Abstract
Since its discovery as a post-translational signal for protein degradation, our understanding of ubiquitin (Ub) has vastly evolved. Today, we recognize that the role of Ub signaling is expansive and encompasses diverse processes including cell division, the DNA damage response, cellular immune signaling, and even organismal development. With such a wide range of functions comes a wide range of regulatory mechanisms that control the activity of the ubiquitylation machinery. Ub attachment to substrates occurs through the sequential action of three classes of enzymes, E1s, E2s, and E3s. In humans, there are 2 E1s, ∼35 E2s, and hundreds of E3s that work to attach Ub to thousands of cellular substrates. Regulation of ubiquitylation can occur at each stage of the stepwise Ub transfer process, and substrates can also impact their own modification. Recent studies have revealed elegant mechanisms that have evolved to control the activity of the enzymes involved. In this minireview, we highlight recent discoveries that define some of the various mechanisms by which the activities of E3-Ub ligases are regulated.
Keywords: E3 ubiquitin ligase, parkin, ubiquitin, ubiquitin ligase, ubiquitylation (ubiquitination), RING, cullin, ubiquitin regulation, HECT
Introduction
E3 ligases are generally grouped into three classes: 1) really interesting new genes (RINGs; also includes the topologically similar U-box E3s); 2) homologous to the E6-AP C terminus (HECTs); and 3) RING between RINGs (RBRs). Although all carry out the final step of covalent ubiquitylation of target proteins, they differ in both structure and mechanism. In humans, thousands of enzymes and proteins (not just E3s) work in concert to catalyze the regulated transfer of Ub3 to an even more diverse set of substrates. The shear number of potential interactions requires a precise network of protein-protein binding events to occur. Recent reviews have highlighted many of the essential mechanistic requirements for Ub transfer in E3s and other components of the Ub machinery (1–3). This minireview attempts to highlight emerging themes in E3-ligase regulation. The mechanisms discussed here encompass broader thematic areas of regulation but appear to be recurring phenomena in the regulation of E3-Ub ligases.
E3 Regulation by Auto-neddylation
RING E3s are the largest class of E3 ligases with over 600 members in the human genome. RINGs facilitate the direct transfer of Ub from an E2∼Ub conjugate to a substrate (4–6). cullin-RING ligases (CRLs) comprise a superfamily of RING E3s involved in a wide range of cellular functions and are responsible for up to 20% of Ub-dependent protein turnover in cells (7). A variety of mechanisms are employed to regulate CRL activity and have been discussed in detail elsewhere (8). Here we highlight a recently defined “on-off” switch involving the Ub-like protein NEDD8 to control CRL E3 activity.
CRLs are multiprotein complexes in which an elongated, highly helical cullin subunit provides a scaffold for modular assembly of a functional E3 ligase. A RING subunit (Rbx1 or Rbx2) binds to the cullin C-terminal domain where it recruits and activates E2∼Ub conjugates for direct Ub transfer to substrate lysine residues. The cullin N-terminal region binds to select substrate recognition modules that recruit specific substrates to the complex. Before a CRL can catalyze Ub transfer, a conserved cullin lysine close to the RING-binding site must be conjugated to the ubiquitin-like (UBL) protein NEDD8 (Fig. 1a). NEDD8 shares 58% identity with Ub, yet has its own dedicated E1 (NAE) and E2s (Ube2M and Ube2F) for activation and transfer. Efficient neddylation of CRLs requires both the Rbx1 RING domain, which binds activated Ube2M∼NEDD8, and a co-E3 DCN1, which simultaneously binds the cullin subunit and the acetylated N terminus of Ube2M. Coordinated interactions between Ube2M∼NEDD8 and Rbx1/DCN1 position the complex to neddylate one and only one cullin lysine residue (9). As Fig. 1a depicts, the covalent attachment of NEDD8 to the cullin subunit induces a large rearrangement of its C-terminal domain that repositions the Rbx1 RING in an orientation optimal for recruiting E2∼Ub conjugates and modifying substrates (10). Thus, the RING subunit of CRLs is multifunctional: it can both facilitate neddylation and promote Ub transfer. Which of the two functions it performs is determined by the overall conformation of the CRL complex, which is in turn dictated by the attachment of NEDD8.
FIGURE 1.
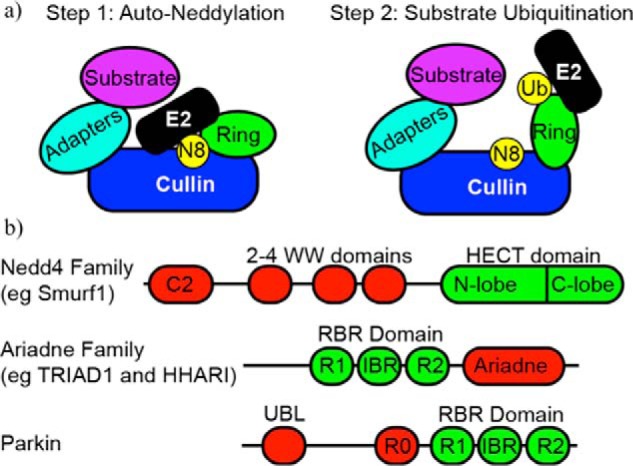
Domain architecture of regulated E3 ligases. a, left, schematic of a cullin ligase bound to an E2∼Nedd8 conjugate. The RING domain of the cullin is positioned to allow specific neddylation. Right, upon neddylation, the RING adopts a new conformation that allows for interaction with an incoming E2∼Ub conjugate that can facilitate substrate ubiquitylation. b, domain architecture of the Nedd4, Ariadne family, and Parkin E3 ligases. IBR, in between ring domain.
In addition to its known role as a CRL-specific regulator, NEDD8 is proposed to have a variety of non-CRL targets in both plants and animals (11, 12). A HECT-type Ub ligase in humans, Smurf1, which is linked to multiple cellular functions including cell growth and mobility, bone generation, and viral autophagy, was recently reported to be activated by neddylation (13). Similar to CRLs, Smurf1 catalyzes its own auto-neddylation, making it the first reported case of a HECT ligase that carries out both Ub and NEDD8 transfer. In contrast to CRLs, Smurf1 neddylates multiple lysine residues throughout its various domains. The result is activation of its Ub ligase activity by promoting the binding of E2 Ub-conjugating enzymes through an as yet undefined mechanism. Smurf1 and other HECTs are distinct from RINGs in that they form an obligate E3∼Ub intermediate through a conserved cysteine residue located in the C-lobe of the HECT domain prior to substrate ubiquitylation (Fig. 1b). Smurf1 auto-neddylation also proceeds via an obligate E3∼NEDD8 intermediate, but this transfer requires a cysteine residue located in the N-lobe of the Smurf1 HECT domain. These findings expand the landscape for how NEDD8 may be used to regulate Ub transfer pathways as well as the mechanisms by which HECT E3s work.
E3 Regulation by Auto-inhibition
It is increasingly clear that many E3s reside in an auto-inhibited state in which a region of the protein outside the catalytic domain prevents access to the active site. The NEDD4 family of HECT E3s, which includes Smurf1 (see above), is in this category. Although they share similar domain architectures with an N-terminal C2 domain, 2–4 WW domains, and a C-terminal HECT domain (Fig. 1b), NEDD4 family members' functions and mechanisms of inactivation and activation vary (14–16). Smurf1 forms an inactive head-to-tail dimer in which the C2 domain of one subunit binds and inhibits the E3 domain in the other (17). Auto-inhibition is released by the binding of various adaptor proteins to the WW domain of Smurf1 (e.g. CKIP-1), the WW domains and the C2 domains (e.g. Cdh1), or even the HECT domain (e.g. CCM2) (18–20). In light of the recent findings discussed above, NEDD8 modification may also lead to the release of auto-inhibitory interactions, although this remains to be tested. In contrast, the activity of Smurf2 is controlled by intramolecular interactions between its C2 and HECT domains (15). Binding of the adaptor protein Smad7 to the Smurf2 WW domains releases the auto-inhibitory state and promotes Ub transfer activity (21).
Phosphorylation is an important mechanism regulating the ligase activity of several E3s, but it has only recently been found to play a role in the release of auto-inhibition. An example of this type of regulation is provided by the E3 ligase Itch (whose name originates from the skin inflammation observed in knock-out mice), which is a member of the HECT NEDD4 family. Itch plays a key role in inflammatory signaling pathways. Interactions between its WW and HECT domains stabilize the auto-inhibited form of Itch. Part of its activation mechanism involves phosphorylation of a proline-rich region, which releases these auto-inhibitory interactions and activates Itch to ubiquitylate JunB, which, in turn, helps prevent the production of cytokines (16). Additionally, an adapter protein, Ndfip1, is required for E2 recruitment to Itch and the transfer of Ub to the inflammatory response activator, Tak1 (22). It remains to be seen whether Ndfip1 binding also releases auto-inhibitory interactions similar to Itch phosphorylation.
The HECT ligase NEDD4.1 is activated by phosphorylation of its HECT and C2 domains by the tyrosine kinase c-Src. Although the auto-inhibitory interactions and site of phosphorylation differ from those observed in Itch, tyrosine phosphorylation disrupts the auto-inhibitory interactions, leading to activation of the ligase (23). Intriguingly, a NEDD4.1 substrate, fibroblast growth factor receptor 1 (FGFR1), is the activator of the NEDD4.1 kinase, c-Src. Ubiquitylation of FGFR1 by NEDD4.1 leads to its removal from the cell surface, thus providing a negative feedback loop for receptor tyrosine kinase signaling. Another closely related HECT ligase, NEDD4.2, is also auto-inhibited via interactions between its HECT and C2 domains, but is activated by calcium binding rather than phosphorylation. Calcium release is a result of phospholipase C activation and serves as a second messenger in a wide variety of cell signaling events. Escobedo et al. (24) showed that calcium binding to the C2 domain in NEDD4.2 prevents interactions between the HECT and C2 domains and results in E3-ligase activation.
The RBR class of E3 ligases uses a unique RING-HECT hybrid mechanism for Ub transfer (25). RBRs contain a RING domain (RING1) that, like traditional RINGs, binds to E2∼Ub conjugates. However, rather than activating the conjugate for direct transfer to a substrate, RBRs behave like HECTs and catalyze Ub transfer from the E2 active site to a catalytic cysteine in a second domain (“RING2”) to form an obligate E3∼Ub intermediate (Fig. 1b) (25). The importance of RBRs in neurological disorders and immune signaling has spurred many functional and structural studies, and these have been recently reviewed (26–28).
The largest family of RBRs is the Ariadne family, defined by an auto-inhibitory Ariadne domain C-terminal to the catalytic RBR domain (Fig. 1b). In a recent crystal structure of human homolog of Ariadne (HHARI), the Ariadne domain interacts with the RBR to conceal the active-site cysteine in the RING2 domain (29). Functional studies revealed that auto-inhibition for the two most conserved members of the Ariadne family, TRIAD1 and HHARI, can be released by binding to neddylated CRLs both in vitro and in vivo (30). Interaction with neddylated CRLs exposes the RING2 active-site cysteine to the same extent as removal of the inhibitory Ariadne domain altogether. In reciprocal fashion, interactions with TRIAD1 or HHARI appear to increase both the ligase activity and the protein levels of neddylated CRLs in cells. These findings hint at a complex regulatory interplay between different classes of E3-ligases.
The RBR E3 Parkin, which if mutated can play a critical role in the onset of juvenile Parkinson disease, provides another example of an auto-inhibited RBR, although its architecture and regulatory mechanisms differ from Ariadne family RBRs. In parkin, the RBR domain is positioned at the C-terminal end of the protein, preceded by a zinc-binding domain, RING0, and a UBL domain (Fig. 1b). In the inactive state, RING0 blocks the E2-binding site on RING1, and a short sequence within the RBR domain called the repressor element is positioned to block the RING2 active-site cysteine (31–33). Regulation of parkin activity involves the kinase PINK1, which recruits parkin to damaged mitochondria and phosphorylates Ser-65 of parkin's UBL domain. The PINK1-dependent phosphorylation induces a conformational change within parkin that enhances its E3 activity to ubiquitylate mitochondrial outer membrane proteins. Intriguingly, PINK1 appears to play multiple roles in parkin activation as it also phosphorylates Ub on Ser-65, the analogous position to the parkin UBL phosphorylation site (34, 35). Phospho-Ub binds to parkin, enhancing its catalytic activity allosterically. Another proposed role for phospho-Ub is that when attached to mitochondrial proteins, it facilitates recruitment of parkin and serves as a mitochondrial anchor. This relationship between kinase and ligase activity results in a feed-forward regulation that helps confine activated parkin activity to damaged mitochondria. Phospho-Ub inhibits deubiquitinases and some Ub chain-building E2 and E3 ligases in vitro, so it is likely a mode of Ub-pathway regulation outside of parkin activation (36).
E3 Regulation by Complex Assembly
E3s are often called to action in response to relatively rare cellular events such as a stalled ribosome, damaged DNA, or instances of epigenetic regulation (37–40). These situations generally require the recruitment of multiple factors (i.e. protein, DNA, RNA), including an active Ub transfer complex to the site of regulation, subsequently generating large supramolecular complexes. This strategy allows the cell to target low-level substrates with high selectivity and dictates where and when ubiquitylation occurs. Recent functional and structural studies reveal that the assembly of Ub transfer complexes on supramolecular complexes involves multivalent interactions that impart an avidity advantage in targeting substrates. An emerging theme in this type of regulation is the importance of contacts between the substrate and Ub machinery that occur distant to the site of catalysis.
Protein translation can stall when an mRNA is truncated or non-stop translation occurs. In such cases, ribosomes can become trapped with a bound peptidyl-tRNA and a nascent polypeptide chain protruding from the ribosome exit tunnel (41–43). When this occurs, ribosomal components must be recycled and the nascent chain must be extracted and destroyed. First, recycling factors split the ribosome into its component 40S and 60S parts, leaving the 60S complex stuck with the associated peptidyl-tRNA and a partly translated nascent chain. Next, the multienzyme ribosome quality control complex (RQC) is assembled on the stalled 60S ribosomal subunit (37, 44, 45). Two RQC components have well characterized roles in Ub transfer: NEMF (Tae2 in yeast) and the RING E3 ligase Listerin (Ltn1 in yeast) (44–46). A model for the assembly of the RQC Ub transfer complex and control of stalled ribosome ubiquitylation has been proposed based on recent cryo-EM studies (Fig. 2a) (46, 47). An essential step in assembly of the RQC is the discrimination of a stalled 60S subunit from functionally competent 60S subunits present at much higher concentrations. This is where NEMF appears to play a critical role (46). NEMF is composed of three globular regions: N- and C-lobes with a middle (M) domain interconnected by coiled-coil segments. After dissociation of the 40S subunit and the associated message, the tRNA attached to the nascent polypeptide is left exposed in the P-site of the 60S subunit. This is an important recognition point for NEMF. Both its N-lobes and its C-lobes appear to recognize the exposed tRNA and also make multiple contacts with nearby ribosomal protein subunits (Fig. 2a) (44, 46, 47). The M-domain also makes multiple contacts with ribosomal proteins and stabilizes the P-stalk of ribosomal RNA. By binding at an interface between 40S and 60S subunits, NEMF effectively prevents premature reassociation of the 40S subunit.
FIGURE 2.
Ubiquitin regulation by supramolecular assembly. a, EM structure (Protein Data Bank (PDB) 3J92) of the 60S ribosome subunit bound to peptidyl-tRNA (orange), NEMF (blue), and Listerin (red). The C-terminal region of Listerin, and specifically the RING domain, is positioned just outside the exit tunnel (orange) to facilitate ubiquitinylation of the nascent chain. The RWD domain of Listerin binds to one interaction surface of the 60S ribosome. NEMF directly interacts with the peptidyl-tRNA and the N terminus of Listerin to help anchor the RQC and prevent reassembly with the 40S subunit. b, structure of the PRC1-nucleosome complex (PDB 4R8P). The members of the PRC1 complex, UbcH5c (green), RING1a (blue), and Bmi1 (gray), sit atop the nucleosome, positioning the active site of the E2 adjacent to Lys-119 of histone H2A (orange). Other histone components, histone H2B 1.1, (yellow), histone H3.2 (dark pink), and histone H4 (maroon), play roles in positioning the complex for proper ubiquitylation. c, the inactive RING domain of Bmi1 makes direct contacts with histones H3.2 and H4 that also stabilize the interaction between PRC1 and the nucleosome. Side chain residues of Lys-62 and Arg-64 hydrogen-bond with Glu-74 in histone H4 and Asp-77 of H3.2, respectively. d, β-sheet residues in UbcH5c that are distant from the active site play a role in positioning the E2 on the NCP. Side chain residues of His-32 and Lys-66 make hydrogen-bonding contacts with backbone phosphate groups from the nucleosome DNA.
Ltn1 is a 200 Å long protein containing numerous HEAT repeats and an RWD (RING finger and WD-domain-containing-proteins and DEAD-like helicases) domain that immediately precedes its C-terminal RING E3 ligase domain. Ltn1 has little affinity for either isolated 60S subunits or intact ribosomes (44). However, the NEMF M-domain serves as an anchor point for the Ltn1 N terminus, allowing the Ltn1 RWD domain to sandwich between two ribosomal proteins to place the RING domain on the rim of the ribosomal exit tunnel (Fig. 2a). Other than its N terminus and RWD domain, the bulk of Ltn1 appears to make little or no contact with the 60S subunit (46, 47). Together, NEMF and Ltn1 provide at least eight different contact points with the 60S subunit and associated peptidyl-tRNA. Individually, each contact point represents a weak interaction, but in combination, they form a stable complex that is specific for an aberrant 60S subunit, prevents reassociation of the 40S subunit, and positions the Ltn1 RING domain where it can recruit E2∼Ub conjugates and facilitate ubiquitylation of the emerging polypeptide chain (44, 46, 47).
A second example of supramolecular complexes regulating Ub transfer involves histone ubiquitylation carried out by the polycomb group (PcG) proteins. The PcG proteins are important multicomponent epigenetic regulators of chromatin structure and establish control of transcription through the addition or removal of various histone post-translational modifications (48, 49). Polycomb repressive complex 1 (PRC1), for example, is responsible for the monoubiquitylation of histone H2A Lys-119, which appears to be a signal for the recruitment of the histone-methyltransferase complex PRC2 (40, 50–54). A complex structure solved by McGinty et al. (55) reveals the importance of multiple types of substrate contacts in the assembly of PRC1 on a nucleosome and subsequent ubiquitylation of histone H2A (Fig. 2b).
PRC1 is assembled around one of two related RING E3 ligases, Ring1a or Ring1b (RNF2). On their own, these RINGs are poor Ub ligases, but their activity increases substantially when they form a heterodimeric complex with Polycomb group RING-finger (PCGF) paralogs, the most widely characterized being Bmi1 (PCG4) (56, 57). Ring1a/b and PCGF paralogs all have RING domains, but only the Ring1 component interacts with E2∼Ub conjugates to facilitate Ub transfer. Although intrinsic Ub transfer activity is enhanced by formation of the Ring1b/Bmi1 heterodimer, it does not efficiently monoubiquitylate isolated H2A. Instead, specific modification only occurs in the context of the nucleosome, implying a role for other histones and/or associated DNA in the assembly and activity of the complex (56–58).
Using a fused E3-E2 construct, McGinty et al. (55) solved a crystal structure of a minimal PRC1 complex bound to a nucleosome core particle (NCP) (Fig. 2a). The structure reveals contacts between the Ring1b subunit of PRC1 and multiple histones. In particular, an interaction shown to be vital for efficient substrate binding and ubiquitylation involves two basic residues in the RING domain, Lys-97 and Arg-98, and an acidic patch on histone H2A. Bmi1 also engages the NCP through hydrogen bonding and van der Waals interactions with histones H3, H4, and H2B. Like Ring1b, two basic residues in Bmi1 (Lys-62 and Arg-64) interact with acidic residues in histone H3 (Glu-77) and histone H4 (Glu-74) that are important for efficient NCP ubiquitylation (Fig. 2b).
Generally thought to be recruited solely by the E3 component of a multiprotein complex, the E2 UbcH5c is also critical for PRC1-NCP complex assembly (55). As expected, UbcH5c binds to Ring1b, in an interaction known to activate the E2∼Ub for transfer to H2A Lys-119. Unexpectedly, UbcH5c also makes direct contact with the NCP, in particular with nucleosomal DNA. Residues in the UbcH5c β-sheet and α-3 helix interact with adjacent DNA phosphate groups (Fig. 2b). The β-sheet of UbcH5 is a widely used protein-protein interaction site, so its involvement in recognizing DNA is particularly striking. Mutation of these E2 residues resulted in a 10-fold decrease in affinity of UbcH5c for the NCP and consequently a decrease in ubiquitylation. As UbcH5c is a particularly promiscuous E2 (59), these networks of novel interactions serve to position the E2 active site directly over Lys-119 of H2A, perfectly poised for Ub transfer to its designated acceptor.
Ring1a and Ring1b can form active E3-Ub ligase complexes with any of six different PCGF paralogs. Functional differences among the different complexes remain to be deciphered but may be linked to substrate targeting, targeting to different genomic loci, and/or the regulation of different genes. Intriguingly, different heterodimeric complexes show large variations in their inherent ability to stimulate the transfer of Ub in simplified reaction systems (57). However, these differences are overcome upon assembly on an NCP, demonstrating again that assembly of E3 ligase supramolecular complexes engages multiple substrate surfaces distant from the site modification, to specify and facilitate ubiquitylation (57).
E3 Regulation by Non-protein Ligand Binding
Small molecules and non-protein ligands also serve as non-covalent regulators of E3 activity. For instance, Turner et al. (81) found that the Saccharomyces cerevisiae E3 Ubr1, associated with regulating protein turnover via the N-end rule pathway, also targets the degradation of the transcriptional regulatory protein Cup9. Cup9 is a transcriptional repressor of the di- and tripeptide transporter Ptr2. Increased levels of dipeptides in the growth medium led to increasing levels in the cell. Elevated peptide levels provide an environmental signal for the Ubr1-dependent ubiquitylation of Cup9 and its subsequent degradation, leading to increased levels of Ptr2 and increased peptide import. The environmental signal is translated into action by the direct binding of dipeptides to Ubr1. This interaction induces a conformational change that alters Ubr1 substrate selectivity toward Cup9. Thus, an environmental signal in the form of a small molecule can directly alter E3 activity via binding and allosteric regulation.
There are also growing numbers of examples in which non-covalent binding of non-protein small molecules or polymers functions to regulate ubiquitylation machinery. For example, the plant hormone auxin regulates many aspects of cell growth and development (60). Auxin binds to the F-box protein, TIR1, and completes the substrate-binding site for auxin-response factor (ARF) transcription factors (61). TIR1 can then deliver an ARF to the SCF E3 ligase, a member of the CRL superfamily, for ubiquitylation and proteasomal degradation (61). In the remaining examples, we highlight new cases where non-protein ligands play critical roles in regulating E3 ligase activities.
In the mid-1950s, thalidomide was introduced as a sedative and prescribed as a treatment for “anxiety, insomnia, gastritis, and tension” (62, 63). Subsequently, thalidomide was prescribed for the treatment of “morning sickness” in pregnant women. It soon became clear that thalidomide was responsible for severe birth defects including deformed limbs, blindness, deafness, and death, leading to its eventual outlawing as a treatment for morning sickness (64, 65). However, recently, thalidomide and related derivatives, lenalidomide and pomalidomide, collectively known as immunomodulatory drugs (IMiDs), have found favor as effective treatments for specific forms of multiple myeloma (66).
A molecular target for thalidomide and other IMiDs is cereblon (CRBN), a receptor that delivers substrates to a CRL E3 ligase complex containing CUL4, RBX1, and DDB1 (CRL4CRBN) (67). Mutations in the CRBN gene are associated with autosomal recessive, non-syndromic mental retardation, and the antiproliferative effects seen with IMiDs in myeloma cells are linked to CRBN expression (68–70). Recent structural studies on the DDB1-CRBN complex bound to thalidomide, lenalidomide, or pomalidomide revealed that IMiD binding to CRBN alters the substrate-binding site and changes target selectivity (Fig. 3) (71). In these studies, Fischer et al. showed that the transcription factor MEIS2, implicated in human development, is an endogenous target for CRL4CRBN and that IMiDs inhibit its degradation. Instead, when bound to thalidomide, lenalidomide, or pomalidomide, the CRL4CRBN complex targets two members of the Ikaros family of transcription factors for ubiquitylation and degradation, IKZF1 and IKZF3. These proteins are essential for B- and T-cell differentiation (72). These results demonstrate that IMiDs are potent small molecular regulators of CRL4CRBN and possess both agonistic and antagonistic properties based on specific substrates (Fig. 3). It raises the intriguing question of whether there are endogenous small molecule regulators for CRBN that the IMiDs are mimicking or whether the drugs have found a serendipitous, yet highly specific binding site. Regardless, the findings serve as an excellent example of how small molecules can regulate ubiquitylation events at the levels of the E3 and substrate.
FIGURE 3.
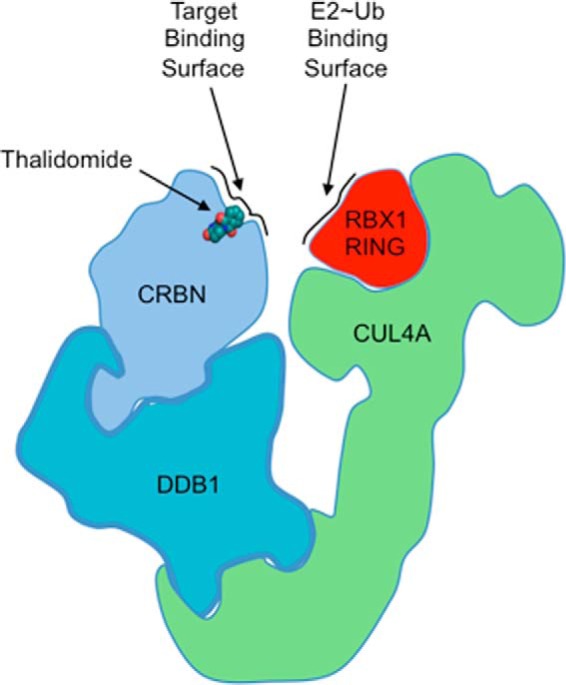
Architectural schematic of the putative CUL4, RBX1, DDB1, CRBN complex (CRL4CRBN) bound to thalidomide (PDB 4C1I for DDB1-CRBNThalidomide complex). Thalidomide occupies a position in the putative CRBN-substrate interface and can positively or negatively modulate target substrate interactions. CRBN sits adjacent to RBX1, bringing E2∼Ub conjugates and substrate together.
Protein poly(ADP-ribosyl)ation (PARylation) is a post-translational modification linked to DNA repair, Wnt signaling, and transcription (73). Attachment of this non-protein polymer has also been linked to ubiquitylation processes (74–76). A recent study into the structural connection between PARylation and ubiquitylation (termed PARylation-dependent ubiquitylation (PARdU)) revealed a novel allosteric on-off switch regulating E3 activity. RNF146 contains an N-terminal RING domain followed by a WWE domain that had previously been shown to bind an internal subunit of PAR polymers known as iso-ADP-ribose (iso-ADPR) (77). Surprisingly, the E3 ligase activity of RNF146 is non-functional in the absence of either PAR or iso-ADPR. By comparing their crystal structure of RNF146 bound to iso-ADPR to an NMR structure of the ligand-free RING, DaRosa et al. (78) observed that the ligand makes contacts to both the RING and the WWE domains (Fig. 4a). Importantly, ligand binding promotes a conformational change in the RING from an E2 binding-incompetent state to an E2 binding-competent one (Fig. 4b). This is the first known example of a RING domain that requires binding of a moiety other than Zn2+ ions to stabilize the active conformation.
FIGURE 4.
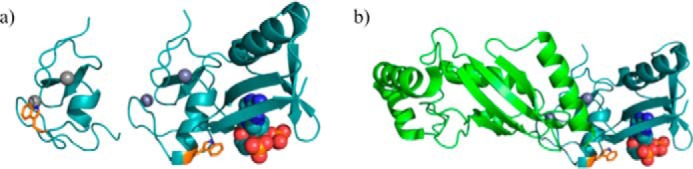
An allosteric switch: the structure of the RNF146-UbcH5a complex. a, left, selected member of the solution ensemble of the RNF146 RING domain showing the E2 binding-incompetent state (PDB 2D8T). Right, crystal structure of the RNF146 RING-WWE domains bound to iso-ADPR (PDB 4QPL). Iso-ADPR binds between the RING and WWE domains. Binding of iso-ADPR results in the repositioning of Trp-66 and the extension of the helical region of the RING domain. b, UbcH5a binds to the RING domain of RNF146 when iso-ADPR binds between the RING and WWE domains. Displacement of Trp-66 allows for the interaction between RING and E2.
RNF146 works in close conjunction with the PAR polymerase Tankyrase (TNKS) (79). The regulatory protein Axin, which is important in Wnt signaling, is among the substrates of TNKS (74, 80). DaRosa et al. (78) show that RNF146 binds directly to TNKS via interactions in the intrinsically disordered RNF146 C-terminal region. Using structure-based mutant forms of RNF146 in cells, they showed that ubiquitylation and degradation of Axin require that RNF146 be able to bind to both PAR and TNKS. The mechanism may guide development of small molecule inhibitors of RNF146, whose overexpression is often associated with lung cancers.
Summary
The recent discoveries surveyed here provide only a glimpse into the diverse regulatory mechanisms used to control E3 ligases. It is, perhaps, not surprising that like other enzymatic pathways ubiquitylation is regulated by E3 auto-inhibition and phosphorylation mechanisms. However, E3 regulation is exceptional in the diversity of other post-translational modifications used, such as neddylation, a signaling pathway closely related to ubiquitylation, and even PARylation. Many types of control mechanisms can converge to regulate a particular E3, which may also include non-covalent binding to a variety of adaptor proteins, protein complexes, or non-protein ligands.
Protein-protein interactions have always been a focal point for investigating Ub transfer pathways. However, many of the important binary interactions exhibit only weak affinities. An important concept governing Ub transfer reactions is the assembly of large macromolecular and supramolecular complexes through extensive networks of secondary interactions among components of the Ub machinery and target substrates. Many of the important interactions that collectively combine to regulate transfer are often distant from the site of catalysis. Identifying and disrupting these important distal interactions may allow for more precise targeting of specific ubiquitylation reactions than targeting the actual E3 catalytic centers. The inability to properly regulate Ub modifications is often associated with cancer and other diseases. However, the interconnected nature of different Ub pathways and similarities between various E3 mechanisms make the Ub pathway a challenging target for drug development. Uncovering the molecular details of E3 activation and inactivation, along with demonstrating that these states can be targeted by small molecules or specialized adapter proteins, provides promising avenues for pursuing cancer and disease treatments.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 GM088055 (to R. E. K.) and R01 GM098503 (to P. S. B.) through the NIGMS and T32 CA080416 (to M. D. S.) through the NCI. The authors declare that they have no conflicts of interest with the contents of this article.
- Ub
- ubiquitin
- CRL
- cullin-RING ligase
- UBL
- ubiquitin-like
- RQC
- ribosome quality control complex
- PcG
- polycomb group
- PCGF
- Polycomb group RING-finger
- NCP
- nucleosome core particle
- IMiD
- immunomodulatory drug
- PAR
- poly(ADP-ribosyl)
- PARylation
- poly(ADP-ribosyl)ation
- iso-ADPR
- iso-ADP-ribose
- NEMF
- nuclear export mediator factor.
References
- 1. Berndsen C. E., Wolberger C. (2014) New insights into ubiquitin E3 ligase mechanism. Nat. Struct. Mol. Biol. 21, 301–307 [DOI] [PubMed] [Google Scholar]
- 2. Mattiroli F., Sixma T. K. (2014) Lysine-targeting specificity in ubiquitin and ubiquitin-like modification pathways. Nat. Struct. Mol. Biol. 21, 308–316 [DOI] [PubMed] [Google Scholar]
- 3. Metzger M. B., Pruneda J. N., Klevit R. E., Weissman A. M. (2014) RING-type E3 ligases: master manipulators of E2 ubiquitin-conjugating enzymes and ubiquitination. Biochim. Biophys. Acta 1843, 47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pruneda J. N., Littlefield P. J., Soss S. E., Nordquist K. A., Chazin W. J., Brzovic P. S., Klevit R. E. (2012) Structure of an E3:E2∼Ub complex reveals an allosteric mechanism shared among RING/U-box ligases. Mol. Cell 47, 933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Plechanovová A., Jaffray E. G., Tatham M. H., Naismith J. H., Hay R. T. (2012) Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature 489, 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dou H., Buetow L., Sibbet G. J., Cameron K., Huang D. T. (2012) BIRC7–E2 ubiquitin conjugate structure reveals the mechanism of ubiquitin transfer by a RING dimer. Nat. Struct. Mol. Biol. 19, 876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soucy T. A., Smith P. G., Milhollen M. A., Berger A. J., Gavin J. M., Adhikari S., Brownell J. E., Burke K. E., Cardin D. P., Critchley S., Cullis C. A., Doucette A., Garnsey J. J., Gaulin J. L., Gershman R. E., Lublinsky A. R., McDonald A., Mizutani H., Narayanan U., Olhava E. J., Peluso S., Rezaei M., Sintchak M. D., Talreja T., Thomas M. P., Traore T., Vyskocil S., Weatherhead G. S., Yu J., Zhang J., Dick L. R., Claiborne C. F., Rolfe M., Bolen J. B., Langston S. P. (2009) An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732–736 [DOI] [PubMed] [Google Scholar]
- 8. Lydeard J. R., Schulman B. A., Harper J. W. (2013) Building and remodelling Cullin–RING E3 ubiquitin ligases. EMBO Rep. 14, 1050–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott D. C., Sviderskiy V. O., Monda J. K., Lydeard J. R., Cho S. E., Harper J. W., Schulman B. A. (2014) Structure of a RING E3 trapped in action reveals ligation mechanism for the ubiquitin-like protein NEDD8. Cell 157, 1671–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duda D. M., Borg L. A., Scott D. C., Hunt H. W., Hammel M., Schulman B. A. (2008) Structural insights into NEDD8 activation of Cullin-RING ligases: conformational control of conjugation. Cell 134, 995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mergner J., Heinzlmeir S., Kuster B., Schwechheimer C. (2015) Deneddylase1 Deconjugates NEDD8 from Non-Cullin Protein Substrates in Arabidopsis thaliana. Plant Cell 27, 741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enchev R. I., Schulman B. A., Peter M. (2015) Protein neddylation: beyond cullin-RING ligases. Nat. Rev. Mol. Cell Biol. 16, 30–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie P., Zhang M., He S., Lu K., Chen Y., Xing G., Lu Y., Liu P., Li Y., Wang S., Chai N., Wu J., Deng H., Wang H.-R., Cao Y., Zhao F., Cui Y., Wang J., He F., Zhang L. (2014) The covalent modifier Nedd8 is critical for the activation of Smurf1 ubiquitin ligase in tumorigenesis. Nat. Commun. 5, 3733. [DOI] [PubMed] [Google Scholar]
- 14. Ingham R. J., Gish G., Pawson T. (2004) The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene 23, 1972–1984 [DOI] [PubMed] [Google Scholar]
- 15. Wiesner S., Ogunjimi A. A., Wang H.-R., Rotin D., Sicheri F., Wrana J. L., Forman-Kay J. D. (2007) Autoinhibition of the HECT-type ubiquitin ligase Smurf2 through its C2 domain. Cell 130, 651–662 [DOI] [PubMed] [Google Scholar]
- 16. Gallagher E., Gao M., Liu Y.-C., Karin M. (2006) Activation of the E3 ubiquitin ligase Itch through a phosphorylation-induced conformational change. Proc. Natl. Acad. Sci. U.S.A. 103, 1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wan L., Zou W., Gao D., Inuzuka H., Fukushima H., Berg A. H., Drapp R., Shaik S., Hu D., Lester C., Eguren M., Malumbres M., Glimcher L. H., Wei W. (2011) Cdh1 regulates osteoblast function through an APC/C-Independent modulation of Smurf1. Mol. Cell 44, 721–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu K., Yin X., Weng T., Xi S., Li L., Xing G., Cheng X., Yang X., Zhang L., He F. (2008) Targeting WW domains linker of HECT-type ubiquitin ligase Smurf1 for activation by CKIP-1. Nat. Cell Biol. 10, 994–1002 [DOI] [PubMed] [Google Scholar]
- 19. Jia L., Yu H. (2011) Cdh1 is a HECT of an activator. Mol. Cell 44, 681–683 [DOI] [PubMed] [Google Scholar]
- 20. Crose L. E. S., Hilder T. L., Sciaky N., Johnson G. L. (2009) Cerebral cavernous malformation 2 protein promotes Smad ubiquitin regulatory factor 1-mediated RhoA degradation in endothelial cells. J. Biol. Chem. 284, 13301–13305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aragón E., Goerner N., Xi Q., Gomes T., Gao S., Massagué J., Macias M. J. (2012) Structural basis for the versatile interactions of Smad7 with regulator WW domains in TGF-β pathways. Structure 20, 1726–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kathania M., Zeng M., Yadav V. N., Moghaddam S. J., Yang B., Venuprasad K. (2015) Ndfip1 regulates Itch ligase activity and airway inflammation via UbcH7. J. Immunol. 194, 2160–2167 [DOI] [PubMed] [Google Scholar]
- 23. Persaud A., Alberts P., Mari S., Tong J., Murchie R., Maspero E., Safi F., Moran M. F., Polo S., Rotin D. (2014) Tyrosine phosphorylation of NEDD4 activates its ubiquitin ligase activity. Sci. Signal. 7, ra95–ra95 [DOI] [PubMed] [Google Scholar]
- 24. Escobedo A., Gomes T., Aragón E., Martín-Malpartida P., Ruiz L., Macias M. J. (2014) Structural basis of the activation and degradation mechanisms of the E3 ubiquitin ligase Nedd4L. Structure 22, 1446–1457 [DOI] [PubMed] [Google Scholar]
- 25. Wenzel D. M., Lissounov A., Brzovic P. S., Klevit R. E. (2011) UBCH7 reactivity profile reveals parkin and HHARI to be RING/HECT hybrids. Nature 474, 105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wenzel D. M., Klevit R. E. (2012) Following Ariadne's thread: a new perspective on RBR ubiquitin ligases. BMC Biol. 10, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smit J. J., Sixma T. K. (2014) RBR E3-ligases at work. EMBO Rep. 15, 142–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dove K. K., Klevit R. E. (2013) Structural Biology: Parkin's serpentine shape revealed in the Year of the Snake. Curr. Biol. 23, R691–R693 [DOI] [PubMed] [Google Scholar]
- 29. Duda D. M., Olszewski J. L., Schuermann J. P., Kurinov I., Miller D. J., Nourse A., Alpi A. F., Schulman B. A. (2013) Structure of HHARI, a RING-IBR-RING ubiquitin ligase: autoinhibition of an Ariadne-family E3 and insights into ligation mechanism. Structure 21, 1030–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelsall I. R., Duda D. M., Olszewski J. L., Hofmann K., Knebel A., Langevin F., Wood N., Wightman M., Schulman B. A., Alpi A. F. (2013) TRIAD1 and HHARI bind to and are activated by distinct neddylated Cullin-RING ligase complexes. EMBO J. 32, 2848–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trempe J.-F., Sauvé V., Grenier K., Seirafi M., Tang M. Y., Ménade M., Al-Abdul-Wahid S., Krett J., Wong K., Kozlov G., Nagar B., Fon E. A., Gehring K. (2013) Structure of Parkin reveals mechanisms for ubiquitin ligase activation. Science 340, 1451–1455 [DOI] [PubMed] [Google Scholar]
- 32. Wauer T., Komander D. (2013) Structure of the human Parkin ligase domain in an autoinhibited state. EMBO J. 32, 2099–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riley B. E., Lougheed J. C., Callaway K., Velasquez M., Brecht E., Nguyen L., Shaler T., Walker D., Yang Y., Regnstrom K., Diep L., Zhang Z., Chiou S., Bova M., Artis D. R., Yao N., Baker J., Yednock T., Johnston J. A. (2013) Structure and function of Parkin E3 ubiquitin ligase reveals aspects of RING and HECT ligases. Nat. Commun. 4, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kane L. A., Lazarou M., Fogel A. I., Li Y., Yamano K., Sarraf S. A., Banerjee S., Youle R. J. (2014) PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 205, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kazlauskaite A., Kondapalli C., Gourlay R., Campbell D. G., Ritorto M. S., Hofmann K., Alessi D. R., Knebel A., Trost M., Muqit M. M. K. (2014) Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem. J. 460, 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wauer T., Swatek K. N., Wagstaff J. L., Gladkova C., Pruneda J. N., Michel M. A., Gersch M., Johnson C. M., Freund S. M., Komander D. (2015) Ubiquitin Ser65 phosphorylation affects ubiquitin structure, chain assembly and hydrolysis. EMBO J. 34, 307–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bengtson M. H., Joazeiro C. A. P. (2010) Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature 467, 470–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Al-Hakim A., Escribano-Diaz C., Landry M.-C., O'Donnell L., Panier S., Szilard R. K., Durocher D. (2010) The ubiquitous role of ubiquitin in the DNA damage response. DNA Repair 9, 1229–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Müller J., Müller C. W. (2014) Structural Biology: enzyme-chromatin complex visualized. Nature 514, 572–573 [DOI] [PubMed] [Google Scholar]
- 40. Blackledge N. P., Farcas A. M., Kondo T., King H. W., McGouran J. F., Hanssen L. L. P., Ito S., Cooper S., Kondo K., Koseki Y., Ishikura T., Long H. K., Sheahan T. W., Brockdorff N., Kessler B. M., Koseki H., Klose R. J. (2014) Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and Polycomb domain formation. Cell 157, 1445–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Doma M. K., Parker R. (2006) Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440, 561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frischmeyer P. A., van Hoof A., O'Donnell K., Guerrerio A. L., Parker R., Dietz H. C. (2002) An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science 295, 2258–2261 [DOI] [PubMed] [Google Scholar]
- 43. van Hoof A., Frischmeyer P. A., Dietz H. C., Parker R. (2002) Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 295, 2262–2264 [DOI] [PubMed] [Google Scholar]
- 44. Brandman O., Stewart-Ornstein J., Wong D., Larson A., Williams C. C., Li G.-W., Zhou S., King D., Shen P. S., Weibezahn J., Dunn J. G., Rouskin S., Inada T., Frost A., Weissman J. S. (2012) A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell 151, 1042–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Defenouillère Q., Yao Y., Mouaikel J., Namane A., Galopier A., Decourty L., Doyen A., Malabat C., Saveanu C., Jacquier A., Fromont-Racine M. (2013) Cdc48-associated complex bound to 60S particles is required for the clearance of aberrant translation products. Proc. Natl. Acad. Sci. U.S.A. 110, 5046–5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shao S., Brown A., Santhanam B., Hegde R. S. (2015) Structure and assembly pathway of the ribosome quality control complex. Mol. Cell 57, 433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lyumkis D., Oliveira dos Passos D., Tahara E. B., Webb K., Bennett E. J., Vinterbo S., Potter C. S., Carragher B., Joazeiro C. A. P. (2014) Structural basis for translational surveillance by the large ribosomal subunit-associated protein quality control complex. Proc. Natl. Acad. Sci. U.S.A. 111, 15981–15986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simon J. A., Kingston R. E. (2009) Mechanisms of Polycomb gene silencing: knowns and unknowns. Nat. Rev. Mol. Cell Biol. 10, 697–708 [DOI] [PubMed] [Google Scholar]
- 49. Johansson C., Schwartz S. (2013) Regulation of human papillomavirus gene expression by splicing and polyadenylation. Nat. Rev. Microbiol. 11, 239–251 [DOI] [PubMed] [Google Scholar]
- 50. Wang H., Wang L., Erdjument-Bromage H., Vidal M., Tempst P., Jones R. S., Zhang Y. (2004) Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873–878 [DOI] [PubMed] [Google Scholar]
- 51. Cao R., Tsukada Y.-i., Zhang Y. (2005) Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol. Cell 20, 845–854 [DOI] [PubMed] [Google Scholar]
- 52. Gao Z., Zhang J., Bonasio R., Strino F., Sawai A., Parisi F., Kluger Y., Reinberg D. (2012) PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol. Cell 45, 344–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Farcas A. M., Blackledge N. P., Sudbery I., Long H. K., McGouran J. F., Rose N. R., Lee S., Sims D., Cerase A., Sheahan T. W., Koseki H., Brockdorff N., Ponting C. P., Kessler B. M., Klose R. J. (2012) KDM2B links the Polycomb Repressive Complex 1 (PRC1) to recognition of CpG islands. Elife 1, e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu X., Johansen J. V., Helin K. (2013) Fbxl10/Kdm2b recruits polycomb repressive complex 1 to CpG islands and regulates H2A ubiquitylation. Mol. Cell 49, 1134–1146 [DOI] [PubMed] [Google Scholar]
- 55. McGinty R. K., Henrici R. C., Tan S. (2014) Crystal structure of the PRC1 ubiquitylation module bound to the nucleosome. Nature 514, 591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buchwald G., van der Stoop P., Weichenrieder O., Perrakis A., van Lohuizen M., Sixma T. K. (2006) Structure and E3-ligase activity of the Ring–Ring complex of Polycomb proteins Bmi1 and Ring1b. EMBO J. 25, 2465–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taherbhoy A. M., Huang O. W., Cochran A. G. (2015) BMI1-RING1B is an autoinhibited RING E3 ubiquitin ligase. Nat. Commun. 6, 7621. [DOI] [PubMed] [Google Scholar]
- 58. Bentley M. L., Corn J. E., Dong K. C., Phung Q., Cheung T. K., Cochran A. G. (2011) Recognition of UbcH5c and the nucleosome by the Bmi1/Ring1b ubiquitin ligase complex. EMBO J. 30, 3285–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brzovic P. S., Klevit R. E. (2006) Ubiquitin Transfer from the E2 perspective: why is UbcH5 so promiscuous? Cell Cycle 5, 2867–2873 [DOI] [PubMed] [Google Scholar]
- 60. Dharmasiri N., Dharmasiri S., Estelle M. (2005) The F-box protein TIR1 is an auxin receptor. Nature 435, 441–445 [DOI] [PubMed] [Google Scholar]
- 61. Tan X., Calderon-Villalobos L. I. A., Sharon M., Zheng C., Robinson C. V., Estelle M., Zheng N. (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446, 640–645 [DOI] [PubMed] [Google Scholar]
- 62. Bartlett J. B., Dredge K., Dalgleish A. G. (2004) The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat. Rev. Cancer 4, 314–322 [DOI] [PubMed] [Google Scholar]
- 63. Shortt J., Hsu A. K., Johnstone R. W. (2013) Thalidomide-analogue biology: immunological, molecular and epigenetic targets in cancer therapy. Oncogene 32, 4191–4202 [DOI] [PubMed] [Google Scholar]
- 64. McBride W. G. (1961) Thalidomide and congenital abnormalities. Lancet 278, 1358 [Google Scholar]
- 65. Lenz W., Pfeiffer R. A., Kosenow W., Hayman D. J. (1962) Thalidomide and congenital abnormalities. Lancet 279, 45–46 [Google Scholar]
- 66. Singhal S., Mehta J., Desikan R., Ayers D., Roberson P., Eddlemon P., Munshi N., Anaissie E., Wilson C., Dhodapkar M., Zeddis J., Siegel D., Crowley J., Barlogie B. (1999) Antitumor activity of thalidomide in refractory multiple myeloma. New Engl. J. Med. 341, 1565–1571 [DOI] [PubMed] [Google Scholar]
- 67. Ito T., Ando H., Suzuki T., Ogura T., Hotta K., Imamura Y., Yamaguchi Y., Handa H. (2010) Identification of a primary target of thalidomide teratogenicity. Science 327, 1345–1350 [DOI] [PubMed] [Google Scholar]
- 68. Higgins J. J., Pucilowska J., Lombardi R. Q., Rooney J. P. (2004) A mutation in a novel ATP-dependent Lon protease gene in a kindred with mild mental retardation. Neurology 63, 1927–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lopez-Girona A., Mendy D., Ito T., Miller K., Gandhi A. K., Kang J., Karasawa S., Carmel G., Jackson P., Abbasian M., Mahmoudi A., Cathers B., Rychak E., Gaidarova S., Chen R., Schafer P. H., Handa H., Daniel T. O., Evans J. F., Chopra R. (2012) Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26, 2326–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhu Y. X., Braggio E., Shi C.-X., Bruins L. A., Schmidt J. E., Van Wier S., Chang X.-B., Bjorklund C. C., Fonseca R., Bergsagel P. L., Orlowski R. Z., Stewart A. K. (2011) Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 118, 4771–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fischer E. S., Böhm K., Lydeard J. R., Yang H., Stadler M. B., Cavadini S., Nagel J., Serluca F., Acker V., Lingaraju G. M., Tichkule R. B., Schebesta M., Forrester W. C., Schirle M., Hassiepen U., Ottl J., Hild M., Beckwith R. E. J., Harper J. W., Jenkins J. L., Thomä N. H. (2014) Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 512, 49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Krönke J., Udeshi N. D., Narla A., Grauman P., Hurst S. N., McConkey M., Svinkina T., Heckl D., Comer E., Li X., Ciarlo C., Hartman E., Munshi N., Schenone M., Schreiber S. L., Carr S. A., Ebert B. L. (2014) Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gibson B. A., Kraus W. L. (2012) New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 13, 411–424 [DOI] [PubMed] [Google Scholar]
- 74. Huang S. M. A., Mishina Y. M., Liu S., Cheung A., Stegmeier F., Michaud G. A., Charlat O., Wiellette E., Zhang Y., Wiessner S., Hild M., Shi X., Wilson C. J., Mickanin C., Myer V., Fazal A., Tomlinson R., Serluca F., Shao W., Cheng H., Shultz M., Rau C., Schirle M., Schlegl J., Ghidelli S., Fawell S., Lu C., Curtis D., Kirschner M. W., Lengauer C., Finan P. M., Tallarico J. A., Bouwmeester T., Porter J. A., Bauer A., Cong F. (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 [DOI] [PubMed] [Google Scholar]
- 75. Levaot N., Voytyuk O., Dimitriou I., Sircoulomb F., Chandrakumar A., Deckert M., Krzyzanowski P. M., Scotter A., Gu S., Janmohamed S., Cong F., Simoncic P. D., Ueki Y., La Rose J., Rottapel R. (2011) Loss of tankyrase-mediated destruction of 3BP2 is the underlying pathogenic mechanism of cherubism. Cell 147, 1324–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Guettler S., LaRose J., Petsalaki E., Gish G., Scotter A., Pawson T., Rottapel R., Sicheri F. (2011) Structural basis and sequence rules for substrate recognition by tankyrase explain the basis for cherubism disease. Cell 147, 1340–1354 [DOI] [PubMed] [Google Scholar]
- 77. Wang Z., Michaud G. A., Cheng Z., Zhang Y., Hinds T. R., Fan E., Cong F., Xu W. (2012) Recognition of the iso-ADP-ribose moiety in poly(ADP-ribose) by WWE domains suggests a general mechanism for poly(ADP-ribosyl)ation-dependent ubiquitination. Genes Dev. 26, 235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. DaRosa P. A., Wang Z., Jiang X., Pruneda J. N., Cong F., Klevit R. E., Xu W. (2015) Allosteric activation of the RNF146 ubiquitin ligase by a poly(ADP-ribosyl)ation signal. Nature 517, 223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Callow M. G., Tran H., Phu L., Lau T., Lee J., Sandoval W. N., Liu P. S., Bheddah S., Tao J., Lill J. R., Hongo J.-A., Davis D., Kirkpatrick D. S., Polakis P., Costa M. (2011) Ubiquitin ligase RNF146 regulates tankyrase and axin to promote Wnt signaling. PLoS ONE 6, e22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang Y., Liu S., Mickanin C., Feng Y., Charlat O., Michaud G. A., Schirle M., Shi X., Hild M., Bauer A., Myer V. E., Finan P. M., Porter J. A., Huang S.-M. A., Cong F. (2011) RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat. Cell Biol. 13, 623–629 [DOI] [PubMed] [Google Scholar]
- 81. Turner G. C., Du F., Varshavsky A. (2000) Peptides accelerate their uptake by activating a ubiquitin-dependent proteolytic pathway. Nature 405, 579–583 [DOI] [PubMed] [Google Scholar]



