Summary
Within a 3D tissue, cells need to integrate signals from growth factors, such as BMPs, and the extracellular matrix (ECM) to coordinate growth and differentiation. Here, we use the Drosophila embryo as a model to investigate how BMP responses are influenced by a cell’s local ECM environment. We show that integrins, which are ECM receptors, are absolutely required for peak BMP signaling. This stimulatory effect of integrins requires their intracellular signaling function, which is activated by the ECM protein collagen IV. Mechanistically, integrins interact with the BMP receptor and stimulate phosphorylation of the downstream Mad transcription factor. The BMP-pathway-enhancing function of integrins is independent of focal adhesion kinase, but it requires conserved NPXY motifs in the β-integrin cytoplasmic tail. Furthermore, we show that an α-integrin subunit is a BMP target gene, identifying positive feedback between integrin signaling and BMP pathway activity that may contribute to robust cell fate decisions.
Graphical Abstract
Highlights
-
•
Drosophila embryos lacking integrin function have disrupted BMP responses
-
•
Collagen IV activates integrin signaling to enhance levels of the pMad transducer
-
•
Integrins bind BMP receptors and promote pMad levels after BMP receptor activation
-
•
BMP activates expression of an α-integrin, representing a positive feedback loop
During development, cells receive information from the extracellular matrix via integrin receptors, in addition to growth factor signals from other cells. Sawala et al. show that BMP-responsive transcription in the Drosophila embryo is not simply a readout of the BMP concentration but also requires integrin-mediated enhancement of BMP signal transduction.
Introduction
Two key requirements for the success of multicellular life are the ability of cells to adhere to each other, via a secreted protein network called the extracellular matrix (ECM), and to communicate with each other, sometimes over long distances, through the release of signaling molecules. In addition to providing structural support to tissues, the ECM has evolved to regulate intercellular signaling pathways, for example by binding to growth factors and regulating their distribution or activity in the extracellular space (Hynes, 2009). The ECM can also signal through its own adhesion receptors, primarily integrins, to initiate intracellular signaling events that converge with growth factor signaling pathways, thus allowing cells to integrate information about ECM composition and mechanical properties with biochemical signals (Giancotti and Ruoslahti, 1999).
Although crosstalk between integrin signaling and growth-factor-activated receptor tyrosine kinase signaling has been studied in some detail (Alam et al., 2007), comparably little is known about how the ECM influences intracellular signaling through bone morphogenetic proteins (BMPs), a highly conserved family of growth factors with diverse roles during development and disease (Wu and Hill, 2009). In the canonical signaling pathway, BMPs assemble complexes of type I and type II receptors, leading to activation of the type I receptor Ser/Thr kinase domain and phosphorylation of a Smad transcription factor (Mad in Drosophila) (Wu and Hill, 2009). Phospho-Mad (pMad) associates with a second Smad transcription factor (Medea in Drosophila), and the pMad/Medea complex accumulates in the nucleus to regulate transcription of BMP target genes. Using the Drosophila model, we have recently shown that the ECM molecule collagen IV directly binds BMPs and regulates their movement across tissues (Sawala et al., 2012; Wang et al., 2008). Several findings indicate that collagen IV may also act locally to enhance BMP signal reception. For example, collagen IV is required for local activation of BMP signaling at the tip of developing renal tubules (Bunt et al., 2010). Furthermore, collagen IV can enhance the effect of BMPs in tissue culture, where long-range movement is unlikely to be important (Paralkar et al., 1992).
To understand how the local ECM environment impacts BMP responses, we investigated a role for integrins, which are collagen IV receptors (Khoshnoodi et al., 2008). We find that maximal levels of BMP pathway activation in vivo are only achieved in the presence of integrin signaling, which functions downstream of collagen IV to potentiate signaling through the canonical Smad pathway.
Results
Integrins Are Required for Peak Levels of BMP Signaling in the Early Drosophila Embryo
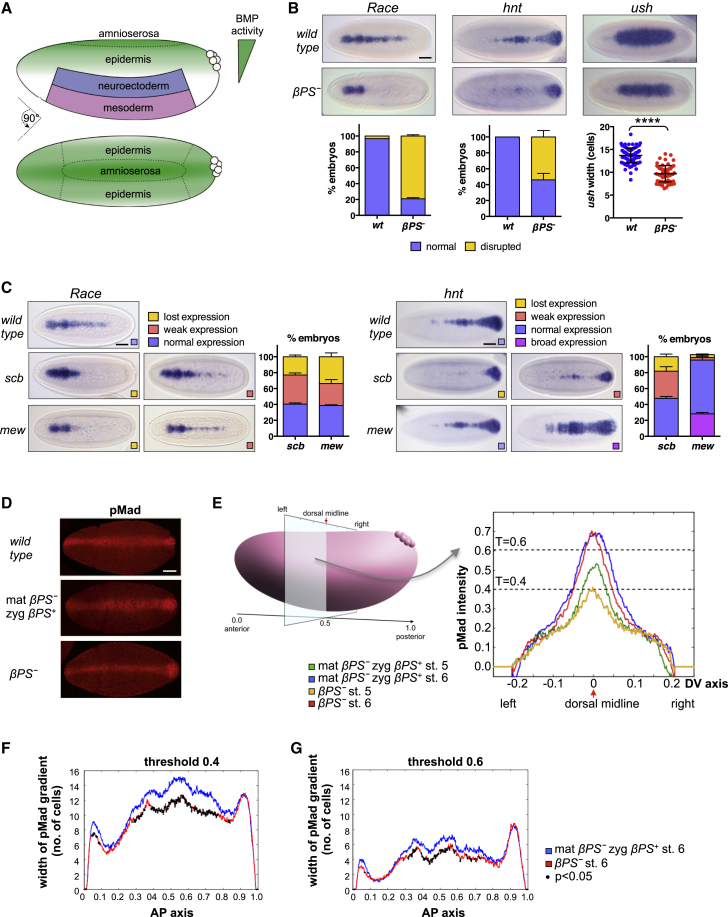
We examined a role for integrins in BMP pathway activation in the early Drosophila embryo, where a BMP activity gradient specifies cell fates along the dorsoventral axis (Figure 1A). Integrin receptors are expressed on the cell surface as α/β heterodimers (Leptin et al., 1989). As βPS is the only β-integrin expressed in the early blastoderm embryo (Figure S1A), we induced homozygous germline clones for a null allele of βPS, mysXG43 (Leptin et al., 1989), and analyzed BMP target gene expression in embryos lacking maternal and zygotic βPS expression and therefore all integrin function (from now on referred to as βPS− embryos). In wild-type embryos, the high threshold BMP target genes Race (Ance in FlyBase) and hindsight (hnt) are expressed in a narrow stripe along the dorsal midline (presumptive amnioserosa), where peak signaling occurs, while the lower threshold gene u-shaped (ush) is expressed in a broader dorsal domain (Figure 1B). By contrast, in βPS− embryos, Race and hnt expression is lost in the presumptive amnioserosa, and ush expression is significantly (p < 0.0001) narrower (Figure 1B), characteristic of embryos with reduced levels of BMP signaling. The BMP defect is rescued by a paternal wild-type copy of βPS (data not shown). Expression of the major BMP ligand in the early embryo, dpp, and of sog, which encodes a critical extracellular BMP regulator (Wu and Hill, 2009), is unaffected in βPS− embryos (Figure S2A). Together, these data indicate that integrins are required for normal levels of BMP signaling in the early embryo.
Figure 1.
Loss of Integrin Expression Causes Defects in BMP Signaling Responses in the Early Embryo
(A) Early Drosophila embryo showing patterning of the dorsal ectoderm by a gradient of BMP activity.
(B) RNA in situ hybridizations of wild-type or maternal/zygotic mysXG43 mutant (βPS−) embryos showing expression of the BMP target genes Race, hnt, and ush. For Race and hnt quantification, n = 3, >15 embryos per genotype in each experiment; error bars represent SEM. For ush width, individual measurements are shown with mean ± SD (n = 92 for wild-type and n = 69 for βPS−). ∗∗∗∗p < 0.0001 (unpaired t test).
(C) RNA in situ hybridizations for Race and hnt in mew (mewM6) and scb (scb5J38) mutant embryos. Phenotypes were classified as “lost,” “weak,” or “broad” (see Supplemental Experimental Procedures and Figure S2B for details) and were counted on embryo samples collected from heterozygous mutant stocks (n = 3, >60 embryos counted per genotype in each experiment; error bars represent SEM).
(D) pMad immunostaining of wild-type, maternal βPS− zygotic βPS+ (mat βPS− zyg βPS+) and maternal/zygotic βPS− (βPS−) embryos. All scale bars represent 50 μm.
(E–G) Quantification of the pMad gradient in mat βPS−, zyg βPS+, and βPS− embryos. (E) Mean pMad intensity along the dorsal-ventral axis at 0.5 embryo length. Threshold lines indicate the width of the pMad gradient plotted in (C) and (D). (F and G) Mean width of the pMad gradient at thresholds 0.4 (C) and 0.6 (D) along the anterior-posterior axis for stage 6 embryos. Black dots indicate significant differences (p < 0.05) between maternal βPS− zyg βPS+ and βPS−.
All embryo images show dorsal views of stage 6 embryos, anterior to the left. See also Figures S1 and S2.
As βPS can form receptors with several α-integrins (Brown, 2000), we examined BMP phenotypes in α-integrin mutant embryos. Out of the five α-integrin genes, we found that multiple edematous wings (mew) and scab (scb) are expressed in partially overlapping domains in the dorsal ectoderm (Figures S1B and S1C), where BMP signaling is active. In addition, mew expression extends ventrally into the neuroectoderm, overlapping with the expression domain of sog (Figure S1D). Embryos mutant for mew or scb exhibit defects in the expression of BMP target genes (Figure 1C), with differences in the phenotypes for mew and scb mutants reflecting their different expression patterns in the embryo. For Race, the expression is preferentially lost in the posterior in scb mutant embryos, whereas it is lost uniformly along the anterior-posterior axis in mew mutants (compare embryos classified as “weak” in Figure 1C). These results suggest that integrin receptors involving both mew and scb gene products enhance BMP signaling in the early embryo. The posteriorly expressed target gene hnt, like Race, is partially or fully lost in the scb mutant but infrequently lost in mew mutants (Figure 1C). Also, in a subset of mew mutants, Race, hnt, or ush expression is broadened, in extreme cases resembling embryos that lack Sog (Figures 1C and S2C). This likely represents a function of mew in regulating Sog distribution, as previously described in the developing wing (Araujo et al., 2003), in addition to its integrin signaling function described below. This second function may lead to a slightly flatter BMP gradient in mew mutants, thus explaining the broader hnt expression observed (Figure 1C), in contrast to the loss of Race, a gene that is absolutely dependent on the highest BMP levels.
We next quantified the pMad gradient in βPS− embryos (Umulis et al., 2010) To circumvent staining variability between samples, we only compared βPS− embryos to siblings that were maternal βPS− zygotic βPS+, as these embryos could be processed together throughout. Maternal βPS− zygotic βPS+ embryos show no defect in BMP target gene expression or pMad activation (Figure 1D; data not shown), making them a valid control. In both βPS− and control embryos, peak pMad levels increase markedly between stage 5 and stage 6 (Figure 1E), consistent with previous reports of pMad gradient dynamics (Rushlow et al., 2001). At stage 5, pMad levels are considerably lower in βPS− embryos than controls, resulting in a narrower gradient and a reduced peak (Figure 1E). At stage 6, peak pMad levels at the dorsal midline reach similar levels in βPS− and control embryos (Figure 1E); however, the dorsal domains over which the gradient reaches a threshold of 0.4 and 0.6 are significantly narrower (Figures 1F and 1G). In wild-type embryos, these thresholds correspond to the width of ush (∼14 cells; see Figure 1A) and Race/hnt (four to six cells; see Figure 3D) expression, respectively, consistent with the reduction in pMad levels leading to disrupted BMP target gene expression in βPS− embryos. Although we cannot rule out additional effects on non-canonical BMP signal transduction or BMP-independent effects, our data demonstrate that integrins are required for the timely and robust formation of the pMad gradient in the early embryo.
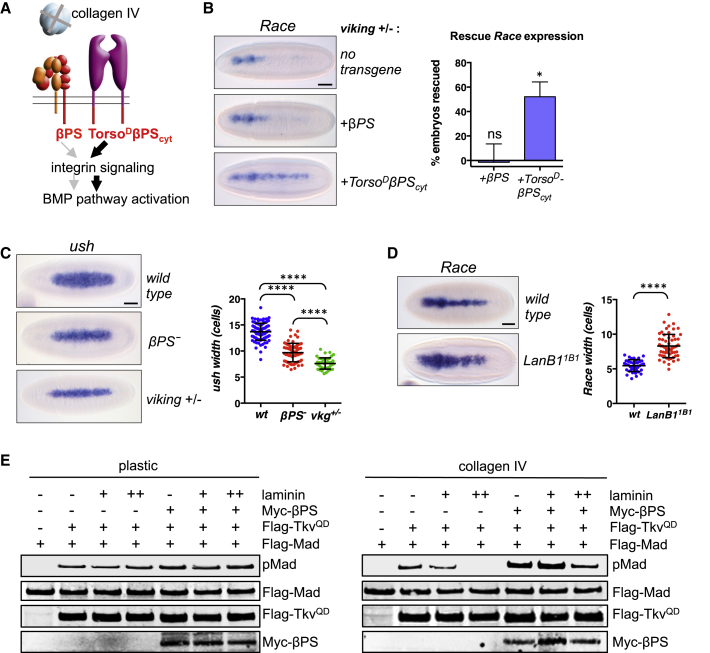
Figure 3.
Activation of Integrin Signaling Can Partially Restore BMP Signaling Defects in Collagen IV Mutant Embryos
(A) Diagram showing how a potential loss of integrin signaling in collagen IV mutant embryos may reduce BMP signaling (left), which would be predicted to be rescued by constitutively active integrin signaling (right).
(B) Expression of TorsoDβPScyt, but not wild-type βPS, can partially restore expression of Race in collagen IV (viking) mutant embryos. Race expression patterns were classified as normal, weak, or lost (n = 3, >70 embryos counted per genotype in each experiment; error bars represent SEM). Asterisks denote significant difference from no transgene control (i.e., 0% rescue); ∗p < 0.05 (t test). See Supplemental Experimental Procedures for details of rescue quantification.
(C) The ush expression pattern is narrower in collagen IV (viking) mutant embryos than in embryos lacking β integrin (βPS−). ush width shown as individual measurements and mean ± SD, n > 45 for each genotype; ∗∗∗∗p < 0.0001 (ordinary one-way ANOVA).
(D) Maternal/zygotic LanB11B1 mutant embryos show a broadened Race expression pattern. Race width shown as individual measurements and mean ± SD, n > 35 for each genotype; ∗∗∗∗p < 0.0001 (Welch’s test). Scale bars represent 50 μm.
(E) Western blot of pMad and transfected Flag-Mad (total Mad), Flag-TkvQD, and Myc-βPS in S2R+ cells which were plated on either plastic or collagen IV and treated with increasing levels of laminin, as indicated.
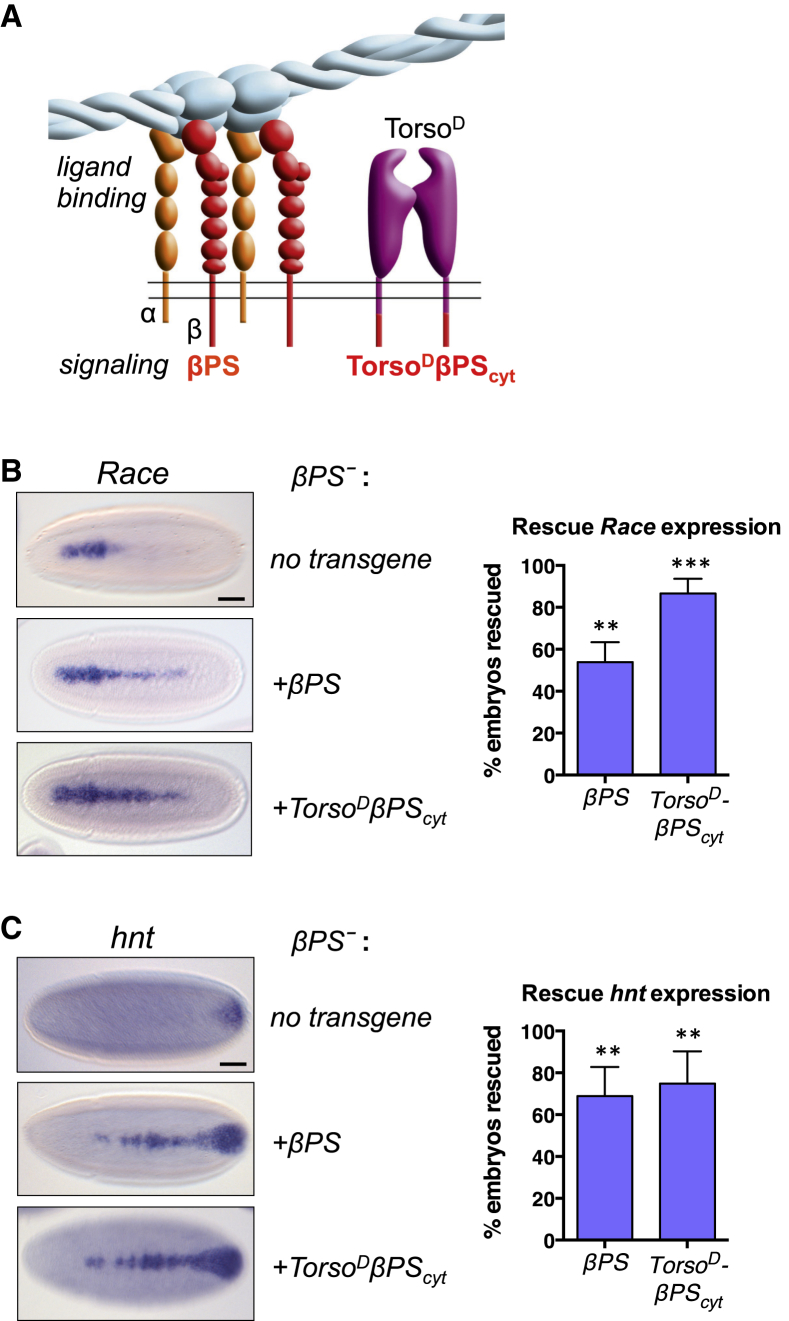
The Signaling Function of Integrins Is Sufficient for Their Role in BMP Pathway Activation
To test if integrins exert their effect on BMP signaling through their extracellular ligand binding activity or by activating intracellular signaling pathways, we made use of a chimeric receptor, TorsoDβPScyt, in which the cytoplasmic domain of βPS is fused to the transmembrane and extracellular domains of a constitutively active form of the Torso receptor (Martin-Bermudo and Brown, 1999). This integrin signaling construct is unable to bind integrin ligands, but it is capable of signaling due to TorsoD-mediated clustering of the βPS cytoplasmic domain (Figure 2A). We used the Gal4/UAS-system to express wild-type βPS or TorsoDβPScyt transgenes in βPS− embryos and tested for their ability to rescue the BMP phenotype. Zygotic expression of wild-type βPS restores Race expression in ∼50% of embryos (Figure 2B). Rescue in only half of the embryos likely reflects the proportion expressing the Gal4 transcription factor maternally and zygotically, as opposed to maternally only. The TorsoDβPScyt integrin signaling construct was also able to rescue Race and hnt induction in βPS− embryos (Figures 2B and 2C), demonstrating that the signaling function of integrins is sufficient for their role in promoting BMP signaling. Overexpression of βPS or TorsoDβPScyt in a wild-type background does not broaden Race or hnt expression (Figure S3), suggesting that whereas integrin signaling is required to augment BMP signaling, it is not limiting with respect to this function in a wild-type context. We conclude that integrin signaling is permissive for generating peak levels of BMP pathway activation.
Figure 2.
Integrin Signaling Is Sufficient to Rescue the BMP Phenotype in βPS− Integrin Mutant Embryos
(A) TorsoDβPScyt construct, which can mimic integrin signaling in the absence of ligand binding.
(B and C) Overexpression of βPS and of TorsoDβPScyt in maternal/zygotic mysXG43 mutant (βPS−) embryos can rescue expression of the peak threshold BMP target genes Race (B) and hnt (C). Rescue was quantified as the percentage increase in embryos with a wild-type expression pattern relative to a no transgene control (n = 3, > 50 embryos counted per genotype in each experiment; error bars are SEM). For details, see Supplemental Experimental Procedures. Asterisks denote significant difference from no transgene control (i.e., 0% rescue); ∗∗p < 0.01, ∗∗∗p < 0.001 (t test). Scale bars represent 50 μm.
See also Figure S3.
Integrin Signaling Acts Downstream of Collagen IV to Enhance BMP Activity
We have shown previously that collagen IV mutant embryos have disrupted BMP signaling due to an altered extracellular BMP distribution (Wang et al., 2008). If collagen IV acts as an integrin ligand, a loss of integrin signaling may contribute to the defect in BMP signaling observed in collagen IV mutant embryos (Figure 3A). To test this, we examined whether TorsoDβPScyt, which induces integrin signaling independent of ECM ligands, can rescue BMP target gene expression in embryos with reduced levels of collagen IV (Figure 3A), due to a mutation in viking, one of two collagen IV genes in Drosophila (Wang et al., 2008). As shown in Figure 3B, overexpression of TorsoDβPScyt restores Race induction in ∼50% of embryos, whereas wild-type βPS, which requires an ECM ligand for activation of signaling, has no effect. This result suggests that integrin signaling may act downstream of collagen IV to enhance BMP activity. The partial rescue of Race expression observed with TorsoDβPScyt in collagen IV mutant embryos, as compared to βPS− embryos (Figure 2B), is consistent with a dual role for collagen IV in promoting BMP signaling in the early embryo: to activate integrin signaling and promote extracellular BMP gradient formation (Sawala et al., 2012; Wang et al., 2008). In agreement with this, a reduction in collagen IV leads to a more severe decrease in BMP signaling than the loss of integrins, based on the width of ush expression (Figure 3C).
To exclude the possibility that the loss of integrin signaling in collagen IV mutants is due to a general loss of ECM integrity, we examined BMP signaling in embryos lacking expression of laminin, an ECM protein that interacts with the collagen IV network and can act as an integrin ligand in Drosophila (Brown, 2000). In contrast to integrin and collagen IV mutants, embryos lacking β-laminin show a variable expansion of the Race expression domain (Figure 3D). We speculate that this expanded Race expression is due to competition between collagen IV and laminin for binding to integrins, with only collagen IV interactions leading to BMP-promoting signaling events. In support of this, we find that addition of laminin to Drosophila cells plated on a collagen IV substrate, but not when plated on plastic, inhibits pMad accumulation in a dose-dependent manner following pathway stimulation, and this effect is reduced by co-transfecting additional βPS (Figure 3E, right panel). Together, these results suggest that engagement of integrin receptors by collagen IV, but not laminin, promotes BMP responses in the early Drosophila embryo.
Integrin Signaling Acts Downstream of BMP Receptor Activation to Enhance pMad Accumulation
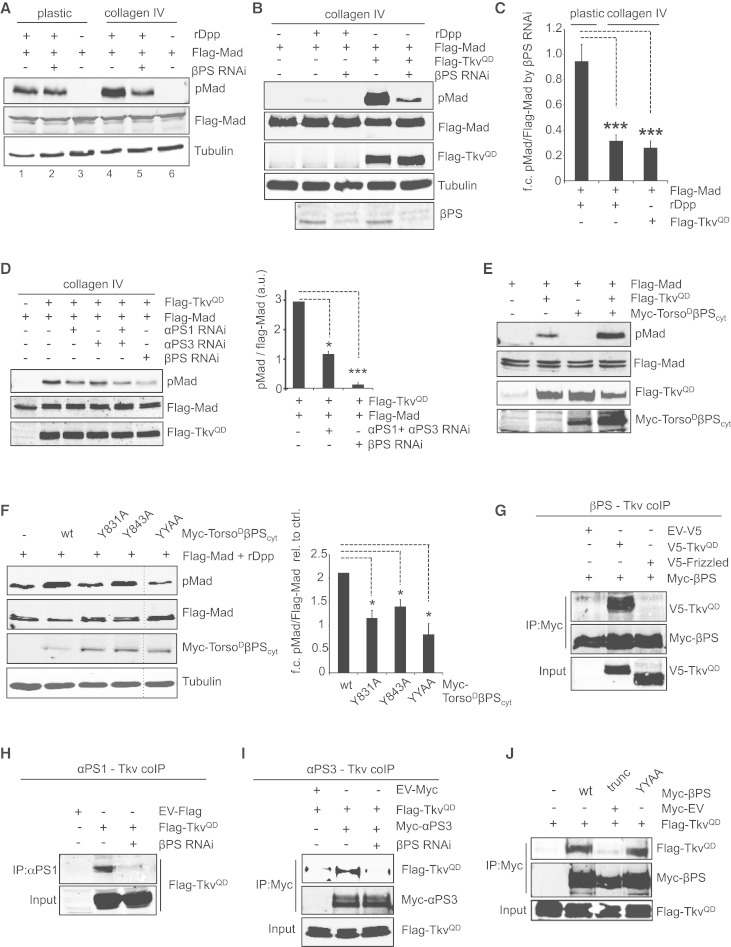
We next investigated the molecular link between integrin signaling and enhanced BMP signal transduction. A key signaling protein linking integrin to growth factor pathways is focal adhesion kinase (FAK) (Giancotti and Ruoslahti, 1999). However, we did not detect any defect in BMP signaling in embryos lacking Drosophila FAK (Figure S4A), suggesting that integrin signaling enhances BMP signal transduction through an FAK-independent mechanism.
To further dissect this mechanism, we developed a Drosophila tissue culture assay. Treatment of S2R+ cells with the BMP ligand Dpp induces pMad, and this is enhanced when cells are plated on collagen IV (Figure 4A). RNAi-mediated knockdown of βPS abolishes the stimulatory effect of collagen IV on pMad induction, whereas it has no effect on pMad levels in plastic-plated cells (Figures 4A and 4C). As shown in Figures 4B and 4C, βPS RNAi in collagen IV-plated cells also reduces pMad induction by a constitutively active form of the BMP receptor Thickveins (Tkv), TkvQD, indicating that collagen IV/integrin signaling acts downstream of Tkv activation. The effects of collagen IV/integrins on pMad levels do not coincide with changes in the levels of total Mad (transfected Flag-Mad) (Figures 4A and 4B), suggesting that they are not mediated via an effect on Mad stability. In terms of the α-integrin requirement, knockdown of both mew and scb together, but not either subunit alone, also reduces pMad accumulation (Figure 4D), suggesting that both α-integrins can function with βPS to stimulate pMad.
Figure 4.
Molecular Mechanism of Integrin-Signaling-Mediated Enhancement of the BMP Pathway
(A) Western blot for Flag-Mad, pMad, and tubulin in S2R+ cells transfected with Flag-Mad, which were plated on either plastic or collagen IV and treated with rDpp and βPS RNAi, as indicated.
(B) Western blot for Flag-Mad, pMad, Flag-TkvQD, βPS, and tubulin in cells transfected with Flag-Mad and plated on collagen IV. BMP signaling was activated by co-transfection of Flag-TkvQD or treatment with rDpp, and cells were treated with βPS RNAi as indicated.
(C) Quantification of the experiments in (A) and (B), showing fold reduction in pMad activation by βPS RNAi on plastic- or collagen-IV-plated cells. pMad levels were normalized to total Mad (Flag-Mad) in each sample. n = 3–6; ∗∗∗p < 0.001 (one-way ANOVA).
(D) Western blot as described in (B) with quantitation, but cells were also treated with αPS1 and/or αPS3 RNAi.
(E) Western blot for Flag-Mad, pMad, Flag-TkvQD, and Myc-TorsoDβPScyt in cells transfected with Flag-Mad, Flag-TkvQD, and Myc-TorsoDβPScyt and plated on plastic.
(F) As in (E), except cells were transfected with Flag-Mad and Myc-tagged wild-type or mutant TorsoDβPScyt, and treated with rDpp. Quantitation shows increase in pMad (n = 4); ∗p < 0.05, ∗∗p < 0.001 (paired t tests).
(G–I) Co-immunoprecipitation experiments between Flag-TkvQD and the βPS (G), αPS1 (H), and αPS3 (I) integrin subunits. Frizzled is a negative control.
(J) Co-immunoprecipitation between Flag-TkvQD and Myc-βPS forms with truncation of the cytoplasmic tail or both NPXY motifs mutated.
All error bars show SEM. See also Figure S4.
Consistent with our in vivo rescue experiments (Figures 2 and 3), the positive effect of collagen IV/integrins on both Dpp- or TkvQD-induced pMad accumulation can be mimicked by expression of TorsoDβPScyt (Figures 4E and 4F), confirming that integrin signaling mediates the effect on pMad observed in S2R+ cells and demonstrating that integrin signaling acts cell autonomously to enhance BMP responses. We next used the TorsoDβPScyt construct to identify residues in the βPS cytoplasmic domain required for integrin-enhanced pMad accumulation. Two conserved NPXY motifs in the β-integrin cytoplasmic tail are important for the recruitment of a variety of integrin-binding proteins (Legate et al., 2009). Mutation of tyrosine in the first (Y831) or second (Y843) NPXY motif to alanine, either alone or in combination, did not affect expression levels of TorsoDβPScyt (Figure 4F) but compromised its ability to promote pMad phosphorylation (Figure 4F). These results suggest that both NPXY motifs in the βPS cytoplasmic tail are important for its enhancement of BMP signaling.
To gain further mechanistic insight, we tested if integrin receptors interact with Tkv. Indeed, we found that Tkv, but not a control transmembrane protein (Frizzled), can co-immunoprecipitate with βPS, mew (αPS1) and scb (αPS3) (Figures 4G–4I). The interaction between αPS1 or αPS3 and Tkv is abrogated by βPS RNAi (Figures 4H and 4I), consistent with integrin surface presentation being dependent on functional integrin receptors (Leptin et al., 1989). We next truncated the βPS cytoplasmic tail after residues involved in stabilization of the integrin α-β heterodimer (Figure S4B). A similar truncation in mammalian β3 integrin does not disrupt cell-surface expression (O’Toole et al., 1991), a result we confirmed for our βPS-trunc construct (Figure S4C). As shown in Figure 4J, truncation of the βPS cytoplasmic tail abrogates the interaction with Tkv, suggesting that the integrin-Tkv interaction is mediated intracellularly and therefore could be relevant for the integrin-mediated enhancement of Tkv-mediated Mad phosphorylation. By contrast, mutation of the NPXY motifs in the βPS did not impair its binding to Tkv (Figure 4J). As the NPXY motifs are required for integrin-signaling mediated enhancement of pMad (Figure 4F), this suggests that binding of integrins to Tkv alone is not sufficient to enhance pMad accumulation (see Discussion). Together, our data suggest that integrin receptors can form complexes with BMP receptors, enhancing their ability to phosphorylate Mad.
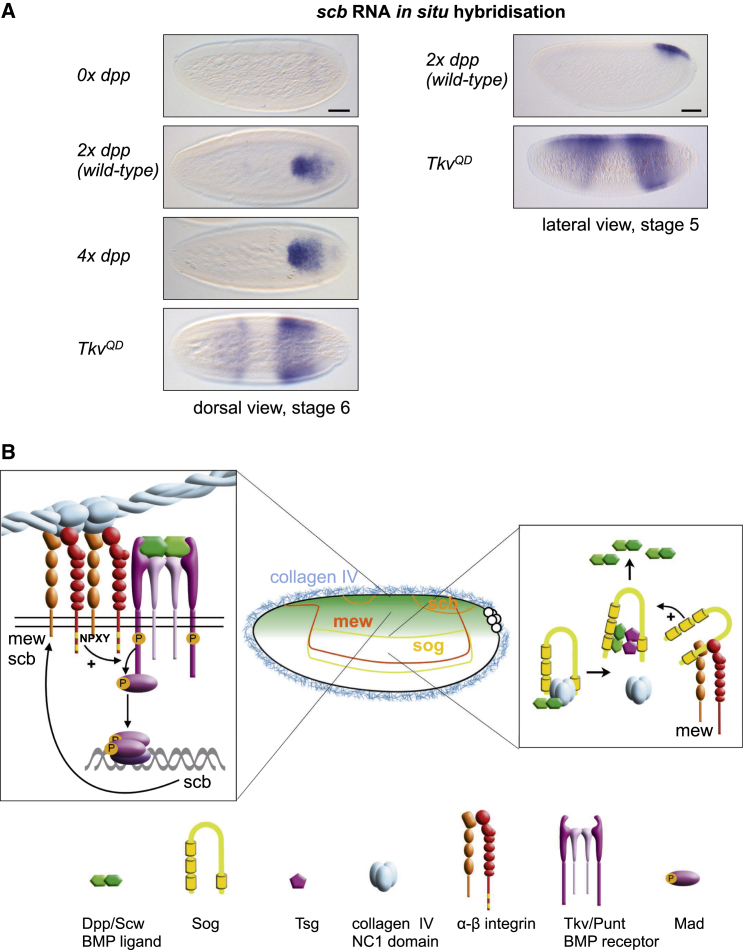
The α-Integrin Subunit scb/αPS3 Is Itself a BMP Target Gene
The spatial and temporal expression pattern of scb is consistent with this α-integrin encoding gene being positively regulated by BMP signaling. Therefore we examined the scb expression pattern in embryos with increased and decreased levels of BMP signaling. We found that scb expression is expanded ventrally in embryos with four copies of dpp or overexpressing the activated BMP receptor, tkvQD, and lost in dppHin37 mutant embryos (Figure 5A). The lack of scb expression in the central region of the embryo can be explained at least in part by a repressive input from the gap gene transcription factor Krüppel, as scb expression expands into the central region in Krüppel mutant embryos (Figure S5). Overall, these data demonstrate that scb is indeed a BMP target gene and identify a positive feedback loop in which BMP-dependent induction of scb expression further enhances BMP signal transduction via the activation of integrin signaling.
Figure 5.
A Positive Feedback Loop Potentiates Integrin-BMP Synergy
(A) RNA in situ hybridizations for scb in wild-type embryos and in embryos with altered levels of BMP signaling. Scale bars represent 50 μm.
(B) Model of integrin-BMP synergy. See Discussion for details.
See also Figure S5.
Discussion
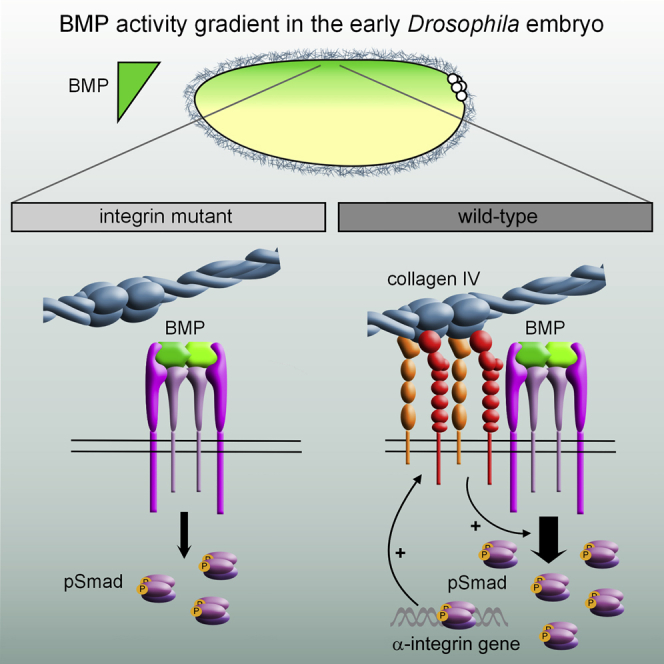
Our study reveals that BMP-responsive transcription during Drosophila embryonic dorsal-ventral axis patterning is not simply a readout of the BMP signal but instead requires synergy between BMP and integrin signaling. Our data support a model whereby collagen IV activation of integrin signaling augments pMad levels (Figure 5B). Consistent with collagen IV acting as an integrin ligand, which is well documented in vertebrates (Khoshnoodi et al., 2008), collagen-IV-directed rotations of follicle epithelia during Drosophila oogenesis also require integrins (Haigo and Bilder, 2011). Thus, we envisage two functions for collagen IV in regulating BMP signaling (Figure 5B): (1) collagen IV shapes the BMP gradient through a direct collagen IV-Dpp interaction, as we have described previously (Sawala et al., 2012; Wang et al., 2008); and (2) collagen IV activates integrin signaling, which amplifies the BMP signal by forming complexes with BMP receptors and enhancing induction of pMad. In addition, we show specificity for collagen-IV-induced integrin activation, as laminin, which can bind both αPS1- and αPS3-containing integrin receptors (Brown, 2000; Schöck and Perrimon, 2003), does not promote BMP responses, possibly due to activation of a distinct signaling complex that does not increase pMad. Instead, our data support competition between collagen IV and laminin for integrin binding, suggesting that the relative levels of laminin, collagen IV, and integrins in a particular developmental context will impact the extent of BMP pathway activation.
Mechanism of Integrin-Enhanced BMP Signal Transduction
Our data indicate that collagen IV/integrin signaling in the early embryo promotes activation of canonical Smad-dependent signal transduction in a mechanism that is independent of FAK but may involve an association of integrins and BMP receptors. Several recent studies have also reported interactions between integrins and BMP receptors, leading to inhibition of Smad phosphorylation (North et al., 2015) or enhanced Smad phosphorylation either via a BMP-ligand-independent, shear-stress-induced activation of FAK/ERK MAPK signaling (Zhou et al., 2013) or via extracellular domain interactions that increase the ligand binding affinity of the BMP receptor (Tian et al., 2012). Our data show that collagen IV/integrin signaling enhances pMad levels via a distinct mechanism, as it is independent of FAK and involves intracellular domain interactions downstream of BMP receptor activation.
We identify two conserved NPXY motifs in the βPS cytoplasmic domain as important sites for integrin-BMP synergy, but these motifs are not required for βPS binding to Tkv, suggesting that binding of integrins to BMP receptors is not sufficient for promoting Mad phosphorylation. One possibility is that the NPXY motifs are required for the recruitment of factors that facilitate or stabilize Mad phosphorylation. NPXY motifs are also involved in regulating the endocytosis and recycling of integrin receptors (Margadant et al., 2012). As some evidence suggests that Smad phosphorylation by BMP receptors occurs preferentially on endosomes (Shi et al., 2007), integrin-facilitated endocytosis and/or recycling of BMP receptors could enhance Mad phosphorylation.
Roles of αPS1/mew and αPS3/scb in Integrin-Mediated Enhancement of BMP Signaling
Previously, integrins have been implicated in regulating Sog distribution in ovarian follicle cells and the pupal wing (Araujo et al., 2003; Negreiros et al., 2010). Although the low penetrance sog mutant-like phenotype we observed in mew mutant embryos is also consistent with a function of mew/αPS1 in modulating Sog levels (Figure 5B), the major role of integrins in the early embryo appears to involve their signaling function, as we can rescue the integrin-null phenotype with the TorsoDβPScyt signaling-activated transgene. Consistent with this, our RNAi knockdown data suggest that integrin signaling through both αPS1 and αPS3 can promote Tkv-mediated Mad phosphorylation and both αPS1 and αPS3 bind Tkv. In the early embryo, due to their different expression patterns and their distinct additional roles of promoting Sog function (αPS1) or providing positive feedback on BMP signaling (αPS3), we expect αPS1 and αPS3 to act only partially redundantly. Indeed, we observe disrupted BMP target gene expression in mew and scb single mutants, with distinct spatial pattern of Race defects. As BMP target gene expression in the central region of the embryo (expressing only αPS1) is more sensitive than the regions in the anterior and posterior (expressing both αPS1 and αPS3) to genetic perturbations, such as overexpression of Sog (Winstanley et al., 2015), we speculate that integrin signaling through both αPS1 and αPS3 may contribute to robustness of the pMad gradient in vivo.
Integrin Signaling as a Positive Feedback Loop for BMP Activity
Our data reveal that expression of scb is induced by BMP signaling in the early embryo, identifying a positive feedback loop in which activation of scb allows integrin signaling, which further increases BMP responses at the dorsal midline. Positive feedback, involving a transcriptional mechanism, has previously been implicated in the conversion of the BMP activity gradient into a spatially bistable pattern of gene expression that subdivides the dorsal ectoderm into distinct tissues (Wang and Ferguson, 2005). Recently, the tumor necrosis factor α ligand eiger has been identified in the positive feedback circuit, but additional genes are thought to be involved (Gavin-Smyth et al., 2013). scb may represent such an additional BMP target gene.
In summary, our findings reveal that interpretation of the BMP embryonic morphogen gradient requires not only a response to the extracellular BMP concentration but also integrin-mediated enhancement through positive feedback and integration of context-specific information from the ECM. We predict that the integrin-BMP synergy identified here may have wide-ranging implications during other development or disease contexts where the functions of BMPs, collagen IV, and integrins converge, such as stem cell fate decisions or angiogenesis.
Experimental Procedures
Fly Strains, Crosses, Embryo Collection, and Phenotype Analysis
Embryos were collected, aged, and fixed with formaldehyde using standard procedures. Details of fly strains, crosses, and phenotype analysis are provided in Supplemental Experimental Procedures.
RNA In Situ Hybridization and Immunostaining
RNA in situ hybridizations were performed using digoxigenin-UTP-labeled exonic RNA probes. Fluorescent RNA in situ hybridizations were performed as previously described (Kosman et al., 2004) using digoxigenin-UTP-labeled probes for exon 5 of mew and full-length sog and a biotin-UTP-labeled probe for exon 3 of scb. Antibodies used were sheep anti-digoxigenin (1:400, Roche), mouse anti-biotin (1:400, Invitrogen), donkey anti-mouse-immunoglobulin G (IgG)-Alexa 488 (1:500, Invitrogen), and donkey anti-sheep-IgG-Alexa 555 (1:500, Invitrogen). For immunostaining, antibodies used were rabbit anti-pMad (1:250, gift from P. ten Dijke), chicken anti-β-Gal (1:1,000, Abcam), anti-rabbit-IgG-Alexa 594 (1:400, Invitrogen), and anti-chicken-IgG-488 (1:400, Invitrogen). Immunostained embryos were mounted in Prolong (Invitrogen). Detailed protocols are available on request. For details of pMad quantification, see Supplemental Experimental Procedures.
Drosophila S2 Cell Culture Experiments
Drosophila S2R+ cells were cultured at 25°C in insect medium (PAA Laboratories) with 10% FBS (PAA Laboratories) and 1% penicillin/streptomycin, plated on human collagen IV or plastic, and transiently transfected using Effectene (QIAGEN). Where appropriate, expression was induced with 500 μΜ CuSO4 24 hr post-transfection. Cells were harvested 72h after transfection or Cu-induction and lysed in NP40 buffer (150 nM NaCl, 20 mM Tris-Cl [pH 8], 1% NP-40, EDTA, 0.1% protease and phosphatase inhibitor) for SDS-PAGE/western blotting. For RNAi treatment, cells were serum-starved for 1 hr and treated with 10 μg/ml double-stranded RNA for 2 hr at 25°C, then 10% FBS was added to the medium. For stimulation with Dpp, transfected cells were serum-starved for 1 hr, followed by addition of 3 nM recombinant Dpp (rDpp) (R&D Systems) for 2 hr before lysis. Protein bands were quantified using Li-COR Imager (Li-COR Biosciences) and ImageJ. For details of DNA transfections, collagen IV plating, laminin competition, co-immunoprecipitation, and immunofluorescence experiments, see Supplemental Experimental Procedures.
Author Contributions
A.S. designed and performed in vivo experiments, analyzed the data, and wrote the manuscript. M.S. performed tissue culture experiments and analyzed the data. C.S. generated some DNA/RNA reagents and performed some in situ hybridizations. S.G.W. performed fluorescent in situ hybridization experiments. H.L.A. designed experiments, analyzed the data, and wrote the manuscript.
Acknowledgments
We thank David Umulis (Purdue University) for the pMad quantification and Pat Caswell for helpful discussions. We also thank Nick Brown, Ruth Palmer, Norbert Perrimon, Peter ten Dijke, and Jean-Paul Vincent for DNA, antibodies, or fly stocks; Lisa Deignan for providing dpp mutant and tkvQD-overexpressing embryos; and Victoria Coyne for providing Kr mutant embryos. This work was supported by a Wellcome Trust program grant to H.L.A. (092005/Z/10/A) and a Wellcome Trust PhD studentship to A.S. (083271/Z/07/Z).
Published: August 27, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2015.08.012.
Supplemental Information
References
- Alam N., Goel H.L., Zarif M.J., Butterfield J.E., Perkins H.M., Sansoucy B.G., Sawyer T.K., Languino L.R. The integrin-growth factor receptor duet. J. Cell. Physiol. 2007;213:649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- Araujo H., Negreiros E., Bier E. Integrins modulate Sog activity in the Drosophila wing. Development. 2003;130:3851–3864. doi: 10.1242/dev.00613. [DOI] [PubMed] [Google Scholar]
- Brown N.H. Cell-cell adhesion via the ECM: integrin genetics in fly and worm. Matrix Biol. 2000;19:191–201. doi: 10.1016/s0945-053x(00)00064-0. [DOI] [PubMed] [Google Scholar]
- Bunt S., Hooley C., Hu N., Scahill C., Weavers H., Skaer H. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev. Cell. 2010;19:296–306. doi: 10.1016/j.devcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin-Smyth J., Wang Y.-C., Butler I., Ferguson E.L. A genetic network conferring canalization to a bistable patterning system in Drosophila. Curr. Biol. 2013;23:2296–2302. doi: 10.1016/j.cub.2013.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F.G., Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Haigo S.L., Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–1074. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshnoodi J., Pedchenko V., Hudson B.G. Mammalian collagen IV. Microsc. Res. Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosman D., Mizutani C.M., Lemons D., Cox W.G., McGinnis W., Bier E. Multiplex detection of RNA expression in Drosophila embryos. Science. 2004;305:846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
- Legate K.R., Wickström S.A., Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- Leptin M., Bogaert T., Lehmann R., Wilcox M. The function of PS integrins during Drosophila embryogenesis. Cell. 1989;56:401–408. doi: 10.1016/0092-8674(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Margadant C., Kreft M., de Groot D.J., Norman J.C., Sonnenberg A. Distinct roles of talin and kindlin in regulating integrin α5β1 function and trafficking. Curr. Biol. 2012;22:1554–1563. doi: 10.1016/j.cub.2012.06.060. [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo M.D., Brown N.H. Uncoupling integrin adhesion and signaling: the betaPS cytoplasmic domain is sufficient to regulate gene expression in the Drosophila embryo. Genes Dev. 1999;13:729–739. doi: 10.1101/gad.13.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negreiros E., Fontenele M., Câmara A.R., Araujo H. alphaPS1betaPS integrin receptors regulate the differential distribution of Sog fragments in polarized epithelia. Genesis. 2010;48:31–43. doi: 10.1002/dvg.20579. [DOI] [PubMed] [Google Scholar]
- North H.A., Pan L., McGuire T.L., Brooker S., Kessler J.A. β1-Integrin alters ependymal stem cell BMP receptor localization and attenuates astrogliosis after spinal cord injury. J. Neurosci. 2015;35:3725–3733. doi: 10.1523/JNEUROSCI.4546-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole T.E., Mandelman D., Forsyth J., Shattil S.J., Plow E.F., Ginsberg M.H. Modulation of the affinity of integrin alpha IIb beta 3 (GPIIb-IIIa) by the cytoplasmic domain of alpha IIb. Science. 1991;254:845–847. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- Paralkar V.M., Weeks B.S., Yu Y.M., Kleinman H.K., Reddi A.H. Recombinant human bone morphogenetic protein 2B stimulates PC12 cell differentiation: potentiation and binding to type IV collagen. J. Cell Biol. 1992;119:1721–1728. doi: 10.1083/jcb.119.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow C., Colosimo P.F., Lin M.C., Xu M., Kirov N. Transcriptional regulation of the Drosophila gene zen by competing Smad and Brinker inputs. Genes Dev. 2001;15:340–351. doi: 10.1101/gad.861401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawala A., Sutcliffe C., Ashe H.L. Multistep molecular mechanism for bone morphogenetic protein extracellular transport in the Drosophila embryo. Proc. Natl. Acad. Sci. USA. 2012;109:11222–11227. doi: 10.1073/pnas.1202781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöck F., Perrimon N. Retraction of the Drosophila germ band requires cell-matrix interaction. Genes Dev. 2003;17:597–602. doi: 10.1101/gad.1068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Chang C., Nie S., Xie S., Wan M., Cao X. Endofin acts as a Smad anchor for receptor activation in BMP signaling. J. Cell Sci. 2007;120:1216–1224. doi: 10.1242/jcs.03400. [DOI] [PubMed] [Google Scholar]
- Tian H., Mythreye K., Golzio C., Katsanis N., Blobe G.C. Endoglin mediates fibronectin/α5β1 integrin and TGF-β pathway crosstalk in endothelial cells. EMBO J. 2012;31:3885–3900. doi: 10.1038/emboj.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umulis D.M., Shimmi O., O’Connor M.B., Othmer H.G. Organism-scale modeling of early Drosophila patterning via bone morphogenetic proteins. Dev. Cell. 2010;18:260–274. doi: 10.1016/j.devcel.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.C., Ferguson E.L. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature. 2005;434:229–234. doi: 10.1038/nature03318. [DOI] [PubMed] [Google Scholar]
- Wang X., Harris R.E., Bayston L.J., Ashe H.L. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- Winstanley J., Sawala A., Baldock C., Ashe H.L. Synthetic enzyme-substrate tethering obviates the Tolloid-ECM interaction during Drosophila BMP gradient formation. eLife. 2015;4:e05508. doi: 10.7554/eLife.05508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.Y., Hill C.S. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Zhou J., Lee P.L., Lee C.I., Wei S.Y., Lim S.H., Lin T.E., Chien S., Chiu J.J. BMP receptor-integrin interaction mediates responses of vascular endothelial Smad1/5 and proliferation to disturbed flow. J. Thromb. Haemost. 2013;11:741–755. doi: 10.1111/jth.12159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.