At the most basic level, the heart is an electrically controlled pump. As such, our attention tends to focus on the feed-forward mechanisms by which cardiac excitation, occurring through co-ordinated ion channel activity, leads to the calcium signal necessary for contraction. Yet, it is becoming clear that the heart is intrinsically regulated, including feedback from its mechanical state to ion channel function, electrical conduction, and calcium–myofilament interactions. This essential aspect of intra-cardiac regulation, originally termed Mechano-Electric Feedback, is now more generally known as Mechano-Electric Coupling (MEC).1
MEC might seem to be an evolutionary quirk or physiological epiphenomenon. It is now understood, however, to be important in numerous cardiac diseases, for instance contributing to the electrophysiological effects of non-uniform contraction, the reduced arrhythmia threshold accompanying atrial or ventricular enlargement, and the impact of pulmonary vein loading on atrial fibrillation.2 Yet this says nothing about its importance in the healthy heart. Does MEC play a role in regulating normal cardiac function?
The first evidence that MEC may be important for cardiac auto-regulation came 100 years ago, with Francis Arthur Bainbridge's observation that right-atrial distension in anaesthetized dogs results in an acute increase in heart rate.3 Fifty years later, Klaus Deck showed a similar chronotropic response to stretch in the isolated sinoatrial node of rabbit and cat,4 demonstrating that an intra-cardiac mechanism was involved. Since then, it has been well established that cardiac pacemaker tissue intrinsically responds to acute changes in mechanical load on a beat-by-beat basis, contributing to the matching of cardiac output to venous return via MEC mechanisms.5
The importance of MEC for normal function of the working myocardium, however, has been more elusive. It has long been known that heart muscle adapts to acutely altered mechanical load by a rapid increase of contractile force. This essential homeostatic mechanism, formalized nearly 100 years ago by Ernest Henry Starling as ‘Starling's Law of the Heart’,6 has generally been attributed to enhanced inter-filament interactions due to a stretch-induced increase in calcium sensitivity of myofilaments and conformational changes in the sarcomere. More recently, MEC has been implicated as an additional contributor, through an acute amplitude- and frequency-dependent stretch-induced increase in calcium spark rate.7,8
Opthof et al.9 present data indicating that MEC may also play a role in the regulation of normal cardiomyocyte electrophysiology. Using a Langendorff-perfused, contracting pig heart model, they investigated the effect of physiological intraventricular pressure on ventricular repolarization. By varying left ventricular load and the site of ventricular activation, they demonstrated that normal intraventricular pressure dynamics appear to synchronize local ventricular repolarization. Much like the increase in calcium spark rate with stretch, which may act as a means of mitigating cell-to-cell contractile heterogeneity, this stretch-induced homogenization of repolarization may be an important intrinsic mechanism (combined with intercellular electrotonic interactions) for protecting against repolarization heterogeneity across the heart.
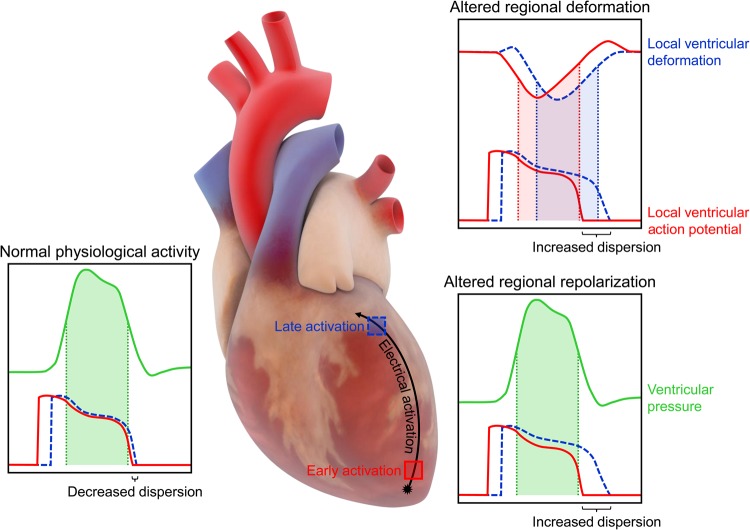
The study involved comparing dispersion of repolarization with activation from two ventricular pacing sites in the presence and absence of left ventricular load controlled by an intraventricular balloon, which showed that the capacity of physiological pressure to synchronize repolarization appears to depend on the temporal relation of global pressure dynamics relative to local action potential phase. The concept that the effects of dynamic ventricular load on the action potential in the isolated heart depend on mechano-electrical timing is not new. In the isolated rabbit heart, artificial transient increases in intraventricular volume cause repolarization during early systole (coinciding with the action potential plateau), cause depolarization during ventricular relaxation (coinciding with the action potential end), and have no effect when applied during an intermediate level of repolarization (∼65%).10 A similar effect has been simulated using a duplex of individually controlled, mechanically interacting cardiac muscles, demonstrating that activation sequence influences electro-mechanical activity, which optimizes myocardial performance by smoothing intrinsic differences in repolarization timing.11 The present work extends these results to the physiological setting, by demonstrating that the effect of normal left ventricular pressure dynamics on repolarization differs between early and late activated regions within a single heartbeat, such that delay of repolarization is larger in early vs. late activated myocardium, leading to a decrease in dispersion of repolarization (Figure 1).
Figure 1.
Effects of cardiac mechano-electric coupling on ventricular repolarization. With normal physiological activity in the healthy heart, the effect of left ventricular pressure on repolarization differs between early and late activated regions, leading to decreased dispersion of repolarization. In cardiac pathologies, where regional ventricular deformation or repolarization is altered, the temporal relation of local action potential phase to mechanical activity or ventricular pressure is shifted, leading to increased dispersion of repolarization.
This finding has clinical implications, both for understanding disease and for the design of novel anti-arrhythmic therapies. For patients in whom regional ventricular deformation or repolarization is altered, causing shifts in the temporal relation of local action potential phase to mechanical activity, an increase in dispersion of repolarization may ensue (Figure 1). For instance, it has been shown that non-uniform deformation of the heart, as occurs in a variety of cardiac pathologies, is associated with increased repolarization heterogeneity in the healthy (rather than diseased) tissue, implicating a role of MEC (rather than electrophysiological remodelling).12 In fact, non-uniformity of deformation is associated with a high susceptibility to ventricular tachyarrhythmias and is one of the best predictors of sudden cardiac death.13 Similarly, there is growing evidence to suggest MEC links between regional heterogeneity in mechanics and repolarization in long QT syndrome,14 as prolongation of repolarization beyond the end of ventricular relaxation exaggerates repolarization instability, resulting in after-contractions that trigger Torsades de Pointes.15 Understanding the role that non-uniformity of ventricular mechanics plays in arrhythmogenesis may allow for the development of image-based markers of arrhythmogenic risk.
In terms of treatment, cardiac arrhythmias are largely managed by pharmacologically modifying ion channel activity, yet this approach is less effective than hoped. There may be unrealized potential for the stabilization of electrophysiological disturbances by targeting cardiac MEC mechanisms. For example, recent evidence suggests a decrease in the risk for ventricular tachyarrhythmias with cardiac resynchronization therapy.16 The electrical benefits of this mechanically targeted therapy may in part represent an improved temporal relation between local electrical activity, mechanical load, and contractile function. Combined with emerging appreciation of cardiac stretch-activated channels as pharmacological targets,17 mechanical heart rhythm management represents an exciting opportunity for translational studies of novel MEC-based anti-arrhythmic treatments aimed at the spatial normalization of repolarization timing.
Funding
The author acknowledges the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Nova Scotia Health Research Foundation, the Heart and Stroke Foundation of Nova Scotia, the Canada Foundation for Innovation, and the Dalhousie Medical Research Foundation for providing funding to support his work on cardiac mechano-electric coupling.
Acknowledgements
The author expresses his gratitude to Dr Peter Kohl for helpful comments on the manuscript.
Conflict of interest: none declared.
References
- 1.Quinn TA, Kohl P, Ravens U. Cardiac mechano-electric coupling research: fifty years of progress and scientific innovation. Prog Biophys Mol Biol 2014;115:71–75. [DOI] [PubMed] [Google Scholar]
- 2.Quinn TA. The importance of non-uniformities in mechano-electric coupling for ventricular arrhythmias. J Interv Card Electrophysiol 2014;39:25–35. [DOI] [PubMed] [Google Scholar]
- 3.Bainbridge FA. The influence of venous filling upon the rate of the heart. J Physiol 1915;50:65–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deck KA. Dehnungseffekte am spontanschlagenden, isolierten Sinusknoten. Pflugers Arch Gesamte Physiol Menschen Tiere 1964;280:120–130. [PubMed] [Google Scholar]
- 5.Quinn TA, Kohl P. Mechano-sensitivity of cardiac pacemaker function: pathophysiological relevance, experimental implications, and conceptual integration with other mechanisms of rhythmicity. Prog Biophys Mol Biol 2012;110:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz AM. Ernest Henry Starling, his predecessors, and the ‘Law of the Heart’. Circulation 2002;106:2986–2992. [DOI] [PubMed] [Google Scholar]
- 7.Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RA, Garny A, Morphew MK, Hoenger A, Lederer WJ, Kohl P. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res 2009;104:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prosser BL, Ward CW, Lederer WJ. X-ROS signalling is enhanced and graded by cyclic cardiomyocyte stretch. Cardiovasc Res 2013;98:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opthof T, Meijborg VMF, Belterman CN, Coronel R. Synchronization of repolarization by mechano-electrical coupling in the porcine heart. Cardiovasc Res 2015;108:181–187. [DOI] [PubMed] [Google Scholar]
- 10.Zabel M, Koller BS, Sachs F, Franz MR. Stretch-induced voltage changes in the isolated beating heart: importance of the timing of stretch and implications for stretch-activated ion channels. Cardiovasc Res 1996;32:120–130. [PubMed] [Google Scholar]
- 11.Markhasin VS, Solovyova O, Katsnelson LB, Protsenko Y, Kohl P, Noble D. Mechano-electric interactions in heterogeneous myocardium: development of fundamental experimental and theoretical models. Prog Biophys Mol Biol 2003;82:207–220. [DOI] [PubMed] [Google Scholar]
- 12.Opthof T, Sutton P, Coronel R, Wright S, Kallis P, Taggart P. The association of abnormal ventricular wall motion and increased dispersion of repolarization in humans is independent of the presence of myocardial infarction. Front Physiol 2012;3:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taggart P, Sutton PM. Cardiac mechano-electric feedback in man: clinical relevance. Prog Biophys Mol Biol 1999;71:139–154. [DOI] [PubMed] [Google Scholar]
- 14.Odening KE, Jung BA, Lang CN, Cabrera Lozoya R, Ziupa D, Menza M, Relan J, Franke G, Perez Feliz S, Koren G, Zehender M, Bode C, Brunner M, Sermesant M, Foll D. Spatial correlation of action potential duration and diastolic dysfunction in transgenic and drug-induced LQT2 rabbits. Heart Rhythm 2013;10:1533–1541. [DOI] [PubMed] [Google Scholar]
- 15.ter Bekke RM, Volders PG. Arrhythmogenic mechano-electric heterogeneity in the long-QT syndrome. Prog Biophys Mol Biol 2012;110:347–358. [DOI] [PubMed] [Google Scholar]
- 16.Barsheshet A, Wang PJ, Moss AJ, Solomon SD, Al-Ahmad A, McNitt S, Foster E, Huang DT, Klein HU, Zareba W, Eldar M, Goldenberg I. Reverse remodeling and the risk of ventricular tachyarrhythmias in the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy). J Am Coll Cardiol 2011;57:2416–2423. [DOI] [PubMed] [Google Scholar]
- 17.Reed A, Kohl P, Peyronnet R. Molecular candidates for cardiac stretch-activated ion channels. Glob Cardiol Sci Pract 2014;2014:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]