Abstract
Background
The risk of pancreatitis with sitagliptin use in routine care remains to be established in older patients. We aimed to determine this risk in older adults who were newly prescribed sitagliptin versus an alternative hypoglycemic agent in the outpatient setting.
Methods
In a population-based retrospective cohort study in Ontario from 2010 until 2012 involving adults aged 66 years and older, we studied those who were newly prescribed sitagliptin or an alternative hypoglycemic agent. Our primary outcome of interest was a hospital encounter (emergency department visit or hospital admission) with acute pancreatitis within 90 days. We used inverse probability of treatment weighting to balance the 2 groups and logistic regression with a robust variance estimate to calculate odds ratios (ORs) and 95% confidence intervals (CIs).
Results
A total of 57 689 patients (mean age 74 yr) were newly prescribed sitagliptin, and 83 405 patients (mean age 75 yr) were given an alternative hypoglycemic agent (metformin, glyburide, gliclazide or insulin) during the study period. After weighting, there were no significant differences in measured baseline characteristics between groups. In the weighted sample, sitagliptin was not associated with an increased risk of a hospital encounter with pancreatitis compared with alternative hypoglycemic agents (weighted total 46 of 57 689 patients taking sitagliptin [0.08%] v. 48 of 55 705 patients taking alternative hypoglycemic agents [0.09%], absolute risk difference –0.01% [95% CI –0.05% to 0.02%], OR 0.92 [95% CI 0.55 to 1.55]).
Interpretation
Older adults newly prescribed sitagliptin in routine care were not at a substantially higher risk of pancreatitis than those prescribed alternative hypoglycemic agents. These findings are reassuring for those who use or prescribe sitagliptin in the management of type 2 diabetes.
Sitagliptin, a dipeptidyl-peptidase-4 (DPP-4) inhibitor, reduces blood glucose by decreasing glucagon secretion and blocking the breakdown of glucagon-like peptide-1, an incretin hormone that stimulates insulin secretion in a glucose-dependent fashion.1 Sitagliptin was the first DPP-4 inhibitor approved on the Ontario’s general benefits formulary in June of 2010. Because of its relative potency (it decreases glycosylated hemoglobin by up to 1% as monotherapy) and low risk of hypoglycemia,2 sitagliptin use has increased substantially over recent years (more than 700 000 prescriptions in Ontario from June 2010 to June 2012).3
Despite its benefits, sitagliptin has been linked with pancreatitis in case reports, animal studies and postmarketing drug surveillance studies.4–7 It has been postulated that incretin drugs (including DPP-4 inhibitors and glucagon-like peptide-1 agonists) might promote pancreatitis by increasing pancreatic mass, modifying enzyme secretion, disturbing acinar architecture, promoting inflammation, or increasing ductal turnover and metaplasia.6,8 As pancreatitis can lead to morbidity and mortality, warnings of the association have been published by regulatory agencies, pharmaceutical companies and diabetes association guidelines (Appendix 1, available at www.cmajopen.ca/content/3/2/E172/suppl/DC1).
In real-practice observational studies, however, the link between DPP-4 inhibitors and pancreatitis has been inconsistently described, and studies have been limited in their collection of baseline covariates, drug use and health care use.5,7,9 Studies have also used self-reported outcomes,7,9,10 and have been limited to younger populations, making results less generalizable to older adults.5,11 We thus aimed to examine the risk of acute pancreatitis with sitagliptin therapy in routine care in a large, representative population of older adults.
Methods
Study design and setting
We conducted a population-based retrospective cohort study involving adults aged 66 years and older from June 2010 to December 2012 using linked (via unique, encoded identifiers) health care databases in Ontario, Canada. Ontario has about 1.8 million adults aged 65 years and older who have comprehensive universal health care. This includes coverage for outpatient prescription medications, physician services, hospital admissions and diagnostic tests.12
This study was conducted at the Institute for Clinical Evaluative Sciences according to a prespecified protocol that was approved by the research ethics board at Sunnybrook Health Sciences Centre (Toronto, Canada). Informed consent was not required. Our report follows guidelines for the description of observational studies.13
Data sources
We obtained patient characteristics, drug use, covariate information and outcome data using records from 6 databases. Vital statistics were obtained from the Registered Persons Database of Ontario, which holds demographic information on all residents who have been issued a health card. The Ontario Drug Benefit Program database contains accurate records of all drug formulary medications dispensed to those aged 65 years and older, with an error rate of less than 1%.14 Diagnostic and procedural information on hospital admissions and emergency department visits was abstracted from the Canadian Institute for Health Information’s Discharge Abstract Database and the National Ambulatory Care Reporting System database, respectively. Covariate information was derived from the Ontario Health Insurance Plan database, which includes health claims for inpatient and outpatient physician services. We used the Institute for Clinical Evaluative Sciences Physician Database to determine prescriber information. Previous studies have used these databases to research adverse drug events and outcomes.15–19 A subpopulation in southwestern Ontario had outpatient glycosylated hemoglobin measurements available before a new hypoglycemic agent prescription.20
With the exception of prescriber information (missing in about 9.6%) and income quintile (missing in about 0.4%), databases were complete for all variables used. International Statistical Classification of Diseases and Related Health Problems, 10th revision (post-2002) and Canadian Classification of Health Interventions (post-2002) codes were used to assess baseline comorbidities and investigations in the 5 years before the hypoglycemic agent prescription (Appendix 2, available at www.cmajopen.ca/content/3/2/E172/suppl/DC1). Codes used to assess the outcome of acute pancreatitis are detailed in Appendix 3 (available at www.cmajopen.ca/content/3/2/E172/suppl/DC1).
Patient selection
To mimic routine practice, we studied older adults newly prescribed sitagliptin or an alternative hypoglycemic agent (metformin, glyburide, gliclazide or insulin) between June 2010 (when sitagliptin was first openly listed on the province’s formulary) and December 2012. The date of the new hypoglycemic drug prescription served as the index date (cohort entry date or start time for follow-up).
In the sitagliptin group we excluded the following patients: those in their first year of eligibility for prescription drug coverage (aged 65 yr) to avoid incomplete medication records; those with a hospital discharge in the 2 days before or on the index date to ensure these were new sitagliptin prescriptions (in Ontario, patients continuing a medication initiated in hospital typically have their medication dispensed on the same day or the day after discharge); those with a code for anesthesia or an epidural in the 30 days before the index date to exclude those with a recent surgery, a risk factor for pancreatitis; those with evidence of a pancreas transplant or pancreatectomy in the 5 years before the index date, to exclude those with surgical manipulation of the pancreas; those with a prescription for 1 or more DPP-4 inhibitors in the 1 year prior (to define new use); and those prescribed saxagliptin or a sitagliptin–metformin combination pill (to restrict to sitagliptin use only).
In patients prescribed an alternate hypoglycemic agent, we applied similar exclusion criteria with differences as follows. We excluded those initiated on metformin without evidence of a code for diabetes in the Ontario Diabetes Database,21 because diabetes itself is a risk factor for pancreatitis, and metformin can be prescribed for indications other than diabetes;5,7 those with a prescription for the same hypoglycemic agent in the 1 year prior (to define new use); those with a prescription for a DPP-4 inhibitor in the 1 year prior (to compare mutually exclusive groups); and those who were previously entered in the sitagliptin cohort. A patient could enter the cohort only once.
Exposure
For the primary analysis, we used an “intention to treat” exposure definition. Following a new hypoglycemic agent prescription, patients were followed until they experienced the primary outcome (defined below), reached 90 days of follow-up or died.
Outcomes
The primary outcome was a hospital encounter (emergency department visit or hospital admission) with acute pancreatitis within 90 days of the index date. We chose 90 days of follow-up to avoid crossover in drug therapy that could occur with longer periods of follow-up, because prescriptions covered by Ontario’s drug plan are prescribed at no more than 100-day intervals, and because previous reports have suggested that patients may be at risk for pancreatitis within this time frame of study.6,11,22
Statistical analysis
We used standardized differences to compare baseline characteristics between groups. This metric describes differences between group means relative to the pooled standard deviation and is considered meaningful if greater than 10%.23
The propensity score was derived from a logistic regression model with 29 baseline covariates incorporated into the score based on prior recommended methods (Appendix 4, available at www.cmajopen.ca/content/3/2/E172/suppl/DC1).24 Inverse probability of treatment weights were calculated using the propensity model to create a sample in which the distribution of measured baseline covariates was independent of treatment assignment.24
For the reference group, we considered those prescribed an alternative hypoglycemic agent. We expressed the risk of acute pancreatitis in relative and absolute terms. To calculate odds ratios (ORs) and 95% confidence intervals (CIs), we fit a logistic regression model using a robust variance estimate, accounting for inverse probability of treatment weights.
We conducted all analyses with SAS version 9.3 including analyses we undertook after knowledge of the primary results. We interpreted 2-tailed p values less than 0.05 as significant.
Sensitivity analyses
In time-to-event analysis we extended follow-up beyond 90 days, terminating the observation period for reasons of death, discontinuation of the study hypoglycemic agent, receipt of a nonstudy hypoglycemic agent or the last date of available records (Mar. 31, 2013). We also restricted our cohort to patients accrued after June 2011 to address the possibility of incomplete DPP-4 inhibitor records before June 2010, because sitagliptin became available on the general access formulary in June 2010. Differences in diagnostic testing between groups were evaluated over the course of follow-up by investigating the proportion of patients with amylase and lipase tests. Finally, we examined separately the risk of pancreatitis in patients taking metformin, sulfonylurea and insulin, and in those where the relevant hypoglycemic agent was prescribed as monotherapy or in the setting of another hypoglycemic agent or agents.
Results
Baseline characteristics
Patient selection is presented in Figures 1 and 2. There were 57 689 patients newly prescribed sitagliptin and 83 405 patients newly prescribed an alternative hypoglycemic agent over the course of study. Before weighting, patients given sitagliptin were younger and taking more medications, and in the years prior, had fewer hospital visits and more diagnostic testing than patients given alternative hypoglycemic agents. After weighting, there were 57 689 in the sitagliptin group and 55 705 in the alternative hypoglycemic agent group, and characteristics were similar between the groups (standardized difference less than 10%). Baseline characteristics are outlined in Tables 1–3 and Appendix 5 (available at www.cmajopen.ca/content/3/2/E172/suppl/DC1).
Figure 1:
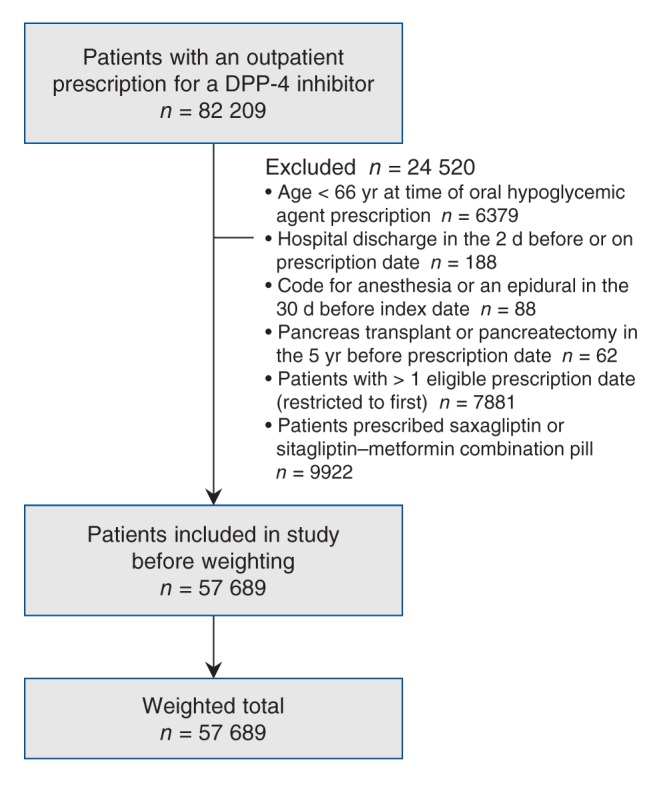
Flow diagram representing patient selection in the sitagliptin cohort. Note: DPP-4 = dipeptidyl-peptidase-4.
Figure 2:
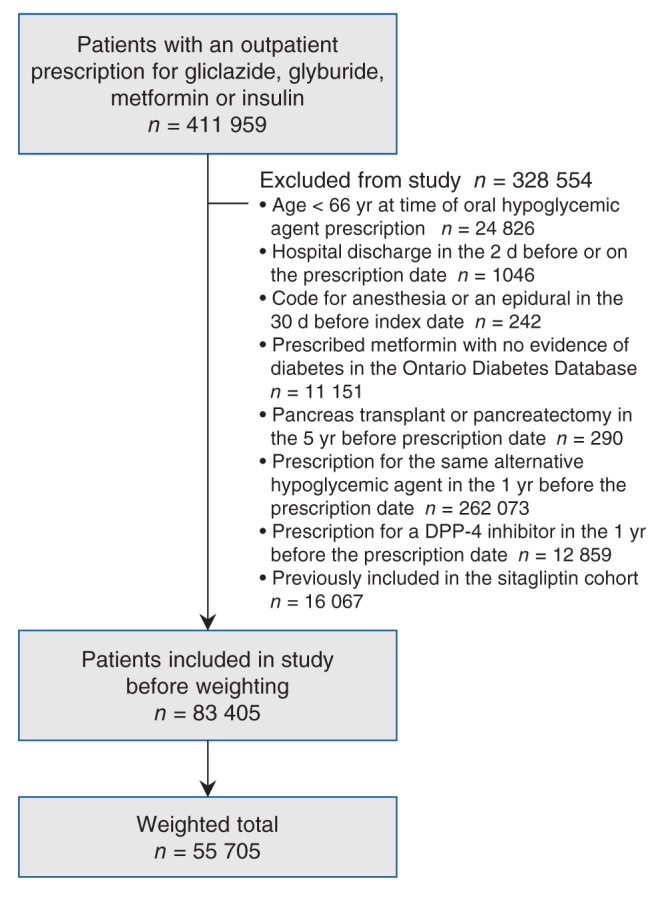
Flow diagram representing patient selection in the alternative hypoglycemic agent cohort. Note: DPP-4 = dipeptidyl-peptidase-4.
Table 1: Characteristics in patients given sitagliptin or an alternative hypoglycemic agent, before and after propensity weighting.
| Characteristic | No. (%) of patients* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before weighting |
After weighting† |
|||||||||
| Sitagliptin n = 57 689 |
Alternative hypoglycemic agent n = 83 405 |
Standardized difference, %‡ | Sitagliptin n = 57 689 |
Alternative hypoglycemic agent n = 55 705 |
Standardized difference, %‡ | |||||
| Age at index date, yr |
||||||||||
| Mean |
73.98 |
75.07 |
17 |
73.98 |
74.07 |
2 |
||||
| Median |
73 |
74 |
73 |
73 |
||||||
| Standard deviation |
6.25 |
6.93 |
6.25 |
5.20 |
||||||
| 66–70 |
21 105 |
(36.58) |
26 880 |
(32.23) |
9 |
21 105 |
(36.58) |
20 171 |
(36.21) |
1 |
| 71–75 |
15 829 |
(27.44) |
21 282 |
(25.52) |
4 |
15 829 |
(27.44) |
15 199 |
(27.28) |
0 |
| 76–80 |
11 228 |
(19.46) |
16 397 |
(19.66) |
0 |
11 228 |
(19.46) |
10 661 |
(19.14) |
1 |
| 81–85 |
6 431 |
(11.15) |
11 112 |
(13.32) |
7 |
6 431 |
(11.15) |
6 350 |
(11.40) |
1 |
| 86–90 |
2 461 |
(4.27) |
5 828 |
(6.99) |
12 |
2 461 |
(4.27) |
2 678 |
(4.81) |
3 |
| > 90 |
635 |
(1.10) |
1 906 |
(2.29) |
9 |
635 |
(1.10) |
646 |
(1.16) |
1 |
| Female sex |
27 584 |
(47.82) |
40 312 |
(48.33) |
1 |
27 584 |
(47.82) |
26 279 |
(47.18) |
1 |
| Rural location |
5 997 |
(10.40) |
12 396 |
(14.86) |
13 |
5 997 |
(10.40) |
6 275 |
(11.26) |
3 |
| Long-term care facility |
1 446 |
(2.51) |
5 581 |
(6.69) |
20 |
1 446 |
(2.51) |
1 594 |
(2.86) |
2 |
| Income quintile§ |
||||||||||
| 1 |
12 582 |
(21.81) |
18 233 |
(21.86) |
0 |
12 582 |
(21.81) |
12 447 |
(22.34) |
1 |
| 2 |
13 048 |
(22.62) |
18 029 |
(21.62) |
2 |
13 048 |
(22.62) |
12 134 |
(21.78) |
2 |
| 3 |
11 601 |
(20.11) |
16 572 |
(19.87) |
1 |
11 601 |
(20.11) |
11 150 |
(20.02) |
0 |
| 4 |
10 860 |
(18.83) |
16 320 |
(19.57) |
2 |
10 860 |
(18.83) |
10 845 |
(19.47) |
2 |
| 5 |
9 419 |
(16.33) |
13 878 |
(16.64) |
1 |
9 419 |
(16.33) |
8 894 |
(15.97) |
1 |
| Missing |
179 |
(0.31) |
373 |
(0.45) |
2 |
179 |
(0.31) |
234 |
(0.42) |
2 |
| Prescribing physician |
||||||||||
| Endocrinology |
4 813 |
(8.34) |
3 042 |
(3.65) |
20 |
4 813 |
(8.34) |
5 047 |
(9.06) |
3 |
| General practice |
42 925 |
(74.41) |
66 305 |
(79.50) |
12 |
42 925 |
(74.41) |
40 948 |
(73.51) |
2 |
| Internal medicine |
2 454 |
(4.25) |
2 281 |
(2.73) |
8 |
2 454 |
(4.25) |
2 407 |
(4.32) |
0 |
| Nephrology |
342 |
(0.59) |
778 |
(0.93) |
4 |
342 |
(0.59) |
360 |
(0.65) |
1 |
| Other |
1 830 |
(3.17) |
2 252 |
(2.70) |
3 |
1 830 |
(3.17) |
1 727 |
(3.10) |
0 |
| Missing | 5 325 | (9.23) | 8 169 | (9.79) | 2 | 5 325 | (9.23) | 5 216 | (9.36) | 0 |
*Unless stated otherwise. †All patients identified before weighting were included in the analyses. The number of patients indicated represents a weighted total. ‡Standardized differences are less sensitive to sample size than traditional hypothesis tests. They provide a measure of the difference between groups divided by the pooled standard deviation; a value greater than 10% is interpreted as a meaningful difference between the groups. §Income was categorized into fifths of average neighbourhood income on the index date.
Table 3: Medication and laboratory data in patients given sitagliptin or an alternative hypoglycemic agent, before and after propensity weighting.
| Medication and laboratory data | No. (%) of patients* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before weighting |
After weighting† |
|||||||||
| Sitagliptin n = 57 689 |
Alternative hypoglycemic agent n = 83 405 |
Standardized difference, %‡ | Sitagliptin n = 57 689 |
Alternative hypoglycemic agent n = 55 705 |
Standardized difference, %‡ | |||||
| Medication use before the index date§ |
||||||||||
| Diuretics |
18 516 |
(32.10) |
28 090 |
(33.68) |
3 |
18 516 |
(32.10) |
18 644 |
(33.47) |
3 |
| Anti-inflammatories |
10 816 |
(18.75) |
13 157 |
(15.77) |
8 |
10 816 |
(18.75) |
10 268 |
(18.43) |
1 |
| Glucocorticoids |
11 297 |
(19.58) |
16 121 |
(19.33) |
1 |
11 297 |
(19.58) |
11 350 |
(20.38) |
2 |
| Sulfonamides |
859 |
(1.49) |
1 647 |
(1.97) |
4 |
859 |
(1.49) |
1 029 |
(1.85) |
3 |
| Tetracyclines |
85 |
(0.15) |
152 |
(0.18) |
1 |
85 |
(0.15) |
128 |
(0.23) |
2 |
| Lipid-lowering drugs |
43 829 |
(75.97) |
51 532 |
(61.79) |
31 |
43 829 |
(75.97) |
42 210 |
(75.77) |
0 |
| Estrogen therapy |
601 |
(1.04) |
886 |
(1.06) |
0 |
601 |
(1.04) |
689 |
(1.24) |
2 |
| β-blockers |
18 780 |
(32.55) |
25 985 |
(31.16) |
3 |
18 780 |
(32.55) |
19 343 |
(34.72) |
5 |
| Azathioprine |
74 |
(0.13) |
139 |
(0.17) |
1 |
74 |
(0.13) |
67 |
(0.12) |
0 |
| Acetaminophen |
2 981 |
(5.17) |
4 455 |
(5.34) |
1 |
2 981 |
(5.17) |
2 871 |
(5.15) |
0 |
| Methyldopa |
92 |
(0.16) |
129 |
(0.15) |
0 |
92 |
(0.16) |
111 |
(0.20) |
1 |
| Tamoxifen |
65 |
(0.11) |
84 |
(0.10) |
0 |
65 |
(0.11) |
42 |
(0.08) |
1 |
| Angiotensin-converting enzyme inhibitors |
26 098 |
(45.24) |
32 599 |
(39.09) |
12 |
26 098 |
(45.24) |
25 536 |
(45.84) |
1 |
| Angiotensin receptor blockers |
19 645 |
(34.05) |
21 037 |
(25.22) |
19 |
19 645 |
(34.05) |
18 335 |
(32.91) |
2 |
| Aliskiren |
1 394 |
(2.42) |
951 |
(1.14) |
10 |
1 394 |
(2.42) |
979 |
(1.76) |
5 |
| Codeine |
5 667 |
(9.82) |
7 468 |
(8.95) |
3 |
5 667 |
(9.82) |
5 693 |
(10.22) |
1 |
| Mesalamine |
55 |
(0.10) |
63 |
(0.08) |
1 |
55 |
(0.10) |
43 |
(0.08) |
1 |
| Metronidazole |
730 |
(1.27) |
1 129 |
(1.35) |
1 |
730 |
(1.27) |
774 |
(1.39) |
1 |
| Sulindac |
37 |
(0.06) |
41 |
(0.05) |
1 |
37 |
(0.06) |
28 |
(0.05) |
1 |
| Valproic acid |
41 |
(0.07) |
94 |
(0.11) |
1 |
41 |
(0.07) |
53 |
(0.10) |
1 |
| Amiodarone |
336 |
(0.58) |
639 |
(0.77) |
2 |
336 |
(0.58) |
377 |
(0.68) |
1 |
| Lamivudine |
12 |
(0.02) |
12 |
(0.01) |
0 |
12 |
(0.02) |
≤ 5 |
— |
|
| Omeprazole |
2 343 |
(4.06) |
3 519 |
(4.22) |
1 |
2 343 |
(4.06) |
2 639 |
(4.74) |
3 |
| Erythromycin |
43 |
(0.07) |
54 |
(0.06) |
0 |
43 |
(0.07) |
32 |
(0.06) |
1 |
| Hypoglycemic agents prescribed in the 120 d before index date¶ |
||||||||||
| Insulin |
4 505 |
(7.81) |
3 164 |
(3.79) |
17 |
4 505 |
(7.81) |
4 091 |
(7.34) |
2 |
| Gliclazide |
17 142 |
(29.71) |
4 566 |
(5.47) |
67 |
17 142 |
(29.71) |
14 734 |
(26.45) |
7 |
| Glyburide |
13 807 |
(23.93) |
12 681 |
(15.20) |
22 |
13 807 |
(23.93) |
14 847 |
(26.65) |
6 |
| Metformin |
43 135 |
(74.77) |
20 987 |
(25.16) |
14 |
43 135 |
(74.77) |
41 592 |
(74.66) |
0 |
| Pioglitazone |
5 812 |
(10.07) |
1 863 |
(2.23) |
33 |
5 812 |
(10.07) |
5 949 |
(10.68) |
2 |
| Repaglinide |
341 |
(0.59) |
194 |
(0.23) |
6 |
341 |
(0.59) |
228 |
(0.41) |
3 |
| Rosiglitazone |
2 015 |
(3.49) |
524 |
(0.63) |
20 |
2 015 |
(3.49) |
1 956 |
(3.51) |
0 |
| Hypoglycemic agents prescribed on the index date** |
||||||||||
| Insulin |
1 010 |
(1.75) |
≤ 5‡‡ |
— |
1 010 |
(1.75) |
0 |
19 |
||
| Gliclazide |
6 232 |
(10.80) |
5 578 |
(6.69) |
15 |
6 232 |
(10.80) |
0 |
49 |
|
| Glyburide |
2 982 |
(5.17) |
6 198 |
(7.43) |
9 |
2 982 |
(5.17) |
0 |
33 |
|
| Metformin |
14 174 |
(24.57) |
1 324 |
(1.59) |
73 |
14 174 |
(24.57) |
0 |
81 |
|
| Pioglitazone |
652 |
(1.13) |
409 |
(0.49) |
7 |
652 |
(1.13) |
771 |
(1.38) |
2 |
| Repaglinide |
61 |
(0.11) |
27 |
(0.03) |
3 |
61 |
(0.11) |
13 |
(0.02) |
3 |
| Rosiglitazone |
89 |
(0.15) |
51 |
(0.06) |
3 |
89 |
(0.15) |
105 |
(0.19) |
1 |
| Hypoglycemic agents prescribed in the 1 yr to 120 d before the index date†† |
||||||||||
| Insulin |
4 671 |
(8.10) |
5 272 |
(6.32) |
37 |
4 671 |
(8.10) |
4 213 |
(7.56) |
2 |
| Gliclazide |
17 175 |
(29.77) |
5 886 |
(7.06) |
61 |
17 175 |
(29.77) |
14 249 |
(25.58) |
9 |
| Glyburide |
17 038 |
(29.53) |
15 101 |
(18.11) |
27 |
17 038 |
(29.53) |
17 053 |
(30.61) |
2 |
| Metformin |
45 376 |
(78.66) |
27 777 |
(33.30) |
103 |
45 376 |
(78.66) |
41 580 |
(74.64) |
9 |
| Pioglitazone |
7 023 |
(12.17) |
2 918 |
(3.50) |
33 |
7 023 |
(12.17) |
6 493 |
(11.66) |
2 |
| Repaglinide |
450 |
(0.78) |
372 |
(0.45) |
4 |
450 |
(0.78) |
353 |
(0.63) |
2 |
| Rosiglitazone |
2 981 |
(5.17) |
1 123 |
(1.35) |
22 |
2 981 |
(5.17) |
2 447 |
(4.39) |
4 |
| Recent test for glycosylated hemoglobin levels |
16 413 |
(28.45) |
14 837 |
(26.63) |
4 |
|||||
| Glycosylated hemoglobin level, % |
||||||||||
| Mean |
7.7 |
7.8 |
8 |
|||||||
| Median |
7.4 |
7.5 |
||||||||
| 25th percentile |
6.9 |
6.9 |
||||||||
| 75th percentile |
8.2 |
8.4 |
||||||||
| Standard deviation | 1.3 | 1.2 | ||||||||
*Unless stated otherwise. †All patients identified before weighting were included in the analyses. The number of patients indicated represents a weighted total. ‡Standardized differences are less sensitive to sample size than traditional hypothesis tests. They provide a measure of the difference between groups divided by the pooled standard deviation; a value greater than 10% is interpreted as a meaningful difference between the groups. §Baseline medication use was assessed in the previous 120 days. There were no prescriptions for pentamidine, flucytosine, clomiphene, clozapine, acarbose, acetohexamide, chlorpropamide, glimepiride, nateglinide or tolbutamide. There were less than 1% prescriptions for dapsone, isoniazid, procainamide, methimazole and nelfinavir. ¶Hypoglycemic agent use in the previous 120 days includes hypoglycemic drugs prescribed from –120 to –1 day before the index date where days’ supply covered the index date. **Hypoglycemic agent use on the index date refers to hypoglycemic drugs prescribed on the same day as study drug (index date). ††Hypoglycemic agent use in the previous 365 to 120 days includes those hypoglycemic drugs prescribed from –365 to –120 days before the index date. ‡‡Numbers less than 6 were not reported for privacy reasons.
Beyond the index prescription, 84.7% and 76.1% of patients given sitagliptin and alternative hypoglycemic agents, respectively, filled at least 1 additional prescription for the relevant hypoglycemic agent. The duration of continuous study drug use (defined by the duration of 1 prescription overlapping with a subsequent prescription, allowing for a 10-day grace period between prescriptions), was about 81, 64, 91, 82 and 108 days in the sitagliptin, insulin, glyburide, gliclazide and metformin groups, respectively.
Outcomes
Results for the primary outcome of pancreatitis are presented in Table 4. Sitagliptin use was not associated with a higher 90-day risk of a hospital encounter with pancreatitis than alternative hypoglycemic agent use (weighted total 46 of 57 689 patients taking sitagliptin [0.08%] v. 48 of 55 705 patients taking alternative hypoglycemic agents [0.09%], absolute risk difference –0.01% [95% CI –0.05% to 0.02%], OR 0.92 [95% CI 0.55 to 1.55]).
Table 4: Ninety-day risk of a hospital encounter with acute pancreatitis in patients given sitagliptin or an alternative hypoglycemic agent,* before and after propensity weighting.
| Variable | No. (%) of events |
Absolute risk difference, % (95% CI) |
Odds ratio (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before weighting |
After weighting† |
|||||||||
| Sitagliptin n = 57 689 |
Alternative hypoglycemic agent n = 83 405 |
Sitagliptin n = 57 689 |
Alternative hypoglycemic agent n = 55 705 |
|||||||
| Hospital encounter with acute pancreatitis | 46 | (0.08) | 83.40 | (0.10) | 46 | (0.08) | 48 | (0.09) | –0.01 (–0.05 to 0.02) |
0.92 (0.55 to 1.55) |
Note: CI = confidence interval. *Patients prescribed glyburide, gliclazide, metformin or insulin served as the reference group. †All patients identified before weighting were included in the analysis. The number of patients indicated represents a weighted total.
Sensitivity analyses
Our findings remained robust in sensitivity analyses. Results of the time to event analysis are shown in Table 5. Sitagliptin use was not associated with a higher risk of a hospital encounter with pancreatitis than alternative hypoglycemic agent use (Cox proportional hazards model with inverse probability of treatment weights; hazard ratio [HR] 1.18 [95% CI 0.94 to 1.49]. The analysis of patients newly prescribed sitagliptin or an alternative hypoglycemic agent after June 2011 produced results similar to our primary analysis (Appendix 6, available at www.cmajopen.ca/content/3/2/E172/suppl/DC1). Similar proportions of amylase and lipase tests were noted in the 2 groups (Appendix 7, available at www.cmajopen.ca/content/3/2/E172/suppl/DC1). Appendices 7–9 (available at www.cmajopen.ca/content/3/2/E172/suppl/DC1) illustrate similar low event rates across groups of study.
Table 5: Time-to-event analysis.
| Variable | No. (%) of events,* after weighting |
|||
|---|---|---|---|---|
| Sitagliptin n = 57 689 |
Alternative hypoglycemic agent n = 55 705 |
|||
| Person-years of follow-up |
14 988 |
16 972 |
||
| Days of follow-up, median (IQR) |
65 |
(30–125) |
75 |
(30–129) |
| Censoring events |
||||
| Hospital encounters with pancreatitis |
260 |
(0.45) |
224 |
(0.40) |
| Hazard ratio (95% CI) |
1.18 |
(0.94 to 1.49) |
1.00 |
(reference) |
| Event rate per 1000 person-years |
17.35 |
13.18 |
||
| Censoring events |
||||
| Death |
9 |
(0.02) |
20.95 |
(0.04) |
| Study hypoglycemic agent discontinued |
8579 |
(14.87) |
17 485 |
(31.39) |
| Prescription for a nonstudy hypoglycemic agent | 48 841 | (84.66) | 37 976 | (68.17) |
Note: CI = confidence interval, IQR = interquartile range. *Unless stated otherwise.
Interpretation
In our cohort, the initiation of sitagliptin was not associated with a higher 90-day risk of a hospital encounter with acute pancreatitis than the initiation of metformin, glyburide, gliclazide or insulin in routine care.
The findings of our study are consistent with the results of randomized controlled trials. A recent systematic review and meta-analysis of 55 randomized trials (33 350 patients) found no difference in the risk of pancreatitis between patients prescribed incretin drugs versus patients prescribed a placebo, lifestyle or other oral hypoglycemic agents (OR 1.11, 95% CI 0.57 to 2.17).27 A cardiovascular study of patients randomly assigned to saxagliptin or placebo found that rates of acute pancreatitis were similar in both groups (22 of 8280 patients given saxagliptin [0.3%] v. 16 of 8212 patients given placebo [0.2%], p = 0.42).28
Our results are also consistent with previous observational studies. In a cohort study of 16 276 patients given sitagliptin and 16 281 matched patients given metformin or glyburide, the risk of acute pancreatitis was similar (relative risk 1.0, 95% CI 0.5 to 2.0).29 An additional cohort study noted comparable rates of pancreatitis in patients taking sitagliptin and alternative hypoglycemic agents (adjusted HR 1.0, 95% CI 0.7 to 1.3),5 as did a recent report of 20 748 patients taking incretin drugs and 51 712 patients taking sulfonylureas (adjusted HR 1.00, 95% CI 0.59 to 1.70).30 Further, a study of 1003 cases and 4012 matched controls found that use of incretin drugs in the 6 months prior was not associated with pancreatitis (OR 0.98, 95% CI 0.69 to 1.38).31 Similar results were noted in a recent Chinese case–control study.32
Our results, however, differ from those of published studies that report a higher risk of pancreatitis with use of DPP-4 inhibitors. Studies using the US Food and Drug Administration database have found that patients taking incretin drugs had a higher odds of pancreatitis (OR 6.74, 95% CI 4.61 to 10.00) than patients taking other oral agents,7 and that the odds of pancreatitis in patients taking DPP-4 inhibitors was 20.8 times (95% CI 12.6 to 34.5) the odds of pancreatitis in those taking other hypoglycemic agents.10 A French surveillance study also noted that the rate of exposure to DPP-4 inhibitors was higher in cases of pancreatitis versus non–cases of pancreatitis (67 patients taking DPP-4 inhibitors in 147 cases of pancreatitis v. 421 patients taking DPP-4 inhibitors in 2962 non–cases of pancreatitis, adjusted reporting OR 12.1, 95% CI 7.3 to 20.0).9 These studies, however, may have been subject to reporting bias as events were self-reported and may have been inflated by external factors.7,9
An additional case–control study found that the adjusted odds of acute pancreatitis in those who currently (within 30 d) (OR 2.24, 95% CI 1.36 to 3.68) and who had recently used incretin drugs (past 30 d and less than 2 yr) was higher (OR 2.01, 95% CI 1.37 to 3.18) than in those who had not used incretin drugs.11 This study performed a more limited assessment of baseline covariates and indices of health care use, and, because it was completed in a younger population (mean age 52 yr), may not be fully generalizable to older adults.
Strengths and limitations
Our study has several strengths. Using a large sample of older patients who used sitagliptin in a routine setting (with multiple comorbidities and taking multiple medications), our study complements information from randomized clinical trials by studying an uncommon but important adverse drug reaction. Our study has adequate statistical power, and the results are generalizable to the larger population. Our new-user design allowed us to observe outcomes after the initiation of treatment. Whereas previous studies included self-reported pancreatitis, in our study pancreatitis was documented in hospital records by the treating health care team. Additionally, to echo routine care and make our findings interpretable in clinical practice, we studied patients who were newly prescribed hypoglycemic alternatives to sitagliptin (metformin, a sulfonylurea or insulin) as a comparison group. We also carried out numerous sensitivity analyses and our primary results remained robust.
Our study has some limitations. Prospective data collection with independent outcome adjudication is a preferred methodology to a retrospective database study. We were unable to detect asymptomatic pancreatitis or pancreatitis that did not result in a hospital presentation. We accurately ascertained medications dispensed but had no information on medication use (although we note that most patients filled at least 1 additional prescription for the relevant study drug).
Given the low event rate of pancreatitis in both groups, we were able to rule out a greater than 1.6-fold increase in the risk of pancreatitis in patients newly prescribed sitagliptin compared with patients newly prescribed alternative hypoglycemic agents with adequate statistical power (upper bound of the CI), but could not rule out a smaller increase in risk. The limited number of events also precluded meaningful subgroup analysis.
The duration of our study did not allow us to measure the long-term effects of sitagliptin. However, we feel that our 90 days follow-up period was reasonable given that the mean duration of continuous drug use ranged from 64 to 108 days, previous reports have suggested that pancreatitis can manifest early in the course of drug exposure,6,11,22 and studies to date have not noted that the risk of pancreatitis differs by exposure duration.27,30
The potential for confounding and bias warrants attention. In the current study, we had limited information on factors such as obesity, body mass index and smoking status, which are known to influence the risk of pancreatitis. It is also possible that physicians may have selectively prescribed sitagliptin therapy to healthier people and may have differentially monitored patients for outcomes. However, using propensity score weighting, we obtained good balance on a large number of measured baseline characteristics between the 2 groups. Further, we note that diagnostic testing for pancreatitis was similar among the 2 groups during follow-up.
Conclusion
In older adults, the initiation of sitagliptin did not result in a higher risk of a hospital encounter with pancreatitis than initiation of an alternative diabetic medication (any of metformin, glyburide, gliclazide or insulin). These findings are reassuring for those who use or prescribe sitagliptin in the management of type 2 diabetes.
Supplemental information
For reviewer comments and the original submission of this manuscript, see www.cmajopen.ca/content/3/2/E172/suppl/DC1
Table 2: Comorbidities in patients given sitagliptin or an alternative hypoglycemic agent, before and after propensity weighting.
| Comorbidities* | No. (%) of patients† |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before weighting |
After weighting‡ |
|||||||||
| Sitagliptin n = 57 689 |
Alternative hypoglycemic agent n = 83 405 |
Standardized difference, %§ | Sitagliptin n = 57 689 |
Alternative hypoglycemic agent n = 55 705 |
Standardized difference, %§ | |||||
| Gallstones/biliary stones |
2 163 |
(3.75) |
3 152 |
(3.78) |
0 |
2 163 |
(3.75) |
2 059 |
(3.70) |
0 |
| Calcium disorder |
123 |
(0.21) |
273 |
(0.33) |
2 |
123 |
(0.21) |
154 |
(0.28) |
1 |
| Alcoholism |
240 |
(0.42) |
659 |
(0.79) |
5 |
240 |
(0.42) |
338 |
(0.61) |
3 |
| Tobacco use |
3 128 |
(5.42) |
4 662 |
(5.59) |
1 |
3 128 |
(5.42) |
3 222 |
(5.78) |
2 |
| Pancreatic neoplasm |
101 |
(0.18) |
250 |
(0.30) |
3 |
101 |
(0.18) |
178 |
(0.32) |
3 |
| Endoscopic retrograde cholangiopancreatography |
281 |
(0.49) |
561 |
(0.67) |
2 |
281 |
(0.49) |
334 |
(0.60) |
2 |
| Chronic kidney disease¶ |
6 069 |
(10.52) |
10 321 |
(12.37) |
6 |
6 069 |
(10.52) |
6 714 |
(12.05) |
5 |
| Bile duct neoplasm |
118 |
(0.20) |
272 |
(0.33) |
2 |
118 |
(0.20) |
158 |
(0.28) |
2 |
| HIV |
50 |
(0.09) |
67 |
(0.08) |
0 |
50 |
(0.09) |
33 |
(0.06) |
1 |
| Systemic lupus erythematosus |
739 |
(1.28) |
1 107 |
(1.33) |
0 |
739 |
(1.28) |
659 |
(1.18) |
1 |
| Polyarteritis nodosa |
216 |
(0.37) |
429 |
(0.51) |
2 |
216 |
(0.37) |
248 |
(0.45) |
1 |
| Celiac disease |
78 |
(0.14) |
158 |
(0.19) |
1 |
78 |
(0.14) |
103 |
(0.18) |
1 |
| Obesity |
4 219 |
(7.31) |
5 468 |
(6.56) |
3 |
4 219 |
(7.31) |
3 696 |
(6.63) |
3 |
| Charlson comorbidity index** |
||||||||||
| Mean |
1.13 |
1.22 |
5 |
1.13 |
1.23 |
7 |
||||
| 0–1 |
40 624 |
(70.42) |
57 156 |
(68.53) |
4 |
40 624 |
(70.42) |
37 816 |
(67.89) |
5 |
| 2 |
7 861 |
(13.63) |
10 430 |
(12.51) |
3 |
7 861 |
(13.63) |
8 053 |
(14.46) |
2 |
| > 3 |
9 204 |
(15.95) |
15 819 |
(18.97) |
8 |
9 204 |
(15.95) |
9 836 |
(17.66) |
5 |
| Diabetic retinopathy |
636 |
(1.10) |
842 |
(1.01) |
1 |
636 |
(1.10) |
783 |
(1.41) |
3 |
| Diabetic neuropathy |
576 |
(1.00) |
843 |
(1.01) |
0 |
576 |
(1.00) |
597 |
(1.07) |
1 |
| Peripheral vascular disease |
679 |
(1.18) |
1 259 |
(1.51) |
3 |
679 |
(1.18) |
740 |
(1.33) |
1 |
| Heart failure |
6 606 |
(11.45) |
11 932 |
(14.31) |
9 |
6 606 |
(11.45) |
7 068 |
(12.69) |
4 |
| Coronary artery bypass graft |
1 766 |
(3.06) |
2 553 |
(3.06) |
0 |
1 766 |
(3.06) |
1 781 |
(3.20) |
1 |
| Hypertension |
49 934 |
(86.56) |
64 828 |
(77.73) |
23 |
49 934 |
(86.56) |
48 277 |
(86.67) |
0 |
| Coronary artery disease |
16 299 |
(28.25) |
23 740 |
(28.46) |
0 |
16 299 |
(28.25) |
16 144 |
(28.98) |
2 |
| Myocardial infarction |
1 243 |
(2.15) |
2 479 |
(2.97) |
5 |
1 243 |
(2.15) |
1 381 |
(2.48) |
2 |
| Stroke/transient ischemic attack |
1 377 |
(2.39) |
3 011 |
(3.61) |
7 |
1 377 |
(2.39) |
1 535 |
(2.76) |
2 |
| Dialysis |
1 251 |
(2.17) |
2 992 |
(3.59) |
8 |
1 251 |
(2.17) |
1 545 |
(2.77) |
4 |
| Renal transplant |
30 |
(0.05) |
118 |
(0.14) |
3 |
30 |
(0.05) |
62 |
(0.11) |
2 |
| Hypoglycemia |
770 |
(1.33) |
1 354 |
(1.62) |
2 |
770 |
(1.33) |
818 |
(1.47) |
1 |
| Acute or chronic pancreatitis |
216 |
(0.37) |
467 |
(0.56) |
3 |
216 |
(0.37) |
267 |
(0.48) |
2 |
| Hyperglycemic emergency | 117 | (0.20) | 245 | (0.29) | 2 | 117 | (0.20) | 162 | (0.29) | 2 |
*Comorbidities were assessed by administrative database codes in the previous 5 years. †Unless stated otherwise. ‡All patients identified before weighting were included in the analyses. The number of patients indicated represents a weighted total. §Standardized differences are less sensitive to sample size than traditional hypothesis tests. They provide a measure of the difference between groups divided by the pooled standard deviation; a value greater than 10% is interpreted as a meaningful difference between the groups. ¶We identified individuals with chronic kidney disease using a validated algorithm of diagnosis and physician claim codes. In Ontario, this algorithm identifies patients with a median estimated glomerular filtration rate (eGFR) of 38 mL/min per 1.73 m2 (interquartile range 27 to 52). Its absence identifies patients with a median eGFR of 69 mL/min per 1.73 m2 (interquartile range 56 to 82). See Fleet et al.25 **Charlson comorbidity index was calculated using 5 years of hospital admission data. “No hospital admissions” received a score of 0. See Charlson et al.26
Supplementary Material
Acknowledgements
The authors thank Brogan Inc., Ottawa, for use of its drug product and therapeutic class database; Gamma-Dynacare for use of the outpatient laboratory database; the team at London Health Sciences Centre, St Joseph’s Health Care; the Thames Valley Hospitals for providing access to the Cerner laboratory database; and Salimah Shariff, PhD (from the Institute for Clinical Evaluative Sciences Western), for administrative support.
References
- 1.Engel SS, Williams-Herman DE, Golm GT, et al. Sitagliptin: review of preclinical and clinical data regarding incidence of pancreatitis. IntJClinPract 2010;64:984-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Januvia: stitagliptin tablets [product monograph]. Kirkland (QC): Merck Canada Inc.; 2013. Available: www.merck.ca/assets/en/pdf/products/JANUVIA-PM_E.pdf (accessed 2014 Mar. 13).
- 3.IMS/brogan [homepage on the Internet].2014. Available: www.imshealth.com/portal/site/imshealth? CURRENT_LOCALE=en_ca (accessed 2014 Mar. 13).
- 4.Sue M, Yoshihara A, Kuboki K, et al. A case of severe acute necrotizing pancreatitis after administration of sitagliptin. Clin Med Insights Case Rep 2013;6:23-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. DiabetesCare 2010;33:2349-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matveyenko AV, Dry S, Cox HI, et al. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes 2009;58:1604-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koehler JA, Baggio LL, Lamont BJ, et al. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes 2009;58:2148-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faillie JL, Babai S, Crepin S, et al. Pancreatitis associated with the use of GLP-1 analogs and DPP-4 inhibitors: a case/non-case study from the French Pharmacovigilance Database. ActaDiabetol 2014;51:491-7. [DOI] [PubMed] [Google Scholar]
- 10.Moore, T, Cohen, M, Furberg, C. ISMP QuarterWatch: perspectives on GLP-1 agents for diabetes 2013. Available: www.ismp.org/quarterwatch/pdfs/2012Q3.pdf. (accessed 2014 Apr. 11).
- 11.Singh S, Chang HY, Richards TM, et al. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMAInternMed 2013;173:534-9. [DOI] [PubMed] [Google Scholar]
- 12.Bronskill S, Carter M, Costa A, et al. Aging in Ontario: an ICES chartbook of health service use by older adults Toronto: Institute for Clinical Evaluative Sciences; 2010. Available: www.ices.on.ca/Publications/Atlases-and-Reports/2010/Aging-in-Ontario.aspx. (accessed 2014 Mar. 13).
- 13.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PrevMed 2007;45:247-51. [DOI] [PubMed] [Google Scholar]
- 14.Levy AR, O’Brien BJ, Sellors C, et al. Coding accuracy of administrative drug claims in the Ontario drug benefit database. CanJClinPharmacol 2003;10:67-71. [PubMed] [Google Scholar]
- 15.Patel AM, Shariff S, Bailey DG, et al. Statin toxicity from macrolide antibiotic coprescription: a population-based cohort study. AnnIntern Med 2013;158:869-76. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui NF, Coca SG, Devereaux PJ, et al. Secular trends in acute dialysis after elective major surgery — 1995 to 2009. CMAJ 2012;184:1237-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih AW, Weir MA, Clemens KK, et al. Oral bisphosphonate use in the elderly is not associated with acute kidney injury. Kidney Int 2012;82:903-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhao YY, Weir MA, Manno M, et al. New fibrate use and acute renal outcomes in elderly adults: a population-based study. Ann Intern Med 2012;156:560-9. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi S, Fleet JL, Bailey DG, et al. Calcium-channel blocker-clarithromycin drug interactions and acute kidney injury. JAMA 2013;310:2544-53. [DOI] [PubMed] [Google Scholar]
- 20.Gandhi S, Shariff SZ, Beyea MM, et al. Identifying geographical regions serviced by hospitals to assess laboratory-based outcomes. BMJ Open 2013;3: pii:e001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512-6. [DOI] [PubMed] [Google Scholar]
- 22.Information for Healthcare Professionals — acute pancreatitis and sitagliptin (marketed as Januvia and Janumet) Silver Spring (MD): U.S. Food and Drug Administration; 2009. Available: www.fda.gov/Drugs/DrugSafety/ PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm183764.htm. (accessed 2014 Mar. 13).
- 23.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational studies. Commun Stat Simulat 2009;38:1228-34. [Google Scholar]
- 24.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleet JL, Dixon SN, Shariff SZ, et al. Detecting chronic kidney disease in population-based administrative databases using an algorithm of hospital encounter and physician claim codes. BMC Nephrol 2013;14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, et al. A new method for classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Shen J, Bala MM, et al. Incretin treatment and risk of pancreatitis in patients with type 2 diabetes mellitus: systematic review and meta-analysis of randomized and non-randomised studies. BMJ 2014;348:g2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317-26. [DOI] [PubMed] [Google Scholar]
- 29.Dore DD, Seeger JD, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin 2009;25:1019-27. [DOI] [PubMed] [Google Scholar]
- 30.Faillie JL, Azoulay L, Patenaude V, et al. Incretin based drugs and risk of acute pancreatitis in patients with type 2 diabetes: cohort study. BMJ 2014;348:g2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giorda CB, Picariello R, Nada E, et al. Incretin therapies and risk of hospital admission for acute pancreatitis in an unselected population of European patients with type 2 diabetes: a case-control study. Lancet Diabetes Endocrinol 2014;2:111-5. [DOI] [PubMed] [Google Scholar]
- 32.Chou HC, Chen WW, Hsiao FY. Acute pancreatitis in patients with type 2 diabetes mellitus treated with dipeptidyl peptidase-4 inhibitors: a population-based nested case-control study. Drug Saf 2014;37:521-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


