Background: Carbohydrate metabolism during decidualization is unknown.
Results: Decidual cells undergo glycolysis upon progesterone signals, and the undifferentiated stromal cells consume lactate for proliferation. Inhibition of glycolysis or lactate flux could compromise decidual development.
Conclusion: Warburg-like glycolysis and lactate communication play critical roles during decidualization.
Significance: Our study will be valuable for understanding the mechanism underlying decidualization.
Keywords: carbohydrate metabolism, glycolysis, pregnancy, reproduction, Warburg effect, Warburg-like glycolysis, lactate flux, decidualization, Hif1α, progesterone
Abstract
Decidualization is an essential process of maternal endometrial stromal cells to support pregnancy. Although it is known that enhanced glucose influx is critical for decidualization, the underlying mechanism in regulating glucose metabolism in decidua remains insufficiently understood. Here, we demonstrate that aerobic glycolysis-related genes and factors are all substantially induced during decidualization, indicating the existence of Warburg-like glycolysis in decidua. In vitro, progesterone activates hypoxia-inducible factor 1α (Hif1α) and c-Myc through Pi3k-Akt signaling pathway to maintain aerobic glycolysis in decidualizing cells. Knocking down of pyruvate kinase M2 (Pkm2) attenuates the induction of decidual marker gene. Decidual formation in vivo is also impaired by glycolysis inhibitor 3-bromopyruvate. Besides, lactate exporter monocarboxylate transporter 4 (Mct4) is induced in newly formed decidual cells, whereas lactate importer Mct1 and proliferation marker Ki-67 are complementarily located in the surrounding undifferentiated cells, which are supposed to consume lactate for proliferation. Hif1α activation is required for lactate-dependent proliferation of the undifferentiated cells. Inhibition of lactate flux leads to compromised decidualization and decelerated lactate-dependent proliferation. In summary, we reveal that Warburg-like glycolysis and local lactate shuttle are activated in decidua and play important roles for supporting early pregnancy.
Introduction
Following embryo implantation, endometrial stromal cells transform into decidual cells to support further pregnancy (1). Compromised decidualization is an essential reason for pregnancy failures, 75% of which are associated with the defects that occur during peri-implantation periods in humans (2). Several studies have shown activated glucose metabolism for human decidualization (3, 4). The activity of glycolysis is also promoted in rodent decidua (5, 6). Now, it has been determined that glycolysis plays critical roles during decidualization in both rodents and humans (3, 4, 7, 8). However, how glucose metabolism is regulated in decidua and the functions of glycolysis during decidualization both remain unclear. As carbohydrate metabolism affects many aspects of cell physiology, it is necessary to further understand decidual glucose metabolism during early pregnancy.
A low oxygen environment generally induces glycolysis in normal cells, but many cancer cells favor an accelerated glycolysis no matter whether under hypoxia or normoxia. This phenomenon is termed as the “Warburg effect” (9, 10). Because of the activation of hypoxia-inducible factor 1 (Hif1) even under normoxia, many enzymes involved in glycolysis are predominantly expressed (10–12). Glucose transporter 1 (Glut1) is an efficient pump for glucose uptake (11). Hexokinase 2 (Hk2) binds to mitochondria to rapidly generate glucose 6-phosphate (G6P)2 (13). After a series of catalyzing reactions, most G6Ps are converted to phosphoenolpyruvate, which is the substrate of tetrameric pyruvate kinase M2 (Pkm2). Pkm2 rather than Pkm1 possesses adjustable property by forming dimeric pyruvate kinase, which weakens its catalyzing activity and causes the accumulation of upstream metabolites (14). For instance, when dimeric Pkm2 is predominant, nucleotide synthesis would be improved because more G6P is available for pentose phosphate pathway (PPP) (10), which is directly controlled by G6P dehydrogenase (G6pdh) (15).
Oxidative cells rely mainly on oxidative phosphorylation to acquire energy so that pyruvate is destined to enter mitochondrion where oxygen is consumed. Pyruvate is oxidized by pyruvate dehydrogenase (Pdh) to form acetyl-CoA, which is subsequently involved in the tricarboxylic acid cycle. However, glycolytic cells activate pyruvate dehydrogenase kinase (Pdk), which phosphorylates the E1α subunit of Pdh (Pdhe1α) to compromise Pdh catalytic activity (16). Thus pyruvate oxidation is attenuated, and most pyruvate converts into lactate by lactate dehydrogenase A (Ldha). Furthermore, monocarboxylate transporter 4 (Mct4) is also induced for exporting lactate (17). Because most cancer cells rely on glucose addiction and acidic microenvironment, drugs targeting glycolysis and lactate flux have become a promising therapeutic strategy in oncology (10, 17).
Here we showed that glycolysis is substantially induced in decidualizing cells both in vivo and in vitro. Progesterone activates Hif1α and c-Myc via Pi3k-Akt signaling pathway to render endometrial stromal cells to depend on aerobic glycolysis. Because decidual glycolysis shows similar characteristics as described in the Warburg effect in tumors (9), we consider that decidual cells undergo Warburg-like glycolysis. Additionally, a lactate shuttle exists between decidual cells and the undifferentiated stromal cells. A high lactate level in the extracellular milieu leads to Hif1α stabilization and subsequently improves proliferation of the undifferentiated cells. Disrupting glycolysis or lactate flux impairs decidualization both in vitro and in vivo. Collectively, our findings provide fundamental insights into glycolysis and lactate communication in endometrium during mouse decidualization. In humans, decidualization also requires enhanced glycolysis (4). Abnormal glucose metabolism in human uterus is associated with several pregnancy complications, and also exists in patients with poor fertility (8, 18–20). Thus our study on decidual carbohydrate metabolism would be valuable for understanding the metabolic mechanism underlying mammalian early pregnancy.
Experimental Procedures
Animals and Treatments
All animal protocols were approved by the Animal Care and Use Committee of South China Agricultural University. All of the experiments were carried out in accordance with the approved guidelines by South China Agricultural University. Models of pregnant and pseudopregnant CD1 mice were prepared by mating female mice with fertile and vasectomized males, respectively, as described previously (21). Day 1 is the day of vaginal plug. The implantation sites on day 5 were confirmed by intravenously injecting Chicago blue dye. Artificial decidualization was induced in pseudopregnant mouse by unilateral intrauterine injection of 10 μl of sesame oil on day 4, and the deciduoma was observed on day 8 of pseudopregnancy. Based on previous studies (22, 23), freshly prepared 3-bromopyruvate (1.8 mg/ml, dissolved in saline, injection volume is 3.3 ml/kg, Sigma) or α-cyano-4-hydroxycinnamic acid (CHC, 37 mg/ml, dissolved into saline containing 26% 1 n NaOH, injection volume is 5.4 ml/kg, Sigma) was injected daily intraperitoneally into pregnant or pseudopregnant mice from days 5 to 7. Saline treatment with an equal injection volume served as a control. Uteri were collected on day 8 to record the weights of implantation sites and deciduoma. To measure the weight of decidual tissue at the implantation site on day 8, embryos were dissected from the implantation sites.
Isolation and Treatment of Mouse Endometrial Stromal Cells
Primary endometrial stromal cells were isolated, cultured, and transfected as described previously (21). Briefly, mouse uteri on day 4 of pregnancy were split and digested in Hanks' balanced salt solution containing 1% trypsin (AMRESCO, Solon, OH) and 6 mg/ml dispase (Roche Applied Science) to remove luminal epithelial cells. The remaining uteri were incubated with 0.15 mg/ml collagenase I (Invitrogen). The supernatants from the digested uteri were filtrated through 70-μm wire gauze and centrifuged to collect the stromal cells. In vitro decidualization was induced by 10 nm estradiol-17β plus 1 μm progesterone for 48 h. The cells were treated with 1 μm progesterone for 48 h, 100 μm CoCl2 for 24 h, or 10 mm sodium l-lactate (Sigma) for 48 h. Cells were also treated with RU486 (1 μm, Sigma), LY294002 (15 μm, Cell Signaling, Beverly, MA), 10058-F4 (20 μm, Sigma), or CHC (500 μm). The siRNAs were synthesized by RiboBio Co., Ltd. (Guangzhou, China). The siRNA sequences for each gene were CTTGCAGCTATTCGAGGAA for Pkm2 (Pkm2-siRNA-1), CTGCCATCTACCACTTGC for Pkm2 (Pkm2-siRNA-2), CGACAAGAGTTGCCTGTTA for Pdk1 (Pdk1-siRNA-1), GACAGAATCCGTCGAGAGA for Pdk1 (Pdk1-siRNA-2), CTGATAACGTGAACAAATA for Hif1α (Hif1α-siRNA-1), and CAAGCAACTGTCATATATA for Hif1α (Hif1α-siRNA-2), respectively. Nonspecific siRNA was transfected as the negative control.
Immunohistochemistry
Immunohistochemistry was performed as described previously (21). Briefly, each sample was fixed in 10% buffered formalin, embedded in paraffin, and cut into 7-μm sections. After rehydration, antigen retrieval was performed by microwaving for 10 min in 10 mm sodium citrate buffer (pH 6.0). Endogenous HRP activity was inhibited with 3% H2O2. After blocking with 10% normal horse serum in PBS, sections were incubated with anti-Pkm2 (4053, Cell Signaling), anti-Mct1 (AB3538P, Chemicon, Pittsburgh, PA), anti-Mct4 (AB3314P, Chemicon), anti-Ki-67 (Rm-9106, Thermo), or anti-GFP (sc-8334, Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 °C. The signal was developed by the diaminobenzidine (DAB)-HRP reaction system.
Real-time PCR and Analysis of Pkm Gene Splices
Total RNAs were extracted using TRIzol reagent (Invitrogen), digested with RQ1 deoxyribonuclease I (Promega, Fitchburg, WI), and reverse-transcribed into cDNA with PrimeScript reverse transcriptase reagent kit (TaKaRa, Dalian, China). Then real-time PCR was performed using a SYBR Premix Ex Taq kit (TaKaRa) as described previously (21). The specific primer sequences for each gene are provided in Table 1. Rpl7 was used for normalization. Data from real-time PCR were analyzed using the 2−ΔΔCt method. The assay for the proportion of Pkm1 and Pkm2 expression was performed based on a previous study (24). Briefly, both Pkm1 and Pkm2 transcripts were amplified using a pair of universal primers. After 25 cycles of amplification, PCR products were recovered from agarose gels and then digested with PstI (New England Biolabs, Ipswich, MA). Digested DNA fragments were analyzed by electrophoresis.
TABLE 1.
Primers used in this study
| Gene | ID | Application | Primers (5′-3′) | Products |
|---|---|---|---|---|
| bp | ||||
| Pkm | NM_011099.3 | RT-PCR (Pst I digestion) | ctgaaggcagtgatgtggcc | 442 |
| acacgaaggtcgacatcctc | ||||
| Pkm2 | NM_011099.3 | Real-time PCR | agcacctgattgcccgaga | 158 |
| cacgataatggccccactg | ||||
| Pkm1 | NM_001253883.1 | Real-time PCR | tgtctggagaaacagccaag | 109 |
| tcctcgaatagctgcaagtg | ||||
| Glut1 | NM_011400.3 | Real-time PCR | agccctgctacagtgtat | 135 |
| aggtctcgggtcacatc | ||||
| Hk2 | NM_013820 | Real-time PCR | ggaacccagctgtttgacca | 107 |
| caggggaacgagaaggtgaaa | ||||
| Ldha | NM_010699 | Real-time PCR | tgtctccagcaaagactactgt | 155 |
| gactgtacttgacaatgttggga | ||||
| Pdk1 | NM_172665 | Real-time PCR | gcactccttattgttcgg | 85 |
| ctagcgttctcatagccatc | ||||
| Mct4 | NM_001038653 | Real-time PCR | gtcatcactggcttgggt | 84 |
| aatagggcgacgcttgtt | ||||
| Mct1 | NM_009196.3 | Real-time PCR | tctggttgcggcttgat | 152 |
| gccagtggtcgcttcttgt | ||||
| G6pdh | NM_008062 | Real-time PCR | ctccaatcaactgtcgaacca | 150 |
| ttgtctcgattccagatgggg | ||||
| Dtprp | NM_010088 | Real-time PCR | agccagaaatcactgccact | 119 |
| tgatccatgcacccataaaa | ||||
| Alp | NM_007431 | Real-time PCR | catataacaccaacgctcag | 150 |
| tggatgtgacctcattgc | ||||
| Rpl7 | M29016 | Real-time PCR | gcagatgtaccgcactgagattc | 129 |
| acctttgggcttactccattgata |
Subcellular Fractionation and Western Blot
Cultured cells were scratched in PBS containing 5 mm EDTA. Cytosolic and nuclear fractions were extracted using cytosolic buffer (10 mm HEPES, pH 7.5, 10 mm KCl, 1.5 mm MgCl2, 0.5 mm DTT, 1 mm NaF, and 1 mm glycerol phosphate) and nuclear buffer (20 mm HEPES, pH 7.5, 420 mm NaCl, 1.5 mm MgCl2, 0.5 mm DTT, 1 mm NaF, and 1 mm glycerol phosphate), respectively. The whole cell lysate was prepared using radioimmunoprecipitation assay buffer containing Complete protease inhibitor cocktail (Roche Applied Science). Protein concentration was measured by a BCA reagent kit (Applygen, Beijing, China). Samples were run on a 10% SDS-PAGE and transferred onto PVDF membranes as described previously (21). For analyzing tetrameric Pkm2, the tissue protein lysate was extracted using TG buffer (20 mm HEPES, pH 7.5, 1% Triton X-100, and 10% glycerol) and incubated with 0.01% glutaraldehyde at room temperature for 5 min. The reaction was terminated by adding 1 m Tris buffer (pH 8.0). The primary antibodies used were as follows: anti-Hif1α (AF1935, R&D Systems, Salt Lake City, UT), anti-Akt (9272, Cell Signaling), anti-p-Akt (4060, Cell Signaling), anti-c-Myc (9402, Cell Signaling), anti-p-Pdhe1α (NB110-93479, Novus Biologicals, Littleton, CO), anti-lamin A/C (2032, Cell Signaling), anti-tubulin (2144, Cell Signaling), anti-Pkm2 (4053, Cell Signaling), anti-p-Pkm2 (3827, Cell Signaling), anti-Mct1 (AB3538P, Chemicon), and anti-Mct4 (AB3314P, Chemicon).
Lactate and Cell Proliferation Assay
Lactate level and cell proliferation were measured by a lactate assay kit (K607, BioVision, Milpitas, CA) and the Cell Counting Kit-8 (Sigma) according to the manufacturer's instructions, respectively. The proliferation rate was calculated as optical density value of the sample/average optical density value of the control × 100%.
Statistical Analysis
Values were expressed as mean ± S.D. Equal variance was tested by F-test. If variance was not equal, a t test assuming unequal variances was used. Otherwise, an equal variance t test was performed. In all cases, p < 0.05 was considered significant. All the experiments were run in triplicate and repeated at least three independent times.
Results
Warburg-like Glycolysis during Decidualization
Excess lactate is an evident product of glycolysis (10, 17). We therefore started with the assessment of lactate level in mouse decidua. When compared with the inter-implantation sites, lactate level at the implantation sites was gradually increased from days 5 to 8 of pregnancy (Fig. 1A), indicating that glycolysis is active during the implantation period.
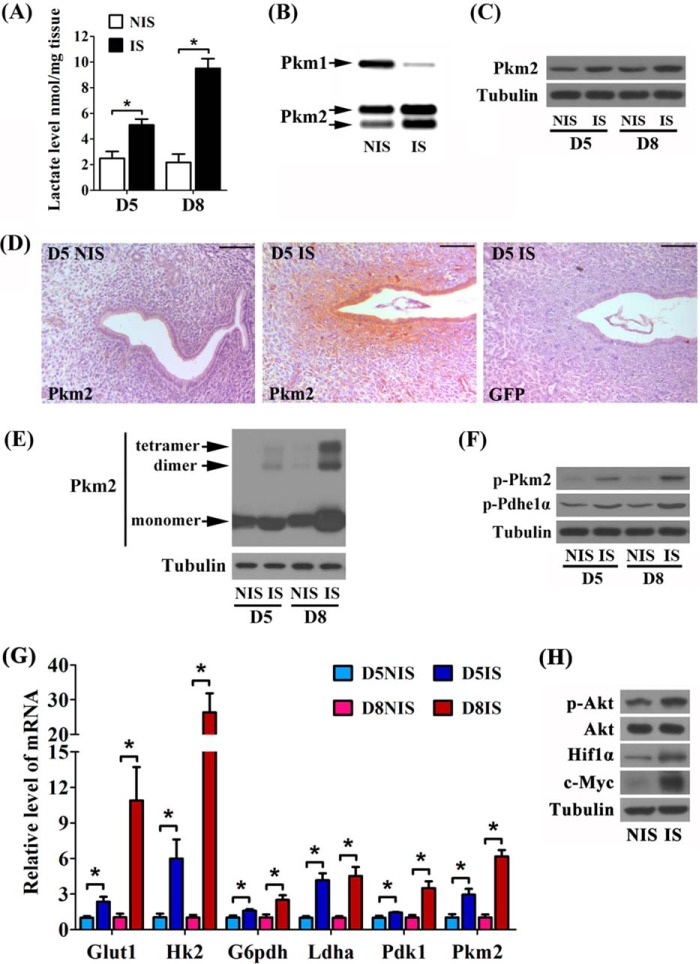
FIGURE 1.
Expression profiles of aerobic glycolysis-associated factors during mouse decidualization. A, lactate level in mouse uteri during decidualization. IS, implantation site; NIS, inter-implantation site; D5, day 5 of pregnancy; D8, day 8 of pregnancy. B, the proportion of Pkm2 and Pkm1 in uterus on day 5. C, decidual Pkm2 protein expression during early pregnancy. D, localizations of Pkm2 in mouse uteri on day 5 of pregnancy. GFP served as control. Bar = 100 μm. E, the tetrameric, dimeric, and monomeric Pkm2 in mouse uteri during early pregnancy shown by SDS-PAGE and Western blot analysis after cross-linking with 0.01% glutaraldehyde at room temperature for 5 min. F, the phosphorylation of Pkm2 and Pdhe1α during decidualization. G, the induction of Glut1, Hk2, G6pdh, Ldha, Pdk1, and Pkm2 mRNA at the implantation sites during the decidualization process. H, the protein expression of p-Akt, Akt, Hif1α, and c-Myc in mouse uteri on day 5 of pregnancy. Values are expressed as mean ± S.D. *, statistical significance (p < 0.05).
Because Pkm2 rather than Pkm1 provides advantages by forming dimeric pyruvate kinase, an alternative splicing switch toward Pkm2 from Pkm1 is therefore favored by glycolytic cells during tumorigenesis (24, 25). Using the approach of exon-specific restriction digestion (24), we found that the proportion of Pkm2 expression was significantly increased at implantation sites, and concomitantly the proportion of Pkm1 expression was clearly reduced (Fig. 1B), suggesting a splicing switch toward Pkm2 during decidualization in vivo. Besides, Pkm2 protein expression was significantly promoted at implantation sites (Fig. 1C). On day 5 of pregnancy, Pkm2 protein signal was mainly localized in the luminal epithelium and subluminal stromal cells around the implanting blastocyst, in which zone the stromal cells were proposed to initiate decidualization (2), whereas no signal was detected at inter-implantation sites (Fig. 1D). To investigate whether decidual Pkm2 is dimeric, the proteins in uterine lysate were cross-linked by 0.01% glutaraldehyde based on the method provided by a previous study (26). Uterine tissues at the implantation sites on day 5 displayed increased expression of monomeric and dimeric Pkm2. On day 8, this increase was further improved, although tetrameric Pkm2 was also manifestly induced at the implantation sites (Fig. 1E). Considering that dimeric Pkm2 can be formed by inducing tyrosine 105-phosphorylated Pkm2 (p-Pkm2) (27), we further showed that the p-Pkm2 level was gradually enhanced at the implantation sites during early pregnancy (Fig. 1F). Collectively, these data suggested that dimeric Pkm2 is induced in decidua, which might be due to enhanced Pkm2 phosphorylation.
In the Warburg effect, tumor cells need to enhance glycolysis to sustain the homeostasis of bioenergy and biomass (10). To investigate whether accelerated glycolysis occurs during decidualization, we examined the relative expression of Pkm2 and several other glycolysis-related genes, such as Glut1 (glucose uptake), Hk2 (converting glucose to G6P), G6pdh (catalyzing G6P for PPP), Ldha (producing lactate), and Pdk1 (repressing Pdh by phosphorylating Pdhe1α). During decidualization, the mRNA levels of these genes at implantation sites were significantly induced on day 5, and these inductions were all substantially enhanced on day 8 (Fig. 1G). Moreover, phosphorylated Pdhe1α (p-Pdhe1α) level was also gradually increased during this process (Fig. 1F), reflecting the repression of Pdh activity and suppressed oxidative metabolism in decidua during early pregnancy.
Although hypoxia triggers glycolysis, Pi3k-Akt signaling pathway controls Hif1α and c-Myc to regulate glycolysis-related gene, and the activation of these factors can induce glycolysis even under normoxia (10, 12). At the implantation sites on day 5, the levels of p-Akt, Hif1α, and c-Myc were substantially increased (Fig. 1H), suggesting that a similar regulatory mechanism may induce decidual glycolysis as in the Warburg effect.
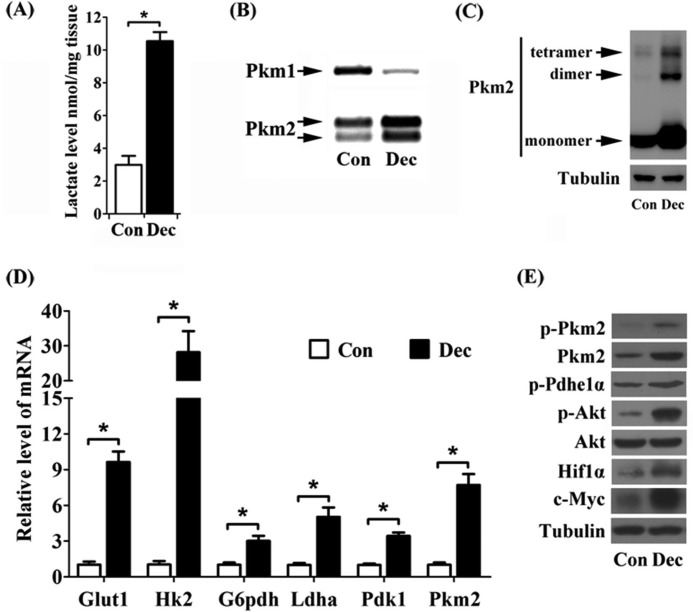
Considering that embryonic metabolism may contribute to these outcomes, and also to validate the independence of decidual glycolysis from the embryo, we employed pseudopregnant mice to induce artificial decidualization. When compared with unstimulated uterine horn, lactate production was significantly improved in oil-induced deciduoma (Fig. 2A). Pkm2 expression and the level of dimeric Pkm2 were both obviously elevated in deciduoma (Fig. 2, B and C). Moreover, the expression levels of glycolysis-related genes, p-Akt, Hif1α, and c-Myc, were noticeably elevated in deciduoma (Fig. 2, D and E). Hence, based on these significant expression profiles of Warburg effect-associated factors at the implantation sites and under artificial decidualization, we suggest that Warburg-like glycolysis exists in decidual cells during early pregnancy.
FIGURE 2.
Warburg-like glycolysis exists in artificial decidualization. A, lactate level in deciduoma (Dec). Con, control horn. B, the proportion of Pkm2 and Pkm1 expression. C, the induction of monomeric, dimeric, and tetrameric Pkm2 in deciduoma. D, real-time RT-PCR analysis of Glut1, Hk2, G6pdh, Ldha, Pdk1, and Pkm2 mRNA expression in deciduoma. E, the protein expression of p-Pkm2, Pkm2, p-Pdhe1α, p-Akt, Akt, Hif1α, and c-Myc in mice uteri. Values are expressed as mean ± S.D. *, statistical significance (p < 0.05).
Progesterone Renders Warburg-like Glycolysis during in Vitro Decidualization
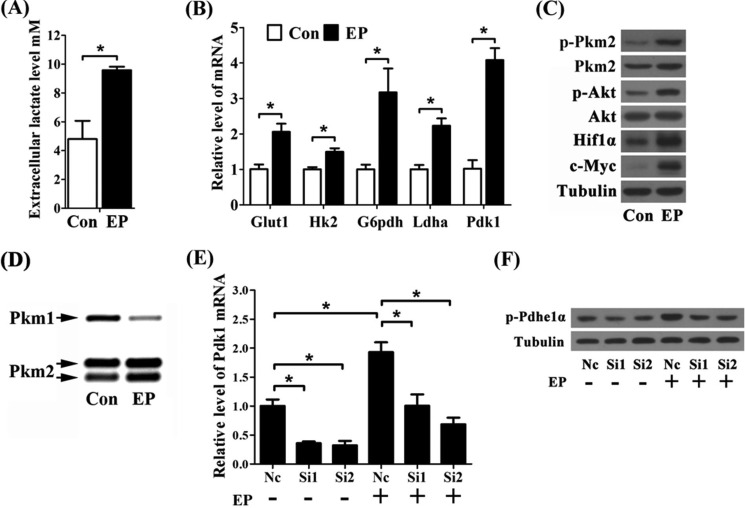
To better understand Warburg-like glycolysis in decidualizing cells, isolated endometrial stromal cells on day 4 of pregnancy were induced for in vitro decidualization. During this process, the extracellular lactate level was significantly increased and reached to nearly 10 mm (Fig. 3A). The expressions of Glut1, Hk2, G6pdh, Ldha, Pdk1, p-Pkm2, and Pkm2, as well as the levels of p-Akt, Hif1α, and c-Myc, were all significantly induced in the decidualizing cells under normoxia culture condition (Fig. 3, B and C). Besides, we also observed that the proportion of Pkm1 expression was decreased in decidualizing cells (Fig. 3D). These data suggested that cultured stromal cells prefer aerobic glycolysis under in vitro decidualization. Moreover, when Pdk1 expression was knocked down by two specific siRNAs in vitro, the induction of p-Pdhe1α was decreased during decidualization (Fig. 3, E and F), implying that Pdk1 is responsible for Pdh inactivation in decidualizing cells.
FIGURE 3.
Warburg-like glycolysis during decidualization in vitro. A, extracellular lactate level during in vitro decidualization. Con, control horn; EP, estrogen plus progesterone. B, the induction of Glut1, Hk2, G6pdh, Ldha, and Pdk1 mRNA during in vitro decidualization. C, the expression of p-Pkm2, Pkm2, p-Akt, Akt, Hif1α, and c-Myc under in vitro decidualization. D, proportion of Pkm2 and Pkm1 expression during in vitro decidualization. E, Pdk1 mRNA expression is silenced after transfection of two specific siRNAs (Si1 and Si2). Nc, nonspecific siRNA. F, Pdk1 knockdown reduces p-Pdhe1α expression during in vitro decidualization. Values are expressed as mean ± S.D. *, statistical significance (p < 0.05).
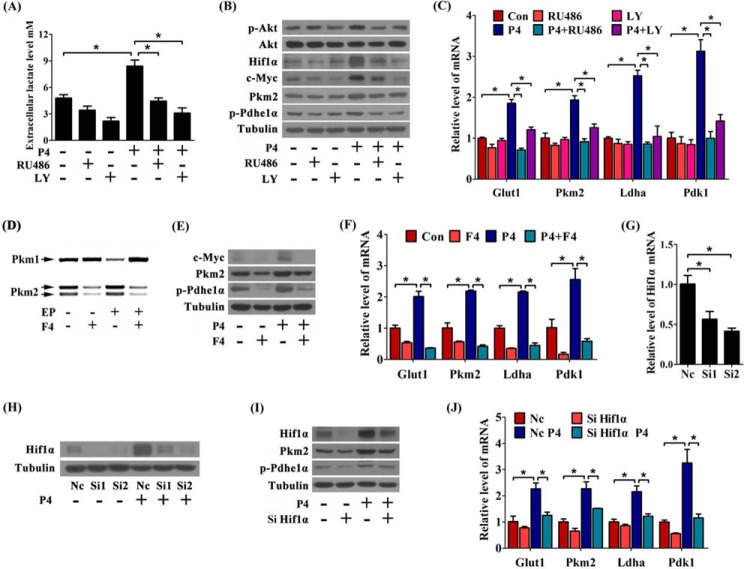
Progesterone and progesterone receptor (PR) are critical for decidualization (1). Our previous work showed that Pi3k-Akt signaling pathway is activated by progesterone via PR in primary endometrial cells (21). In this study, progesterone treatment in vitro significantly increased extracellular lactate production, which was attenuated by PR antagonist RU486 or Pi3k kinase inhibitor LY294002 (Fig. 4A). Progesterone also induced the expression of p-Akt, Hif1α, and c-Myc, and these inductions were abolished by either RU486 or LY294002 (Fig. 4B). Meanwhile, the expression of Glut1, Pkm2, Ldha, Pdk1, and p-Pdhe1α was enhanced by progesterone, and RU486 or LY294002 could abrogate these effects in a similar pattern (Fig. 4, B and C).
FIGURE 4.
Progesterone regulates glycolysis-related genes expression via c-Myc and Hif1α. A, effects of RU486 and LY294002 (LY) on lactate level during progesterone (P4) treatment. B, effects of RU486 and LY294002 on the levels of p-Akt, Akt, Hif1α, c-Myc, Pkm2, and p-Pdhe1α during progesterone treatment. C, RU486 or LY294002 abrogates progesterone induction on the mRNA expression of Glut1, Pkm2, Ldha, and Pdk1. Con, control horn. D, effects of c-Myc inhibitor 10058-F4 (F4) on the proportions of Pkm1 and Pkm2 expression during in vitro decidualization. EP, estrogen plus progesterone. E, effects of 10058-F4 on progesterone induction of c-Myc, Pkm2, and p-Pdhe1α protein level. F, effects of 10058-F4 treatment on the induction of Glut1, Pkm2, Ldha, and Pdk1 mRNA expression under progesterone treatment. G, Hif1α mRNA expression following transfection with two specific Hif1α siRNAs (Si1 and Si2) and the nonspecific siRNA (Nc) for 48 h. H, effects of the two Hif1α siRNAs on Hif1α protein expression. I, Hif1α knockdown abrogates progesterone induction on Pkm2 and p-Pdhe1α in protein levels. Si Hif1α, Hif1α siRNA-2. J, Hif1α knockdown inhibits progesterone-induced mRNA expression of Glut1, Pkm2, Ldha, and Pdk1. Values are expressed as mean ± S.D. *, statistical significance (p < 0.05).
Then we focused on the effects of c-Myc and Hif1α on glycolysis in the stromal cells. 10058-F4, an inhibitor of c-Myc, caused an increase of the proportion of Pkm1 expression during in vitro decidualization (Fig. 4D), consistent with a previous study showing that c-Myc controls the splicing switch toward Pkm2 (24). Moreover, the inductions of Glut1, Pkm2, Ldha, Pdk1, and p-Pdhe1α under progesterone treatment were abrogated by 10058-F4 (Fig. 4, E and F). Additionally, cells were transfected with nonspecific siRNA (Nc) and two siRNAs targeting Hif1α. When compared with Hif1α siRNA-1, Hif1α siRNA-2 (Si2) showed more efficient effects on Hif1α expression in both mRNA and protein levels (Fig. 4, G and H). After transfecting Hif1α siRNA-2, the increases of Glut1, Pkm2, Ldha, Pdk1, and p-Pdhe1α under progesterone treatment were also diminished by silencing Hif1α (Fig. 4, I and J). Collectively, these data suggested that Pi3k-Akt signaling pathway is involved in progesterone-dependent aerobic glycolysis via c-Myc and Hif1α.
Decidualization Relies on Glycolysis
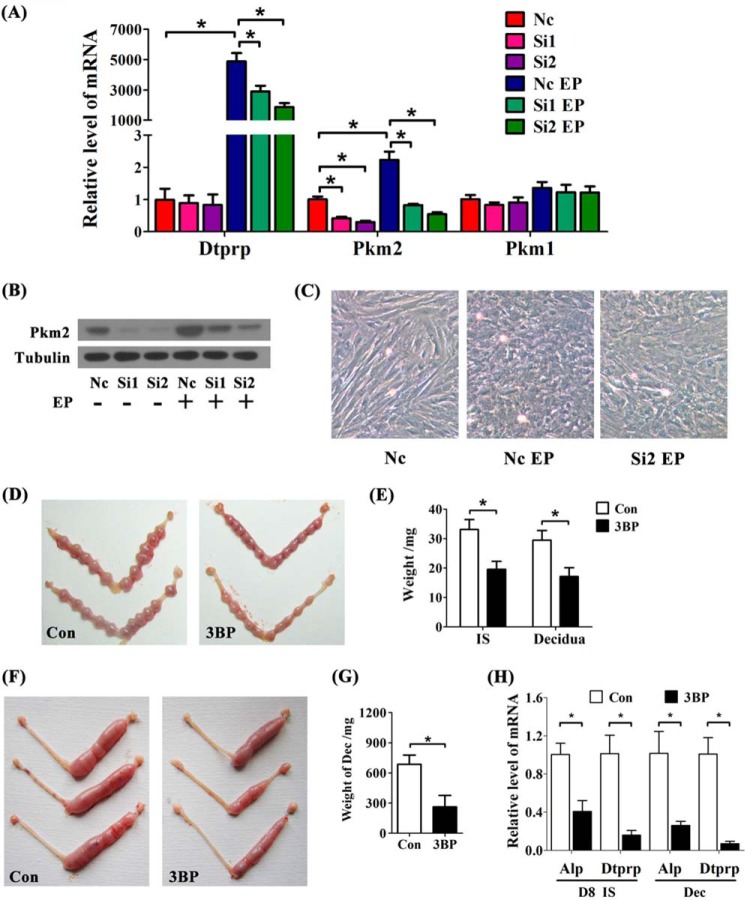
When Pkm2 expression was specifically silenced by two specific siRNAs without affecting Pkm1, the expression of decidua/trophoblast prolactin-related protein (Dtprp), a well known decidualization marker (28), was obviously inhibited (Fig. 5, A and B). Additionally, the transition from fibroblast-like stromal cells to epithelioid decidual cells was attenuated by Pkm2 siRNA-2 (Fig. 5C).
FIGURE 5.
Glycolysis is critical for mouse decidualization. A, the mRNA expression of Dtprp, Pkm2, and Pkm1 when Pkm2 is silenced by two specific siRNAs (Si1 and Si2) during in vitro decidualization. Nc, nonspecific siRNA; EP, estrogen plus progesterone. B, Pkm2 protein level is significantly decreased by the two Pkm2 siRNAs (Si1 and Si2). C, the mesenchymal-to-epithelial transition during in vitro decidualization is impaired by Pkm2 siRNA-2. D, effects of 3-bromopyruvate (3BP) treatment on the size of implantation sites on day 8. Con, control horn. E, the weights of implantation sites and the embryo-free decidua on day 8 of pregnancy under 3-bromopyruvate treatment. IS, implantation site. F, the morphology of deciduoma following 3-bromopyruvate treatment in pseudopregnant mice. G, effects of 3-bromopyruvate on the weight of deciduoma. H, expression of Alp and Dtprp in 3-bromopyruvate-treated mouse uteri. D8, day 8 of pregnancy; Dec, deciduoma. Values are expressed as mean ± S.D. *, statistical significance (p < 0.05).
To examine whether glycolysis is functional for decidualization in vivo, pregnant mice were treated with 3-bromopyruvate, a widely used anti-tumor drug, by significantly inhibiting glycolysis (13, 23). In pregnant mice, 3-bromopyruvate substantially reduced the growth and weight of implantation sites (Fig. 5, D and E) and led to retarded deciduoma (Fig. 5, F and G). Additionally, the expressions of Dtprp and alkaline phosphatase (Alp), two markers for decidualization (28), were both robustly diminished under 3-bromopyruvate treatment (Fig. 5H), suggesting that glycolysis is critical for decidualization and early pregnancy.
Lactate Shuttle during Early Pregnancy
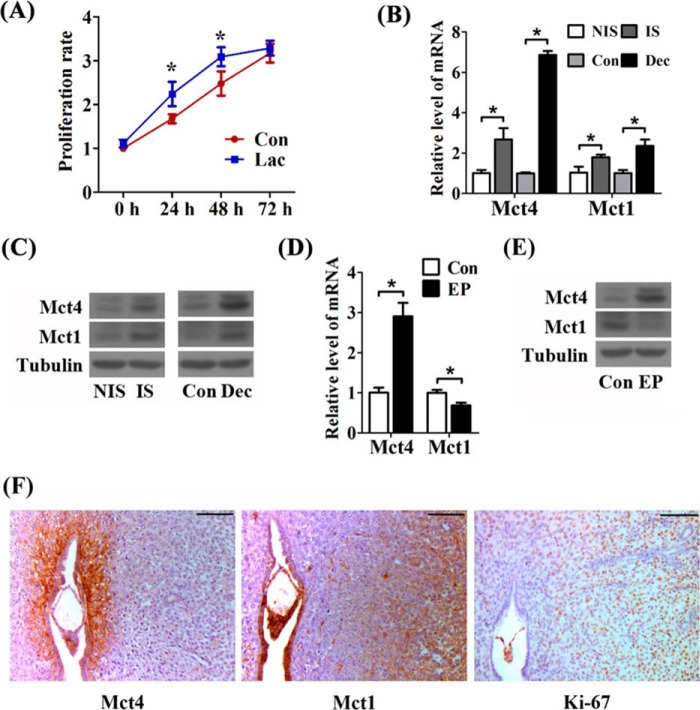
Under glycolysis, endometrial stromal cells have to face an acidic microenvironment during decidualization. Lactate plays multiple roles for tumor survival and growth (17, 29). Herein we found a comparable proliferation rate of the lactate-treated cells (Fig. 6A). The dose of lactate was chosen to be 10 mm based on the lactate level during in vitro decidualization (Fig. 3A). Therefore, we proposed that the undifferentiated stromal cells could utilize excessive lactate for proliferation.
FIGURE 6.
Lactate flux in mouse decidua. A, effects of lactate (Lac) on proliferation of the undifferentiated endometrial stromal cells. Con, control horn. B, the induction of Mct4 and Mct1 in mRNA level at the implantation sites on day 5 and in deciduoma (Dec). NIS, inter-implantation site; IS, implantation site. C, Western blot analysis of Mct4 and Mct1 in mouse uteri on day 5 and in deciduoma. D, the mRNA level of Mct4 and Mct1 during in vitro decidualization. EP, estrogen plus progesterone. E, the protein level of Mct4 and Mct1 expression under in vitro decidualization. F, immunostaining of Mct4, Mct1, and Ki-67 at implantation sites on day 5 of pregnancy. Bar = 100 μm. Values are expressed as mean ± S.D. *, statistical significance (p < 0.05).
Lactate flux widely exists between glycolytic cells (expressing Mct4 for exporting lactate) and oxidative cells (expressing Mct1 for importing lactate) (17, 30, 31). In our study, the levels of Mct1 and Mct4 were both induced at implantation sites and in deciduoma (Fig. 6, B and C). Under in vitro decidualization, there was an up-regulation of Mct4 and a down-regulation of Mct1 (Fig. 6, D and E). These data suggested that a local lactate shuttle properly exists in the decidua. As shown in Fig. 6F, Mct4 was expressed in the implanted embryo and the surrounding decidualizing cells on day 5 of pregnancy. Complementarily, Mct1 protein was detected in the blastocyst, luminal epithelium, and noticeably in the stromal cells, which were away from the Mct4-positive stromal cells. Moreover, the signals of Ki-67 were also detected in blastocyst and the stromal cells beyond the primary decidual zone. Collectively, these data implied that lactate from decidual cells may properly promote proliferation of the undifferentiated cells.
Dual Roles of Hif1α in Lactate Communication
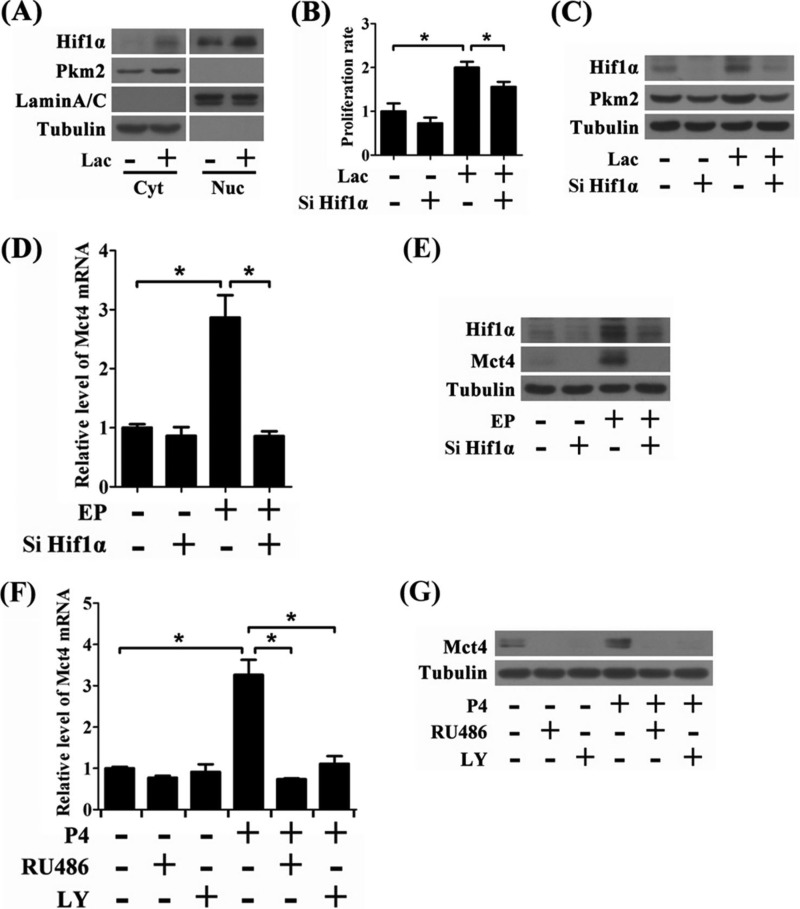
In tumor cells, lactate treatment can benefit Hif1α stabilization (32). As shown in Fig. 7A, when the undifferentiated cells were treated with lactate, Hif1α protein was accumulated in both cytoplasm and nucleus. The expression of Pkm2, one of Hif1α-targeting genes (12), was also increased. Moreover, knockdown of Hif1α could decrease proliferation rate and Pkm2 induction in lactate-treated cells (Fig. 7, B and C). These results suggested that Hif1α contributes to lactate-stimulated proliferation of the undifferentiated stromal cells.
FIGURE 7.
Hif1α regulates lactate shuttle in endometrial stromal cells. A, Hif1α and Pkm2 levels in nuclear and cytoplasmic fractions during lactate treatment. Cyt, cytoplasm; Nuc, nucleus. B, effects of Hif1α siRNA-2 (Si Hif1α) on lactate-induced cell proliferation. C, effects of Hif1α siRNA-2 on the protein level of Pkm2 and Hif1α during lactate treatment. D, effects of Hif1α siRNA-2 on Mct4 mRNA level under in vitro decidualization. EP, estrogen plus progesterone. E, effects of Hif1α knockdown on Mct4 protein level under in vitro decidualization. F, effects of RU486 and LY294002 on Mct4 mRNA level during progesterone (P4) treatment. G, effects of RU486 and LY294002 on Mct4 protein level in progesterone-treated cells. Values are expressed as mean ± S.D. *, statistical significance (p < 0.05).
In glycolytic cells, Hif1α transcriptionally induces Mct4 expression (31). Under in vitro decidualization, Mct4 expression was suppressed by Hif1α siRNA-2 (Fig. 7, D and E). Progesterone-induced Mct4 expression was also diminished by RU486 or LY294002 (Fig. 7, F and G), suggesting that the PR-Pi3k-Akt-Hif1α pathway controls lactate export.
Lactate Communication Benefits Decidualization
To examine the roles of lactate flux during decidualization, pregnant mice and cultured cells were treated with CHC (a specific inhibitor predominantly targeted on Mct1 rather than other Mcts) (33). In the undifferentiated stromal cells, CHC decelerated proliferation under lactate stimulation. However, the inhibitory effect of CHC on proliferation was not evident in decidualizing cells (Fig. 8A), suggesting that the undifferentiated stromal cells are more dependent on lactate consumption than decidual cells. Additionally, during in vitro decidualization or lactate treatment, CHC pretreatment could eliminate Hif1α increase and abrogate the induction of Pkm2 and Mct4 (Fig. 8B). In decidualizing cells, CHC treatment suppressed the induction of glycolysis-related genes and Dtprp expression (Fig. 8C) and hampered the morphological transformation of the stromal cells during in vitro decidualization (Fig. 8D). Collectively, inhibition of lactate communication not only impaired the proliferation of the undifferentiated cells, but also disrupted glycolysis and the differentiation ability in decidualizing cells.
FIGURE 8.
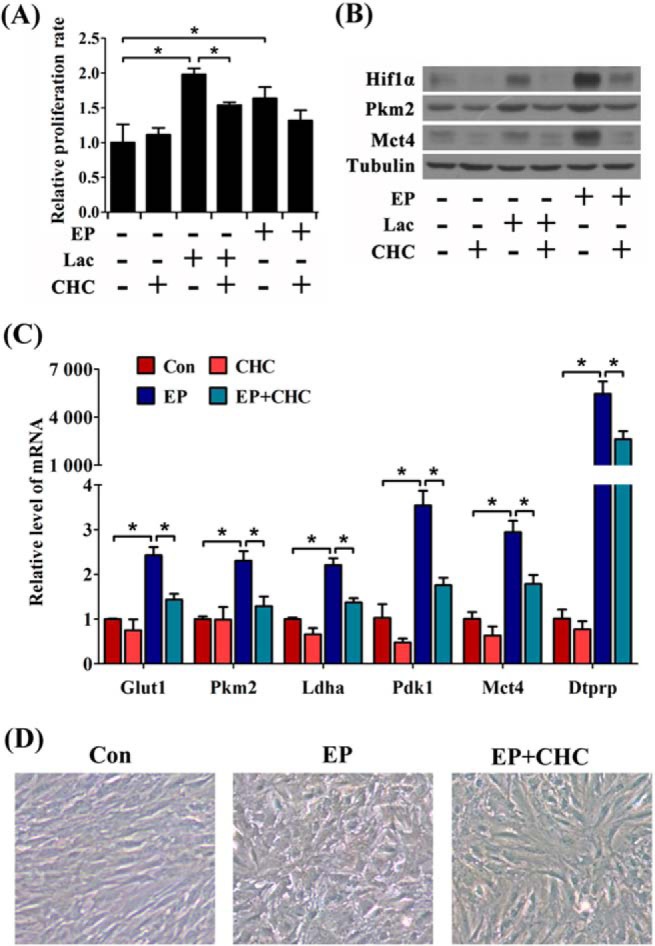
Effects of lactate flux in decidualization in vitro. A, effects of CHC on lactate (Lac)-induced cell proliferation. EP, estrogen plus progesterone. B, effects of CHC on the protein levels of Hif1α, Pkm2, and Mct4 during lactate treatment or in vitro decidualization. C, the inhibitory effects of CHC on the mRNA expressions of Dtprp and glycolysis-related genes under in vitro decidualization. Con, control horn. D, effects of CHC on the morphological changes in the cultured stromal cells during in vitro decidualization. Values are expressed as mean ± S.D. *, statistical significance (p < 0.05).
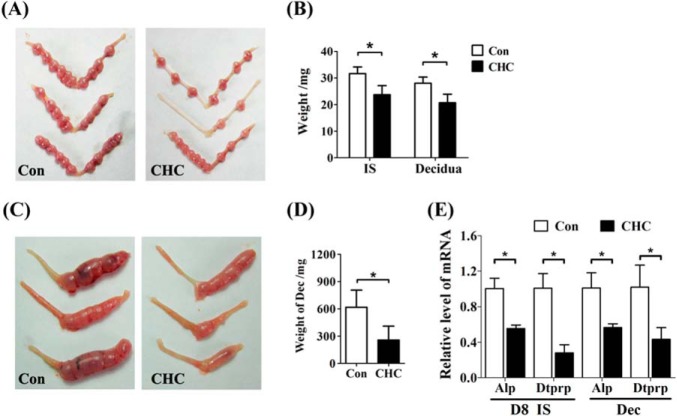
In pregnant mice, CHC administration significantly reduced the size of implantation sites and decreased the weight of decidua during early pregnancy (Fig. 9, A and B). Interestingly, the implanting mouse blastocysts also express Mct4 and Mct1 (Fig. 6F). Lactate is considered as an energy substance for blastocysts (34). Thus we performed artificial decidualization in pseudopregnant mice to eliminate embryonic effects. CHC injection could repress deciduoma formation (Fig. 9, C and D). Expressions of Alp and Dtprp were both decreased in CHC-treated mouse uteri (Fig. 9E). Collectively, these data suggested that lactate flux plays important roles during decidualization.
FIGURE 9.
Functions of lactate flux in decidualization in vivo. A, the morphology of implantation sites on day 8 of pregnancy under CHC treatment. B, effects of CHC treatment on the weight of implantation sites (IS) and the separated decidua. Con, control horn. C, the morphology of deciduoma on day 8 of pseudopregnancy under CHC treatment. D, effects of CHC treatment on the weight of deciduoma. E, effects of CHC treatment on the mRNA levels of Alp and Dtprp in implantation sites and deciduoma. D8, day 8 of pregnancy Values are expressed as mean ± S.D. *, statistical significance (p < 0.05).
Discussion
Based on the increase of lactate production and the induction of glycolysis-related genes, as well as the active effects of Hif1α on glycolysis upon progesterone signaling, we suggest that decidualizing cells undergo Warburg-like glycolysis, the metabolic process of which is briefly depicted in Fig. 10. Several studies also imply that decidual signals improve glycolysis in human endometrial stromal cells, which are also cultured under normoxic conditions. Glut1 is strongly induced and plays important roles during human decidualization (8, 35). Pdk4, which has a similar property as Pdk1, is also induced in human decidualizing cells (36). Besides, an accelerated glycolytic flux is essentially required for decidualization in both mice and humans (4).
FIGURE 10.
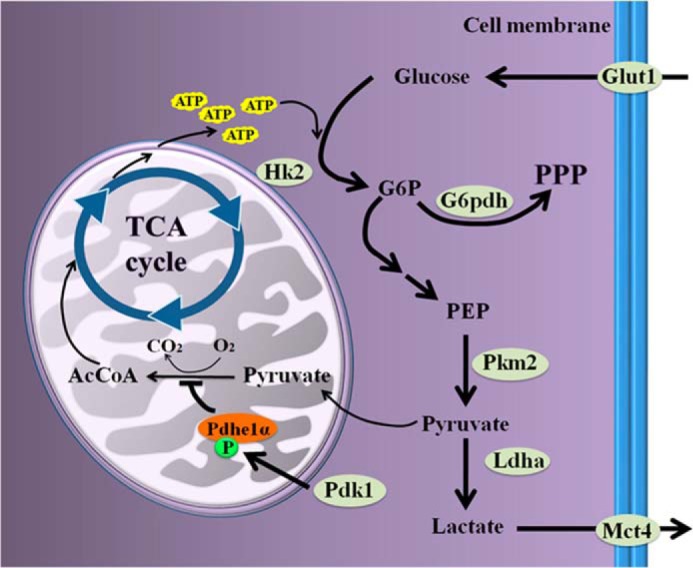
Schematic pathways on the glucose influx in glycolysis. PEP, phosphoenolpyruvate; AcCoA, acetyl-CoA; TCA Cycle, tricarboxylic acid cycle.
In most cases, tumors evolve and become first hypoxic and then acidic (11). During early carcinogenesis, substantial proliferation of oxidative cells leads to insufficient oxygen content in the microenvironment, which induces Hif1α stabilization and subsequently renders these cells with glycolysis (11). Similarly, during the peri-implantation period, mouse endometrial cells widely proliferate under the effects of progesterone and estrogen (37). In humans, partial pressure of oxygen at the interface between mother and embryo is low in the first trimester (38). The endometrial hypoxia thereby contributes to Hif1α activation (39). However, to maintain glycolysis, Hif1α could be stabilized independent of hypoxia once some other signals (including Pi3k-Akt pathway) are activated (11). Our study reveals that Hif1α protein is enhanced by progesterone in the stromal cells under normoxia condition, consistent with an earlier study indicating progesterone-dependent Hif1α induction in mouse uteri during early pregnancy (39). Furthermore, Pi3k-Akt signaling pathway is activated in mouse endometrium during decidualization (21, 35). Thus we propose that mouse decidualizing cells are eventually adapted to aerobic glycolysis due to Hif1α activation under progesterone priming. Similarly, in primary human endometrial stromal cells, estrogen has been proved to evoke a Warburg-like glucose metabolism by inducing dimeric Pkm2 (40).
Dimeric Pkm2 incorporates energy acquisition and biomass production in the Warburg effect (10). Some macromolecular precursor, such as carbon source intermediates, should be piled up for anabolism, especially for nucleotide synthesis, because polyploidization and proliferation occur in decidual cells (2). Our previous work showed that ribonucleotide reductase 2 (Rrm2), a rate-limiting enzyme in deoxynucleotide production for DNA synthesis, is strongly expressed and essential for mouse decidualization (21). Additionally, as the rate-limiting enzyme for PPP, G6pdh was also promoted during decidualization in our study, suggesting that the buildup of G6P is shunted into PPP for nucleic acid synthesis. In human endometrial stromal cells, the supplement of ribonucleotides and deoxyribonucleotides could restore decidualization, which is disturbed by blocking PPP (41). In this study, knocking down Pkm2 or injecting 3-bromopyruvate to disrupt glycolysis can impair decidualization. Consistently, drugs targeting glycolysis could hamper decidualization in both humans and rodents (4). Therefore, Warburg-like glycolysis is essential for decidualizing cells to ensure enough bioenergy and biosynthesis during early pregnancy.
Lactate produced from glycolytic cells can be transported and utilized for oxidative metabolism (17). When we adopted lactate treatment to mimic the acidic environment near the decidual zone, the proliferation of the undifferentiated stromal cells was accelerated. CHC pretreatment reduces lactate-dependent proliferation, implying that the undifferentiated cells can import lactate through Mct1 for proliferation. Besides, the complementary expression patterns of Mct4 and Mct1 in the decidual and the undifferentiated cells, which share a close anatomical relationship in vivo, further support the concept of local lactate flux in the endometrial stroma during post-implantation period.
Successful decidualization is a prerequisite for gestation and essentially related to human fertility. Defects in decidualization cause inadequate placentation and angiogenesis, which could give rise to pregnancy complications, such as preeclampsia (2). As lactate stimulates the migration of human umbilical vein endothelial cells and promotes angiogenesis (30), understanding the glycolysis and lactate flux in decidua might be meaningful for the prediction of preeclampsia in the first trimester. Additionally, patients with idiopathic infertility show decreased Glut1 expression in the endometrial stromal cells, suggesting the influence of dysfunction of endometrial stromal and decidual cells upon glucose metabolism (8, 19). Patients with polycystic ovary syndrome also show disturbed glycolysis and reduced proliferation rate of endometrial stromal cells (20). Disordered glucose metabolism in human endometrium is also involved in the poor fertility of older women or of women taking oral contraceptives (18). Thus our study on carbohydrate metabolism in endometrium may provide new insights into the pathogenesis of metabolism-related diseases affecting human reproductive health.
Author Contributions
R. J. Z. and Z. M. Y. conceived and designed research; R. J. Z., X. W. G., Q. R. Q., T. S. W., X. Y. Z., and J. L. L. performed research; and R. J. Z. and Z. M. Y. analyzed data and wrote the paper. All of the authors reviewed the manuscript.
This work was supported by the National Basic Research Program of China (Grants 2011CB944402 and 2013CB910803) and National Natural Science Foundation of China (Grants 31271602, 31471397 and 31272263). The authors declare that they have no conflicts of interest with the contents of this article.
- G6P
- glucose 6-phosphate
- G6pdh
- G6P dehydrogenase
- PPP
- pentose phosphate pathway
- Pdh
- pyruvate dehydrogenase
- Pdk
- pyruvate dehydrogenase kinase
- Ldha
- lactate dehydrogenase A
- Dtprp
- decidua/trophoblast prolactin-related protein
- Alp
- alkaline phosphatase
- CHC
- α-cyano-4-hydroxycinnamic acid
- PR
- progesterone receptor
- p-Pkm2
- phosphorylated Pkm2
- p-Pdhe1α
- phosphorylated Pdhe1α
- p-Akt
- phosphorylated Akt.
References
- 1. Wang H., Dey S. K. (2006) Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 7, 185–199 [DOI] [PubMed] [Google Scholar]
- 2. Cha J., Sun X., Dey S. K. (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat. Med. 18, 1754–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frolova A. I., Moley K. H. (2011) Quantitative analysis of glucose transporter mRNAs in endometrial stromal cells reveals critical role of GLUT1 in uterine receptivity. Endocrinology 152, 2123–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kommagani R., Szwarc M. M., Kovanci E., Gibbons W. E., Putluri N., Maity S., Creighton C. J., Sreekumar A., DeMayo F. J., Lydon J. P., O'Malley B. W. (2013) Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. PLoS Genet. 9, e1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Surani M. A., Heald P. J. (1971) Changes in enzymes of carbohydrate metabolism in rat uterus during early pregnancy. Acta Endocrinol. (Copenh.) 68, 805–816, 10.1530/acta.0.0680805 [DOI] [PubMed] [Google Scholar]
- 6. Murdoch R. N. (1987) Glycolysis in the mouse uterus during the early post-implantation stages of pregnancy and the effects of acute doses of ethanol. Teratology 35, 169–176 [DOI] [PubMed] [Google Scholar]
- 7. Frolova A., Flessner L., Chi M., Kim S. T., Foyouzi-Yousefi N., Moley K. H. (2009) Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology 150, 1512–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frolova A. I., Moley K. H. (2011) Glucose transporters in the uterus: an analysis of tissue distribution and proposed physiological roles. Reproduction 142, 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warburg O. (1956) On the origin of cancer cells. Science 123, 309–314 [DOI] [PubMed] [Google Scholar]
- 10. Vander Heiden M. G., Cantley L. C., Thompson C. B. (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gatenby R. A., Gillies R. J. (2004) Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 [DOI] [PubMed] [Google Scholar]
- 12. Semenza G. L. (2010) HIF-1: upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 20, 51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mathupala S. P., Ko Y. H., Pedersen P. L. (2009) Hexokinase-2 bound to mitochondria: cancer's stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin. Cancer Biol. 19, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christofk H. R., Vander Heiden M. G., Wu N., Asara J. M., Cantley L. C. (2008) Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452, 181–186 [DOI] [PubMed] [Google Scholar]
- 15. Jiang P., Du W., Wang X., Mancuso A., Gao X., Wu M., Yang X. (2011) p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 13, 310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaplon J., Zheng L., Meissl K., Chaneton B., Selivanov V. A., Mackay G., van der Burg S. H., Verdegaal E. M., Cascante M., Shlomi T., Gottlieb E., Peeper D. S. (2013) A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 498, 109–112 [DOI] [PubMed] [Google Scholar]
- 17. Doherty J. R., Cleveland J. L. (2013) Targeting lactate metabolism for cancer therapeutics. J. Clin. Invest. 123, 3685–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hackl H. (1973) Metabolism of glucose in the human endometrium with special reference to fertility and contraception. Acta Obstet. Gynecol. Scand. 52, 135–140 [DOI] [PubMed] [Google Scholar]
- 19. von Wolff M., Ursel S., Hahn U., Steldinger R., Strowitzki T. (2003) Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy. J. Clin. Endocrinol. Metab. 88, 3885–3892 [DOI] [PubMed] [Google Scholar]
- 20. Kim J. Y., Song H., Kim H., Kang H. J., Jun J. H., Hong S. R., Koong M. K., Kim I. S. (2009) Transcriptional profiling with a pathway-oriented analysis identifies dysregulated molecular phenotypes in the endometrium of patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 94, 1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lei W., Feng X. H., Deng W. B., Ni H., Zhang Z. R., Jia B., Yang X. L., Wang T. S., Liu J. L., Su R. W., Liang X. H., Qi Q. R., Yang Z. M. (2012) Progesterone and DNA damage encourage uterine cell proliferation and decidualization through up-regulating ribonucleotide reductase 2 expression during early pregnancy in mice. J. Biol. Chem. 287, 15174–15192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Del Prete E., Lutz T. A., Scharrer E. (2004) Inhibition of glucose oxidation by α-cyano-4-hydroxycinnamic acid stimulates feeding in rats. Physiol. Behav. 80, 489–498 [DOI] [PubMed] [Google Scholar]
- 23. Cardaci S., Rizza S., Filomeni G., Bernardini R., Bertocchi F., Mattei M., Paci M., Rotilio G., Ciriolo M. R. (2012) Glutamine deprivation enhances antitumor activity of 3-bromopyruvate through the stabilization of monocarboxylate transporter-1. Cancer Res. 72, 4526–4536 [DOI] [PubMed] [Google Scholar]
- 24. David C. J., Chen M., Assanah M., Canoll P., Manley J. L. (2010) HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 463, 364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Christofk H. R., Vander Heiden M. G., Harris M. H., Ramanathan A., Gerszten R. E., Wei R., Fleming M. D., Schreiber S. L., Cantley L. C. (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 [DOI] [PubMed] [Google Scholar]
- 26. Wang H. J., Hsieh Y. J., Cheng W. C., Lin C. P., Lin Y. S., Yang S. F., Chen C. C., Izumiya Y., Yu J. S., Kung H. J., Wang W. C. (2014) JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1α-mediated glucose metabolism. Proc. Natl. Acad. Sci. U.S.A. 111, 279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hitosugi T., Kang S., Vander Heiden M. G., Chung T. W., Elf S., Lythgoe K., Dong S., Lonial S., Wang X., Chen G. Z., Xie J., Gu T. L., Polakiewicz R. D., Roesel J. L., Boggon T. J., Khuri F. R., Gilliland D. G., Cantley L. C., Kaufman J., Chen J. (2009) Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci. Signal. 2, ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Das A., Mantena S. R., Kannan A., Evans D. B., Bagchi M. K., Bagchi I. C. (2009) De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 12542–12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirschhaeuser F., Sattler U. G., Mueller-Klieser W. (2011) Lactate: a metabolic key player in cancer. Cancer Res. 71, 6921–6925 [DOI] [PubMed] [Google Scholar]
- 30. Végran F., Boidot R., Michiels C., Sonveaux P., Feron O. (2011) Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 71, 2550–2560 [DOI] [PubMed] [Google Scholar]
- 31. Ullah M. S., Davies A. J., Halestrap A. P. (2006) The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J. Biol. Chem. 281, 9030–9037 [DOI] [PubMed] [Google Scholar]
- 32. Lu H., Forbes R. A., Verma A. (2002) Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem. 277, 23111–23115 [DOI] [PubMed] [Google Scholar]
- 33. Sonveaux P., Végran F., Schroeder T., Wergin M. C., Verrax J., Rabbani Z. N., De Saedeleer C. J., Kennedy K. M., Diepart C., Jordan B. F., Kelley M. J., Gallez B., Wahl M. L., Feron O., Dewhirst M. W. (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118, 3930–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jansen S., Pantaleon M., Kaye P. L. (2008) Characterization and regulation of monocarboxylate cotransporters Slc16a7 and Slc16a3 in preimplantation mouse embryos. Biol. Reprod. 79, 84–92 [DOI] [PubMed] [Google Scholar]
- 35. Kim S. T., Moley K. H. (2009) Regulation of facilitative glucose transporters and AKT/MAPK/PRKAA signaling via estradiol and progesterone in the mouse uterine epithelium. Biol. Reprod. 81, 188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bombail V., Gibson D. A., Collins F., MacPherson S., Critchley H. O., Saunders P. T. (2010) A role for the orphan nuclear receptor estrogen-related receptor α in endometrial stromal cell decidualization and expression of genes implicated in energy metabolism. J. Clin. Endocrinol. Metab. 95, E224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Q., Kannan A., DeMayo F. J., Lydon J. P., Cooke P. S., Yamagishi H., Srivastava D., Bagchi M. K., Bagchi I. C. (2011) The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331, 912–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodesch F., Simon P., Donner C., Jauniaux E. (1992) Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 80, 283–285 [PubMed] [Google Scholar]
- 39. Daikoku T., Matsumoto H., Gupta R. A., Das S. K., Gassmann M., DuBois R. N., Dey S. K. (2003) Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner: evidence for differential function of HIFs during early pregnancy. J. Biol. Chem. 278, 7683–7691 [DOI] [PubMed] [Google Scholar]
- 40. Salama S. A., Mohammad M. A., Diaz-Arrastia C. R., Kamel M. W., Kilic G. S., Ndofor B. T., Abdel-Baki M. S., Theiler S. K. (2014) Estradiol-17β upregulates pyruvate kinase M2 expression to co-activate estrogen receptor-α and to integrate metabolic reprogramming with the mitogenic response in endometrial cells. J. Clin. Endocrinol. Metab. 99, 3790–3799 [DOI] [PubMed] [Google Scholar]
- 41. Frolova A. I., O'Neill K., Moley K. H. (2011) Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells, preventing decidualization and implantation. Mol. Endocrinol. 25, 1444–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]