 (2%) | 4 (2%) | 98 (3%) | |
| CD4 count (cells/μL), median (IQR)a | 241 (112–389) | 246 (121–402) | 228 (113–384) | 222 (113–393) | 218 (95–354) | 230 (114–390) | 0.14 |
| Haemoglobin (g/dL), median (IQR)a | 10.3 (9.2–11.6) | 10.7 (9.4–12) | 10.7 (9.1–12) | 10.7 (9.5–11.9) | 10.4 (8.6–11.7) | 10.7 (9.3–11.9) | 0.26 |
| BMI (kg/m2), median (IQR) | 20.1 (18.3–22.3) | 20.6 (18.4–23.0) | 21.6 (19.1–24.3) | 20.8 (18.4–23.4) | 20.5 (17.6–22.5) | 20.8 (18.5–23.4) | < 0.001 |
| WHO clinical stage data collection, n (%)c | 362 (96%) | 888 (95%) | 1104 (93%) | 1113 (90%) | 202 (90%) | 3669 (93%) | < 0.001 |
| CD4 data collection, n (%)c | 344 (91%) | 839 (90%) | 863 (72%) | 1062 (86%) | 164 (73%) | 3272 (83%) | < 0.001 |
| Haemoglobin data collection, n (%)c | 250 (66%) | 545 (58%) | 559 (47%) | 623 (51%) | 41 (18%) | 2018 (51%) | < 0.001 |
| BMI data collection, n (%) | 354 (94%) | 748 (80%) | 730 (61%) | 563 (46%) | 53 (24%) | 2448 (62%) | < 0.001 |
| ART eligibility at enrolment, n (%) | < 0.001 | ||||||
| Eligible | 181 (48%) | 599 (64%) | 708 (59%) | 791 (64%) | 135 (60%) | 2414 (61%) | |
| Not eligible | 162 (43%) | 249 (27%) | 212 (18%) | 270 (22%) | 36 (16%) | 929 (23%) | |
| Indeterminate | 34 (9%) | 88 (9%) | 272 (23%) | 169 (14%) | 54 (24%) | 617 (16%) |
aCD4 count and haemoglobin are summarised using median (interquartile range).
bPercentages are computed using the number of patients with a non-missing value.
cCD4, haemoglobin, WHO clinical stage are collected at enrolment. Enrolment data are collected in a window of ± 90 days from date of enrolment.
dTo compare the distribution of study characteristics for participants by year, we employ Kruskal–Wallis tests. Similarly, we use a Spearman rank correlation test for continuous variables by year.
Figure 1.
Bar plot of quarterly client enrolment by sex, FGH Nigeria Cohort, 2009–2013
Figure 2.
Quarterly trends in CD4+ cell count, hemoglobin data collection and advanced disease at enrollment, FGH Nigeria Cohort, 2009–2013. Lowess (locally weighted scatterplot smoothing) curves are displayed along with the quarterly point estimates. Advanced HIV disease is indicated by WHO clinical stage III/IV at enrollment.
Figure 3.
Conditional density plot for trends in antiretroviral treatment (ART) eligibility at enrollment, FGH Nigeria Cohort, 2009–2013. Trends in the proportion of enrollees who are eligible, not eligible, or indeterminately eligible for ART at enrollment based on CD4 count, WHO clinical stage and ART eligibility criteria at time of enrollment. Antiretroviral treatment eligibility criteria change in June 2010 (end of Q2–10).
Discussion
As annual rates of new HIV infections in sub-Saharan Africa grew rapidly from the onset of the epidemic, peaking at 2.7 million new infections in the region in 1998,1 initial efforts to curve the epidemic in Africa were met with scepticism. However, as ART costs declined and HIV programme funding increased through initiatives such as PEPFAR, it is evident that delivery of effective and comprehensive HIV/AIDS programmes in complex, resource-limited settings across Africa is feasible and achievable. The focus is now on sustaining these programmes and improving on the quality of services by learning from successes and challenges that such programmes have faced.
Routinely collected data from our clinics in Niger and Kwara states revealed that women of younger age were disproportionately enrolled compared to men. This finding is consistent with trends observed in other HIV treatment programmes in sub-Saharan Africa.7,10 The sex disparity in enrolment may be partly explained by the preponderance of HIV in women (women account for 58% of all persons living with HIV in sub-Saharan Africa),11 sex differences in health-seeking behaviour, and opportunities available to women to enter into HIV care that are not usually available to men, such as antenatal clinics and PMTCT initiatives.12–14 Future efforts to effectively engage men at every level of the HIV care cascade via evidence-based approaches (e.g., couples testing, involvement in PMTCT, and male-friendly clinics) are warranted.
The majority of our patients were noted to have advanced clinical disease (WHO clinical stage III/IV) at programme inception. This proportion declined over time, consistent with trends observed in HIV programmes in Mozambique, South Africa, Rwanda, and East Africa.7,13,15–17 These findings provide evidence for the effectiveness of our programme in reaching and enrolling HIV-infected individuals at earlier stages of disease. In addition, as the programme matured, the number of enrolled clients increased annually until 2012, possibly reflecting improved access to our services, and increased community confidence of the benefits of HIV testing and treatment.
With each successive year, collection rates for CD4+ cell count and haemoglobin data declined. Overall, 49 and 17% of patients did not have haemoglobin and CD4+ cell counts documented at enrolment, respectively. This finding is problematic, as CD4+ cell count is a determining factor in ART initiation,18,19 and haemoglobin testing is important in establishing the presence of anaemia, a common side effect of ZDV-containing regimens (a standard regimen in our population).20,21 We posit possible explanations for this finding. First, changes in programme leadership in the second year of the programme likely reduced the intensity of logistics coordination for laboratory testing, including CD4+ cell count and haemoglobin. Second, we transitioned experienced FGH clinicians away from direct patient care to technical oversight in the second year of programme implementation, which resulted in less experienced clinicians taking on more direct patient care. Third, it is possible that CD4+ and haemoglobin testing was performed but data not logged in due to large workloads/inability of data entry staff to stay on top of increasing patient volume. These factors could have cumulatively affected the quality of services provided, including data collection and completion rates. Structural barriers associated with clients travelling long distances to access testing services could also play a role. Prior to the end of the 5-year project, we installed point-of-care CD4+ counters in several small feeder sites in order to enable clients to access CD4+ testing services closer to their homes.22,23
Limitations of the study
One of our study's limitations is our inability to determine eligibility for patients missing CD4+ cell counts with WHO stage I and II disease (and III before June 2010), whereas this determination was straightforward for patients having WHO stage IV disease (and III after June 2010) who were all eligible for treatment. Since those patients without CD4+ counts were not included in the denominator, the proportion of patients that we report as ART eligible could be an overestimate. A second limitation is that our findings are drawn from five clinics in rural north central Nigeria, and may therefore not necessarily be generalisable to other parts of Nigeria or Sub-Saharan Africa. Third, the sizeable proportion of missing data may compromise the validity of some of the conclusions we draw here. Although we estimated 1 year retention at 70%, we are unable to definitively assess mortality in our cohort because of poor ascertainment of loss to follow-up, transfer, and death. Nevertheless, our findings provide valuable insight into patient characteristics and trends in patient enrolment into HIV care and treatment in a “real world” rural setting in Africa's most populated country.
Conclusions and Implications
Trends in programme characteristics and enrolment from our experience implementing a comprehensive HIV care programme in rural north-central Nigeria present mixed results. Whereas impressive gains in enrolment scale up occurred over 5 years (>10-fold increase in enrolled patients), the proportions of patients receiving essential clinical monitoring (CD4+ cell count and haemoglobin), which is vital to effective monitoring and evaluation of programme impact, remained suboptimal. Our results highlight the magnitude of challenges facing the successful attainment of goals outlined in the 5-year PEPFAR–GON (Government of Nigeria) partnership framework. The rapid scale-up of HIV programmes in Nigeria is achievable, but maintaining the quality of data collection and documentation is difficult. Addressing the HIV epidemic in resource-constrained settings such as ours requires programmes that are able to scale up quickly and yet remain able to maintain quality of data collection and documentation. Lessons learnt from our experience will contribute to developing such programmes in analogous settings.
Disclaimer Statements
Contributors All authors contributed to this article.
Funding National Institutes of Health (no. NIH R01HD075075, KL2TR000445).
Conflicts of interest No conflicts of interest.
Ethics approval Ethical approval obtained from Vanderbilt University IRB.
Acknowledgements
The authors acknowledge the contributions of the following persons to the implementation of Vanderbilt's HIV programme in Nigeria: Julie Lankford, Ibrahim Sodangi, Robb Reed, Adiba Hassan and Dr. Saidu Ishaq. This publication was made possible by support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health, award number R01HD075075. The findings and conclusions in this paper are those of the author(s) and do not necessarily represent the official position of the National Institutes of Health. CMA is supported in part by Clinician and Translational Science Award (CTSA)/Vanderbilt Clinical &Translational Research Scholars grant (KL2TR000445).
References
- 1.UNAIDS. Special report update: how Africa turned AIDS around. 2013. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/20130521_Update_Africa.pdf (accessed 2014 May 15)
- 2.National Agency for the Control of AIDS (NACA) Federal Republic of Nigeria Global AIDS Response. Country Progress Report, Nigeria, GARPR 2012. Abuja, Nigeria. 2012. Available from: http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/Nigeria%202012%20GARPR%20Report%20Revised.pdf.
- 3.The U.S. President's Emergency Plan for AIDS Relief (PEPFAR) Partnership Framework on HIV/AIDS, 2010–2015. A Memorandum of understanding between the Government of Nigeria and the United States Government to Fight HIV/AIDS in Nigeria. 2014. Available from: http://www.pepfar.gov/documents/organization/148812.pdf (accessed 2014 June 11)
- 4.Measure DHS/ICF International. Nigeria demographic and health survey, 2013. Preliminary Report. Calverton, MD, USA: National Population Commission; 2013. [Google Scholar]
- 5.Federal Ministry of Health, Nigeria (FMOH) National AIDS/STI Control Program. Technical Report 2010 National HIV Sero-prevalence Sentinel Survey. Abuja, Nigeria: Federal Ministry of Health; 2010. Available from: http://www.nigeriaaids.org/documents/2010_National%20HIV%20Sero%20Prevalence%20Sentinel%20Survey.pdf (accessed 2014 June 1) [Google Scholar]
- 6.Aliyu MH, Blevins M, Parrish DD, Megazzini KM, Gebi UI, Muhammad MY et al. Risk factors for delayed initiation of combination antiretroviral therapy in rural North Central Nigeria. J Acquir Immune Defic Syndr. 2014;65((2)):e41–9. doi: 10.1097/QAI.0b013e31829ceaec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geng EH, Hunt PW, Diero LO, Kimaiyo S, Somi GR, Okong P et al. Trends in the clinical characteristics of HIV-infected patients initiating antiretroviral therapy in Kenya, Uganda and Tanzania between 2002 and 2009. J Int AIDS Soc. 2011;14:46. doi: 10.1186/1758-2652-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Federal Ministry of Health, Nigeria (FMOH) National guidelines for HIV and AIDS treatment and care in adolescents and adults. Abuja, Nigeria: Federal Ministry of Health; 2007. [Google Scholar]
- 9.Abimiku AG. Institute of Human Virology, University of Maryland School of Medicine PEPFAR Program (AIDS Care Treatment in Nigeria [ACTION]). Building laboratory infrastructure to support scale-up of HIV/AIDS treatment, care, and prevention: in-country experience. Am J Clin Pathol. 2009;131((6)):875–86. doi: 10.1309/AJCPELMG6GX6RQSM. [DOI] [PubMed] [Google Scholar]
- 10.Cornell M, Grimsrud A, Fairall L, Fox MP, van Cutsem G, Giddy J et al. International Epidemiologic Databases to Evaluate AIDS Southern Africa (IeDEA-SA) Collaboration. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002-2007. AIDS. 2010;24((14)):2263–70. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UNAIDS. Regional fact sheet 2012. 2014. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/2012_FS_regional_ssa_en.pdf (accessed 2014 June 11)
- 12.Centers for Disease Control and Prevention (CDC) Differences between HIV-Infected men and women in antiretroviral therapy outcomes – six African countries, 2004-2012. Morb Mortal Wkly Rep. 2013;62((47)):945–52. [PMC free article] [PubMed] [Google Scholar]
- 13.Lahuerta M, Wu Y, Hoffman S, Elul B, Kulkarni SG, Remien RH et al. Multi-level determinants of late ART initiation in sub-Saharan Africa Team and identifying optimal models of HIV Care in sub-Saharan Africa Collaboration. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006-2011: findings from four sub-saharan African countries. Clin Infect Dis. 2014;58((3)):432–41. doi: 10.1093/cid/cit724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R et al. International epidemiologic databases to evaluate AIDS Southern Africa Collaboration. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med. 2012;9((9)):e1001304. doi: 10.1371/journal.pmed.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon TD, Burlison JR, Blevins M, Shepherd BE, Baptista A, Sidat M et al. Enrolment and programmatic trends and predictors of antiretroviral therapy initiation from President's Emergency Plan for AIDS Relief (PEPFAR)-supported public HIV care and treatment sites in rural Mozambique. Int J STD AIDS. 2011;22:621–7. doi: 10.1258/ijsa.2011.010442. [DOI] [PubMed] [Google Scholar]
- 16.Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96((4)):315–20. [PubMed] [Google Scholar]
- 17.Rich ML, Miller AC, Niyigena P, Franke MF, Niyonzima JB, Socci A et al. Excellent clinical outcomes and high retention in care among adults in a community-based HIV treatment program in rural Rwanda. J Acquir Immune Defic Syndr. 2012;59((3)):e35–e42. doi: 10.1097/QAI.0b013e31824476c4. [DOI] [PubMed] [Google Scholar]
- 18.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF et al. Enhanced CD4+T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368((3)):218–30. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker JD, Bien CH, Easterbrook PJ, Doherty MC, Penazzato M, Vitoria M et al. Optimal strategies for monitoring response to antiretroviral therapy in HIV-infected adults, adolescents, children and pregnant women: a systematic review. AIDS. 2014;28(Suppl 2)):S151–60. [PubMed] [Google Scholar]
- 20.Sartorius BK, Chersich MF, Mwaura M, Meda N, Temmerman M, Newell ML et al. Maternal anaemia and duration of zidovudine in antiretroviral regimens for preventing mother-to-child transmission: a randomized trial in three African countries. BMC Infect Dis. 2013;13:522. doi: 10.1186/1471-2334-13-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parrish DD, Blevins M, Megazzini KM, Shepherd BE, Mohammed MY, Wester CW et al. Haemoglobin recovery among HIV-1 infected patients on zidovudine-based antiretroviral therapy and other regimens in north-central Nigeria. Int J STD AIDS. 2014;25((5)):355–9. doi: 10.1177/0956462413506887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jani SitoeNE, IV, Alfai ER, Chongo PL, Quevedo JI, Rocha BM et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378:1572–9. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 23.Aliyu MH, Blevins M, Audet C, Shepherd BE, Hassan A, Onwujekwe O et al. Optimizing PMTCT service delivery in rural North-Central Nigeria: protocol and design for a cluster randomized study. Contemp Clin Trials. 2013;36((1)):187–97. doi: 10.1016/j.cct.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]