Background: Signaling pathways involved in the sensitization by target receptors of β-amyloid-triggered neurotoxicity have not been identified.
Results: Exposure to β-amyloid in the presence of nicotinic receptors led to ERK activation followed by JNK activation and increased PHF-tau.
Conclusion: β-amyloid neurotoxicity entails activation of discrete intracellular signaling pathways.
Significance: Activation of select signaling pathways by β-amyloid may be an early event in Alzheimer disease.
Keywords: amyloid-beta (AB), c-Jun N-terminal kinase (JNK), extracellular-signal-regulated kinase (ERK), mitogen-activated protein kinase (MAPK), nicotinic acetylcholine receptors (nAChR)
Abstract
Among putative downstream synaptic targets of β-amyloid (Aβ) are signaling molecules involved in synaptic function, memory formation and cognition, such as the MAP kinases, MKPs, CaMKII, CREB, Fyn, and Tau. Here, we assessed the activation and interaction of signaling pathways upon prolonged exposure to Aβ in model nerve cells expressing nicotinic acetylcholine receptors (nAChRs). Our goal was to characterize the steps underlying sensitization of the nerve cells to neurotoxicity when Aβ-target receptors are present. Of particular focus was the connection of the activated signaling molecules to oxidative stress. Differentiated neuroblastoma cells expressing mouse α4β2-nAChRs were exposed to Aβ1–42 for intervals from 30 min to 3 days. The cells and cell-derived protein extracts were then probed for activation of signaling pathway molecules (ERK, JNK, CaMKII, CREB, MARCKS, Fyn, tau). Our results show substantial, progressive activation of ERK in response to nanomolar Aβ exposure, starting at the earliest time point. Increased ERK activation was followed by JNK activation as well as an increased expression of PHF-tau, paralleled by increased levels of reactive oxygen species (ROS). The impact of prolonged Aβ on the levels of pERK, pJNK, and ROS was attenuated by MEK-selective and JNK-selective inhibitors. In addition, the MEK inhibitor as well as a JNK inhibitor attenuated Aβ-induced nuclear fragmentation, which followed the changes in ROS levels. These results demonstrate that the presence of nAChRs sensitizes neurons to the neurotoxic action of Aβ through the timed activation of discrete intracellular signaling molecules, suggesting pathways involved in the early stages of Alzheimer disease.
Introduction
Alzheimer disease (AD)2 is a progressive neurodegenerative disorder. It is characterized by various pathological hallmarks including extracellular plaques composed primarily of beta amyloid (Aβ), intracellular neurofibrillary tangles formed from hyperphosphorylated tau, synaptic loss, and neurodegeneration in brain regions critical for memory processes. The onset of AD is best correlated with increased levels of Aβ (1). There exists evidence indicating that various different intracellular pathways including MAP kinases (MAPK), Fyn and others can be acutely regulated by oligomeric Aβ (2). The roles for these pathways in AD remain to be determined.
All MAPK pathways (the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 pathways) appear to have been activated in vulnerable neurons by Aβ (2), suggesting involvement in the pathophysiology and pathogenesis of AD. Studies conducted on post-mortem tissues have confirmed that the activation of MAPK occurs at a very early stage in AD (3, 4). In model neuronal systems, ERK and one of its downstream effectors CREB (cyclic AMP-regulatory element binding) were activated by Aβ in a manner dependent on calcium and nAChRs (5, 6). Notably, Aβ activation of the ERK pathway has been linked to caspase activation (7) and the pathological hyper-phosphorylation of tau. Studies have also demonstrated that Aβ can regulate CREB and Ca2+/calmodulin-dependent kinase II (CaMKII) by activating calcineurin and protein phosphatase 1, leading to deficits in long-term potentiation (LTP) in the hippocampus (8). ERK/CREB signaling in the hippocampus normally plays a significant role in determining LTP (9, 10). Hence, contextual and spatial memory formation in mammals (11–14) resulting from alterations in ERK signaling by Aβ may contribute to early memory deficits in AD.
A possible link between the JNK signaling pathway and Aβ-induced oxidative stress has been implicated in neuronal compromise (15–16). Indeed, inhibitors for JNK, as well as ERK1/2, and the upstream regulators MEKs (MAP2Ks) have been widely investigated as potential therapeutic drugs for AD (e.g. 17, 18). Interestingly, MAPK signaling is regulated by a group of MAP kinase phosphatases (MKPs) (19), specifically, the dual-specificity MKPs, which can differentially regulate ERK and JNK (20).
In parallel, Fyn, a member of the Src family of kinases, plays a role in the regulation of Aβ production as well as Aβ-induced synaptic deficits and neurotoxicity, perhaps primarily by inducing tyrosine phosphorylation of tau (21). The relation of this signaling pathway to the MAPK pathway in AD remains unclear.
As we have previously found that the presence of a relatively high-affinity target receptor for Aβ sensitizes nerve cells to Aβ toxicity (22), potential roles for select intracellular pathways involved in this sensitization were evaluated. The study focused on the MAPK pathway, assessing the relationship between receptor-linked, Aβ-induced alterations in signaling and oxidative stress.
Experimental Procedures
Cell Culture and Transfection
Hybrid neuroblastoma NG108–15 cells were used as a model nerve cell system, as they are normally devoid of functional nAChRs (22–24). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 15% FBS and hypoxanthine/aminopterin/thymidine (HAT selection). Cells were differentiated on poly-l-lysine (plates) or Cell-Tak (coverslips) with 1 mm dibutyryl cyclic AMP in DMEM in the presence of reduced serum (1% FBS) and penicillin-streptomycin-glutamine for 72 h, as described previously (22–24). pcDNA3.1 expression vectors harboring mouse sequences for α4 and β2 nAChR subunits (1:4 ratio, respectively) were transfected into the differentiated cells using FuGENE HD, a lipid-based transfection reagent, for 48 h. Mock-transfected NG108–15 cells, exposed only to FuGENE HD and not plasmid DNA, were used as controls (22–24).
Immunoblot Analysis
NG108–15 cells were grown and differentiated on poly-l-lysine-coated 10-cm cell culture plates as previously described. Forty-eight hours after transfection, the cells were treated with 100 nm Aβ1–42 for different time-points ranging from 30 min to 3 days in the presence or absence of particular inhibitors. To further distinguish the effects produced by interaction between Aβ1–42 and α4β2 nAChRs, mock-transfected cells (untreated or Aβ-treated) were used as controls. The medium was aspirated, and the cells were washed with PBS. After adding 100 μl of lysis buffer (25 mm HEPES, 200 mm NaCl, 1 mm EDTA, 0.5% digitonin, 0.2% sodium cholate, 0.5% Nonidet P-40, HaltTM Protease Inhibitor Mixture, 0.02% DTT), the cells were scraped gently from the plates sitting on ice and collected in a microcentrifuge tube. The plates were washed with another 100 μl of lysis buffer for maximum cell recovery. The extracted proteins were collected by centrifugation at 13,000 rpm for 20 min at 4 °C. The pellet was discarded and the supernatant (protein) was collected in a fresh microcentrifuge tube. The protein samples were then diluted with 25 μl of SDS sample buffer and 10 μl dithiothreitol to make a total volume of 100 μl. The samples were heated to 95 °C for 5 min, cooled immediately on ice, and then centrifuged. Equal amounts of protein were loaded onto 4–20% Tris-HCl gels and the samples resolved by SDS-PAGE. Proteins were then transferred to preactivated PVDF membrane using the Invitrogen iBlot semi-dry blotting system. Membranes were preactivated by incubating with methanol for 30 s, washing with distilled water for 2 min, and adding transfer buffer for 30 min. After transfer, membranes were soaked in PBS for 5 min and incubated with LI-COR blocking buffer for 2 h at room temperature with shaking. The membranes were then separately incubated with various primary antibodies at dilutions of 1:1000 overnight at 4 °C with gentle shaking. The membranes were washed three times for 10 min each with TBS containing 0.1% Tween (TBST). Bound primary antibodies were detected via IR dye-linked LI-COR secondary antibodies (anti-mouse, anti-rabbit, or anti-rat; 1:5000) for 1 h at room temperature followed by washing the membrane three times with TBST for 10 min each. Immunoreactivity for actin or GAPDH was used as a loading control. Immunoreactive bands in the PVDF membrane were visualized using an Odyssey Infrared Imaging system (LI-COR Biosciences). Bands were compared with protein standards.
Immunocytochemistry
Cells were differentiated on Cell-Tak-coated coverslips and transfected as described (22–24). After various treatments, cells were fixed using freshly prepared 4% paraformaldehyde for 40min and rinsed with PBS for 30 min. To block nonspecific binding, the fixed cells were incubated in TBS containing 5% BSA and 10% normal goat serum for 30 min. Rabbit anti-pERK1/2 and mouse anti-ERK1/2 (1:200) were then added to the cultures and incubated overnight at 4 °C. The cultures were then washed with 10% goat serum in TBS for 30 min, and thereafter, incubated with the fluorescent secondary antibodies (anti-rabbit Alexa-488 and anti-mouse Alexa-633 IgGs, respectively; 1:500) for 30 min at room temperature. The coverslips were finally washed with 10% normal goat serum and TBS, plated onto glass microscope slides and sealed for imaging using a Zeiss LSM 5 Pascal confocal imaging system on a Zeiss Axiovert 200M microscope or a Nikon Cameleon AOTF confocal system.
Reactive Oxygen Species (ROS)/Hoechst Staining
Relative oxidative stress was assessed in live cultures of differentiated cells, transfected as described (22), as changes in the levels of reactive molecular species. Following various treatments, the Image-iT Live Reactive Oxygen Species Detection assay (Life Technologies) was performed in accordance with the manufacturer's protocol. In brief, the cells were incubated with carboxy-H2DCFDA (component A) at 37 °C for 30 min. During the last 5 min of incubation, 2 μg/ml of HOECHST stain (component B) was added. The cells were washed twice with HBS and visualized using an Olympus IX71 epifluorescence microscope at excitation/emission of 495/529 nm (ROS) and 350/461 nm (HOECHST), respectively, linked to a Macrofire camera.
Aβ
Aβ1–42 was purchased from American Peptide. A stock solution (0.1 mm) of Aβ1–42 was prepared by dissolving the solid peptide in double-de-ionized water. The solution was vortexed and sonicated for 15 min to ensure complete solubilization (see Ref. 23). Under these conditions, Aβ1–42 is largely present as stable oligomers, as assessed by SDS-PAGE and native gel analysis (6, 23).
Chemicals
DMEM was from Mediatech, Inc. FBS and normal goat serum were from GIBCO. Cell-Tak was from BD Biosciences (Bedford, MA). Poly-l-lysine was from Sigma. Image iT Live ROS Detection kit was purchased from Life Technologies. Paraformaldehyde was from Fisher. BSA was purchased from Sigma-Aldrich. Anti-phosphoERK, anti-panERK, anti-phosphoJNK, anti-panJNK, anti-phosphoCREB, and anti-phosphoMARCKS were all from Cell Signaling. Anti-phosphoFyn was from BD Transduction Labs. Anti-PHF tau (clone AT8), anti-phosphoCaMKII and anti-panCaMKII were from ThermoFisher (Pierce). Anti-MKP7 was from Santa Cruz Biotechnology. Anti-actin was from Sigma; anti-GAPDH was from Abcam. The FITC-labeled and peroxidase-conjugated secondary antibodies (goat anti-rabbit and goat anti-mouse) were from Jackson ImmunoResearch. The Alexa-488 and Alexa-633 secondary antibodies were purchased from Life Technologies. IRDye® 680 goat anti-rabbit secondary antibodies were from LI-COR. U0126 was from Cell Signaling, while DJNK-1 was the kind gift of Dr. Tessi Sherrin (University of Hawaii). Both reagents were used at concentrations effecting full inhibition, as based on previous reports (25). All common reagents (e.g. buffers, salts, solvents, detergents, etc.) were either from ThermoFisher or Sigma-Aldrich.
Statistical Analysis
Multiple comparisons were subjected to ANOVA, followed by Bonferroni posthoc tests. A minimum of p < 0.05 was used to establish a significant difference. All experiments were replicated at least 3 times.
Results
Differential Activation of MAPK Pathways following Sustained Exposure of Neuronal Cultures Expressing α4β2-nAChRs to Aβ
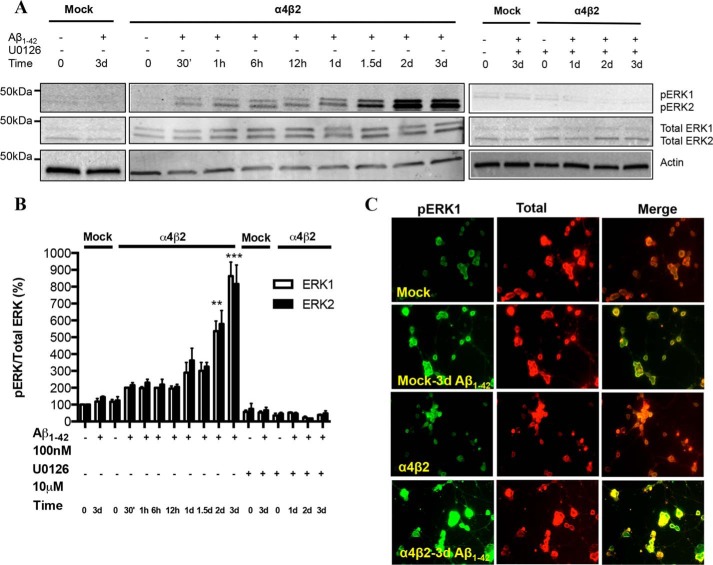
We have previously shown that the presence of α4β2-nAChRs sensitizes the cells to toxicity induced by prolonged exposure to Aβ, as measured by increased ROS production, decreased nuclear integrity, and eventual cell death (22). To investigate the initial downstream effects of receptor-coupled sensitization of Aβ1–42 toxicity, differentiated NG108–15 cells expressing α4β2-nAChRs were exposed to fresh, oligomeric Aβ1–42 at 100 nm (22) for several time-points from 30 min, 1 h, 6 h, 12 h, 1 d, 1.5 d, 2 to 3 days. Mock-transfected cells treated with Aβ1–42 for 0 and 3 days were used as controls. Cell lysates were analyzed via immunoblotting using rabbit anti-phospho-ERK1/2 and mouse anti-ERK1/2 (pan) antibodies. Whereas little if any activation was observed in mock-transfected cells treated with 100 nm Aβ1–42 for 3 days, the levels of pERK1 as well as pERK2 were strongly up-regulated in a time-dependent manner in cells transfected with α4β2-nAChRs, increasing severalfold by 2–3 days, (Fig. 1, A and B; p < 0.001 compared with mock-transfected controls). The cells expressing α4β2-nAChRs had basal levels (t0) of pERK1/2 comparable to the levels found in mock-transfected cells treated with Aβ1–42 for 3 days. Interestingly, an Aβ-induced increase in pERK1 and pERK2 was detectable at 30min out to 12 h for α4β2-nAChR-transfected cells (p < 0.05 compared with baseline), indicating that activation of ERK is a very early event. The increase in pERK induced by Aβ1–42 was inhibited upon co-incubation with U0126, a specific MEK1/2 inhibitor (ERK is downstream of MEK1/2), as assessed by both immunoblot analysis (Figs. 1A and B) and immunostaining (Fig. 1C). The selectivity for ERK activation via α4β2-nAChRs is in accordance with the studies using a transgenic AD mouse model (Tg2576), which showed an age-dependent increase both in nAChR expression as well as in ERK2 activation in the hippocampus and the cortex (5). A similar effect was observed using organotypic hippocampal slices incubated with Aβ (5).
FIGURE 1.
Activation of the ERK MAPK pathway in NG108–15 cells expressing α4β2 nAChRs in response to prolonged exposure to Aβ: time-dependence and sensitivity to a MEK inhibitor. A and B, progressive increases in pERK levels in response to prolonged exposure to 100 nm Aβ1–42 in the absence or presence of U1026, a selective inhibitor of MEK, the upstream regulator of ERK, in cells expressing α4β2-nAChRs or not (Mock: mock-transfected). pERK levels were compared with total ERK as well as actin (loading control). Data are expressed as % (± S.E.) of untreated, mock-transfected controls (n = 3 experiments). C, representative images depicting immunostaining of pERK and total ERK in mock- and α4β2-nAChR-transfected cells treated with Aβ1–42 for 3 days. pERK levels in cells expressing α4β2-nAChRs and treated with Aβ were significantly different in comparison to untreated controls.
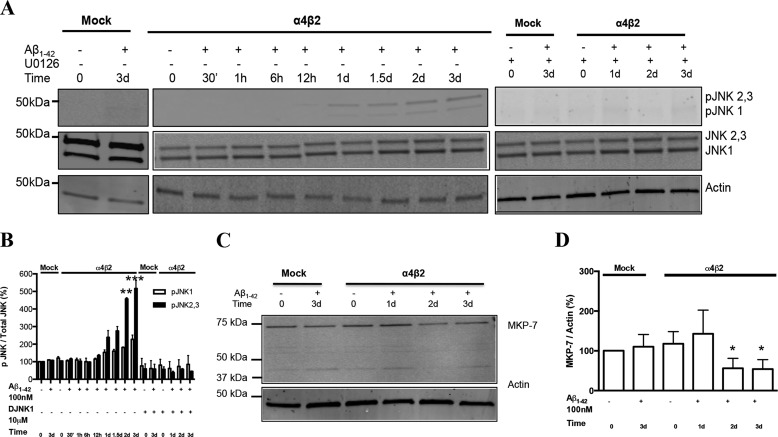
The phosphorylated forms of JNK, another member of the MAPK family, was found to be significantly up-regulated as a result of Aβ1–42 exposure, starting at 1 day and then substantially increasing at 2–3 days. This up-regulation was fully blocked by co-incubation with the JNK peptide inhibitor, DJNK-1 (Fig. 2A). However, the increase in the levels of the neuronal isoforms of pJNK2,3 was dramatically more pronounced than that of pJNK1: over 5-fold as compared around 2-fold, respectively, by 3 days in the presence of the α4β2-nAChRs (Fig. 2B). The basal levels of pJNK1–3 were nominally higher in α4β2-nAChR-transfected cells as compared with mock-transfected cells. This up-regulation is consistent with the recent studies demonstrating the neuroprotective effect of inhibiting JNK activity in rodent AD models (26, 27).
FIGURE 2.
Activation of the JNK MAPK pathway in NG108–15 cells expressing α4β2 nAChRs in response to prolonged exposure to Aβ: time-dependence and sensitivity to JNK inhibitor. A, progressive increases in the levels of pJNK1 as well as pJNK2,3 in response to prolonged exposure to 100 nm Aβ1–42 in the absence or presence of DJNK1, a selective inhibitor of JNK, in cells expressing α4β2-nAChRs or not (Mock: mock-transfected). B, quantification of averaged immunoblot intensities for pJNKs normalized to total JNK, as compared with actin (loading control). Data are expressed as % (± S.E.) of untreated, mock-transfected controls (n = 3 experiments). C and D, expression levels of MKP-7 in differentiated NG108–15 cells treated or not with Aβ1–42. pJNK1 and pJNK2,3 levels in cells expressing α4β2-nAChRs and treated with Aβ for 2 and 3 days were significantly different in comparison to controls.
As MAPK phosphatases (MKPs) regulate MAPK signaling, we determined the levels of MKP in cells treated with Aβ. Aβ-induced up-regulation of ERK and JNK was accompanied by a significant reduction in the levels of MKPs, especially MKP-7, which is JNK-specific (MKP-7, Fig. 2, C and D; MKP-1, not shown), indicating that decreased phosphatase activity may, in part, contribute to the MAPK activation. Under the conditions of the present study, there was no consistent up-regulation of p38 MAPK with prolonged exposure of differentiated NG108–15 cells expressing nAChRs (data not shown).
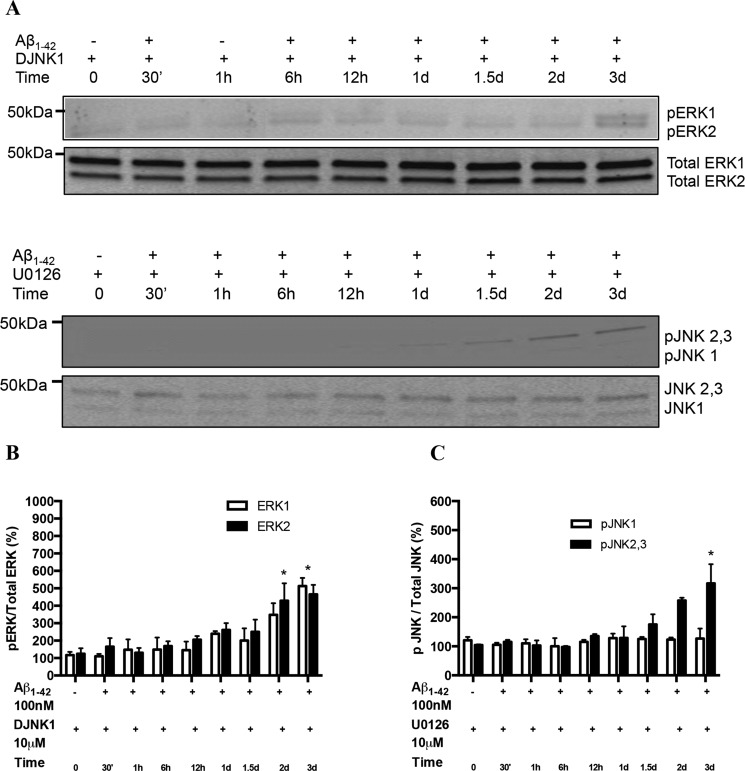
To investigate possible cross-talk between the ERK and JNK pathways, we determined the levels of pERK1/2 against total ERK in cells expressing α4β2-nAChRs and treated or not with Aβ1–42 in the presence of the JNK inhibitor DJNK-1. The levels of pERK1/2 were significantly attenuated (Fig. 3, A and B), particularly after 1d of treatment. A similar trend was observed for pJNK levels when the α4β2-nAChR-expressing cells were co-treated with Aβ1–42 and the MEK inhibitor U0126 (Fig. 3, A and C). The markedly decreased up-regulation suggests a partial interplay between the two pathways.
FIGURE 3.
Interplay between ERK and JNK pathways activated in cells expressing α4β2 nAChRs in response to prolonged exposure to Aβ. A, the levels of pERK1/2 were attenuated in response to prolonged exposure to 100 nm Aβ1–42 in the presence of DJNK1, a selective inhibitor of JNK, in cells expressing α4β2-nAChRs. Similarly, levels of pJNK1 as well as pJNK2,3 were reduced significantly on incubation with U1026, MEK inhibitor. B and C represent the quantification of averaged immunoblot intensities for pERK normalized to total ERK and pJNKs normalized to total JNK, as compared with actin (loading control), respectively. *, p < 0.05 compared with controls not treated with Aβ.
MAPK Pathways in Aβ-induced Oxidative Stress
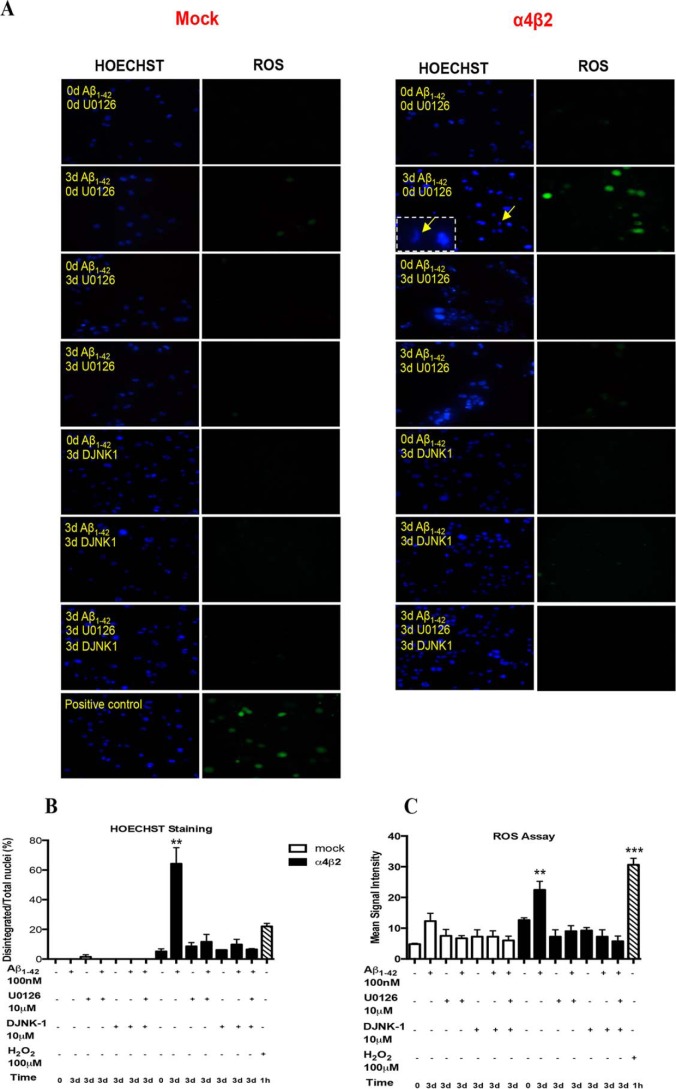
To test the role of MAPK in the sensitization by α4β2-nAChRs of Aβ-induced toxicity, specifically oxidative stress, we determined the levels of ROS and nuclear integrity in the cells, untreated (control), or treated with 100 nm Aβ1–42 for 3 days in the presence or absence of U0126 (MEK inhibitor) or DJNK-1 (JNK inhibitor) or both. Co-treatment with U0126 or DJNK-1 attenuated the number of ROS-positive cells to ∼15% of the values found in the absence of the inhibitors (Fig. 4, A and B) over baseline. A similar trend was observed for the number of disintegrated nuclei (Fig. 4, A and C). Treatment with U0126 or DJNK-1 alone, as separate controls or even co-treatment with both the inhibitors together with Aβ, blocked any significant ROS in either mock or α4β2-nAChR-transfected cells.
FIGURE 4.
Effects of ERK and JNK inhibitors on Aβ-induced oxidative stress in the presence of α4β2 nAChRs. A, alterations in oxidative stress (as changes in ROS) and nuclear integrity (HOESCHT staining) in differentiated NG108–15 cells expressing high-affinity nAChRs (right: α4β2) or not (left: Mock) in response to 3-day treatment with 100 nm Aβ1–42 in the absence (control) or presence of either 10 μm U0126 (MEK inhibitor) or DJNK1 (JNK inhibitor) or both. As a positive control, ROS and nuclear integrity were assessed following treatment with 100 μm hydrogen peroxide. Quantification of HOESCHT (B) and ROS (C) staining is presented as the means (± S.E.) of fluorescent intensity and number (±S.E.) of disintegrated nuclei/total nuclei (magnified example in inset), respectively, for all cells in a given field (3 experiments). Signals in cells expressing α4β2-nAChRs and treated with Aβ in presence of inhibitor were not significantly different from control (mock-transfected) cells treated with Aβ. **, p < 0.01: ***, p < 0.001 compared with controls.
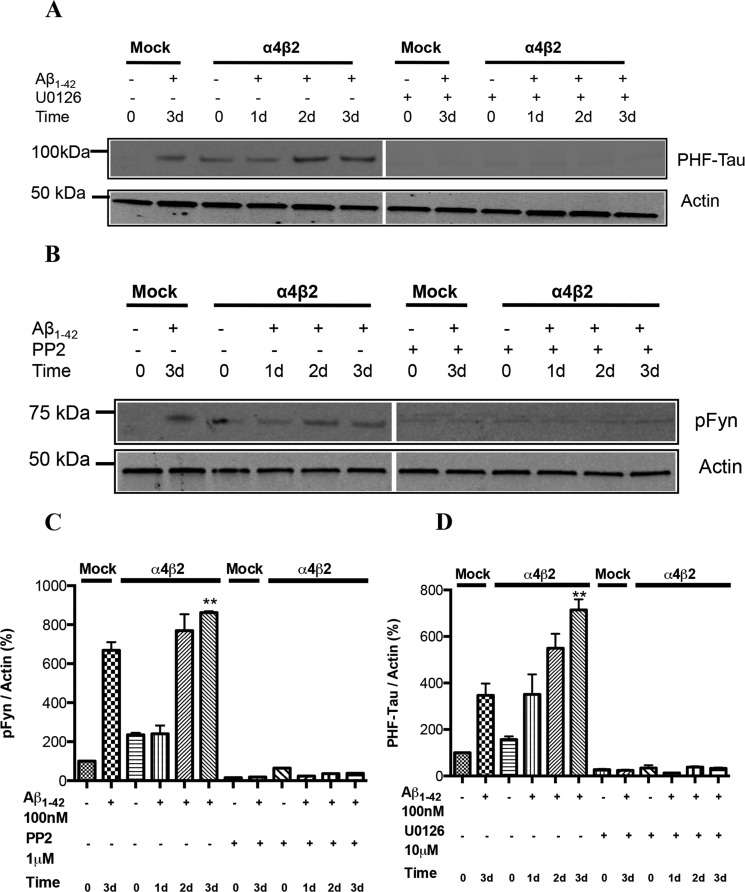
Regulation of PHF-tau and pFyn following Sustained Exposure of Neuronal Cultures Expressing α4β2-nAChRs to Aβ
Mislocalization of hyperphosphorylated tau (and, consequently, PHF-tau) has been widely proposed to be a downstream effector of Aβ toxicity (28, 29). In addition, recent evidence proposes that the pathological mislocalization of tau is correlated with targeting of Fyn to postsynaptic sites at CA1 synapses (30). In accordance with these reports, our studies showed that incubation with Aβ caused a significant up-regulation in the levels of both pFyn and PHF-tau (Fig. 5), which paralleled the time course for MAPK activation. However, the induction of pFyn was nAChR-independent, whereas the extent of the induction of PHF-tau was receptor-dependent. These data suggest that while Fyn may be important if not essential for tau pathology, it is not involved in the nAChR sensitization of Aβ-mediated toxicity.
FIGURE 5.
Alterations in the levels of PHF-Tau and pFyn in response to prolonged exposure to Aβ: receptor-dependence. Progressive increases in PHF-Tau (A) and pFyn (B) in differentiated NG108–15 cells expressing α4β2-nAChRs or not (Mock: mock-transfected) response to prolonged exposure to 100 nm Aβ1–42. Note the significant increases following 3-day treatment with Aβ in the absence of target receptor (Mock). Aβ-induced increases in PHF-Tau were compared in the absence or presence of U1026, a selective inhibitor of MEK, while Aβ-induced increases in pFyn were compared in the absence or presence of PP2, a Src kinase family inhibitor. C and D, quantification of averaged immunoblot intensities, as compared with actin (loading control). Data are expressed as % (± S.E.) of untreated, mock-transfected controls (n = 3 experiments). **, p < 0.01 for PHF-tau or pFyn levels in cells expressing α4β2-nAChRs and treated with Aβ for 2 and 3 days when compared with untreated control.
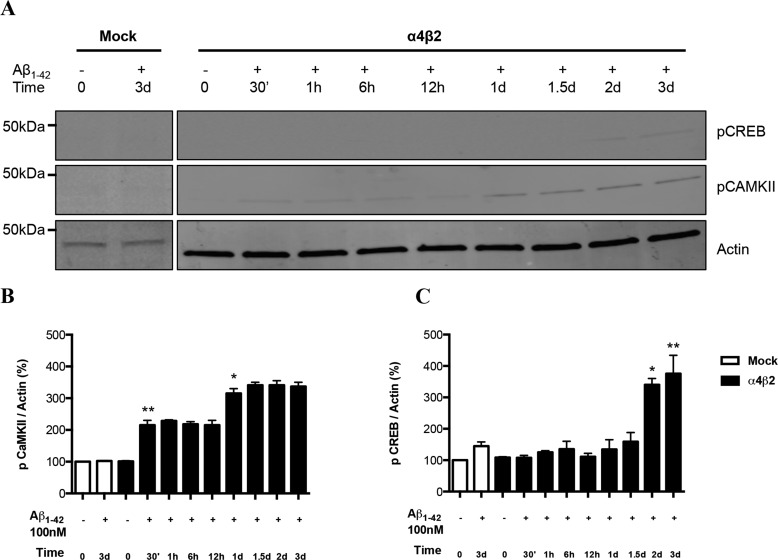
Altered Levels of pCaMKII and pCREB in Comparison to pMARCKS on Prolonged Incubation of Cells Expressing α4β2-nAChRs to Aβ
Activation of nAChRs have been shown to trigger, in turn, the calcium-dependent activation of CaMKII/IV, ERK/MAPK, and CREB in several neuronal models (31–35). Based on these studies and our findings showing altered calcium responses in α4β2-nAChR-transfected cells exposed to Aβ for 3 days (22), we also assessed changes in the levels of CaMKII under same conditions. As expected, the levels of phospho-CaMKII were initially increased at 30 min of Aβ exposure and maintained steady state levels up to 12 h. However, the levels rose up again starting at 1day, indicating that the induction in pCaMKII by Aβ has two phases, with the later phase delayed as compared with pERK. Overall, the expression of pCaMKII appeared to be modest.
Levels of phospho-CREB, a downstream effector of ERK and CaMKII, were shown to increase at a much later time point around day 2–3 in cells expressing α4β2-nAChR, following the second phase increase in pCaMKII (Fig. 6C). There was a slight increase in pCREB levels in mock-transfected cells exposed to Aβ. Unlike pERK and CaMKII, pCREB was not significantly increased at 30 min.
FIGURE 6.
Alterations in the levels of pCaMKII and pCREB Fyn in response to prolonged exposure to Aβ. A, progressive increases in the levels of pCaMKII and pCREB as compared with total CaMKII in differentiated NG108–15 cells expressing α4β2-nAChRs or not (Mock: mock-transfected) response to prolonged exposure to 100 nm Aβ1–42. B and C, quantification of averaged immunoblot intensities, as compared with actin (loading control) for pCaMKII and pCREB, respectively. Data are expressed as % (±S.E.) of untreated, mock-transfected controls (n = 3 experiments). *, p < 0.05 or **, p < 0.01 for pCaMKII or pCREB levels in cells expressing α4β2-nAChRs and treated with Aβ for 2 and 3 days when compared with untreated control.
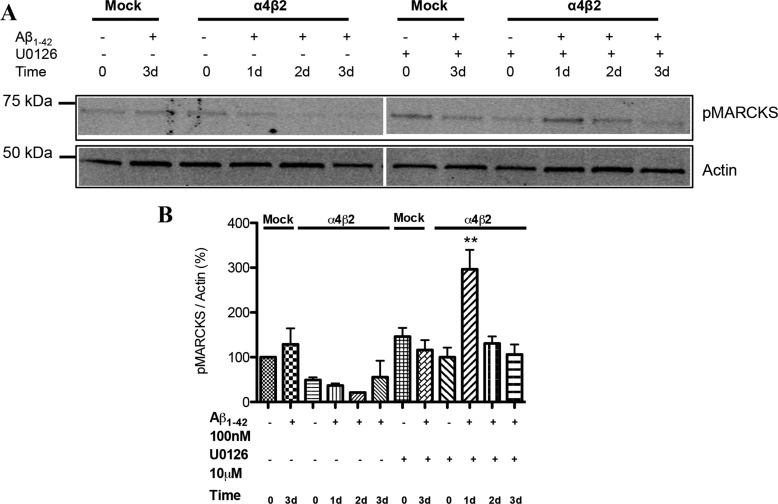
For comparison, alterations in pMARCKS, identified in a preliminary proteomics screen following Aβ treatment, was examined to assess the involvement of the parallel PKC pathway. There was actually a decrease in pMARCKs in receptor-expressing cells as compared with controls (mock-transfected) in response to prolonged Aβ exposure (Fig. 7). Interestingly, inhibition of the ERK pathway led to a substantially enhanced but transient up-regulation of pMARCKS (at day 1), most notably in nAChR-expressing cells. This would indicate that the MAPK pathway may be suppressing the PKC-MARCKS pathway upon exposure to Aβ.
FIGURE 7.
Alterations in the levels of pMARCKS in response to prolonged exposure to Aβ: down-regulation and the impact of ERK inhibition. A, progressive decreases in the levels of pMARCKS in differentiated NG108–15 cells expressing α4β2-nAChRs or not (Mock: mock-transfected) response to prolonged exposure to 100 nm Aβ1–42 in the absence (control) or presence of 10 μm U0126 (MEK inhibitor). Progressive decreases in the levels of Western blot micrograph showing the altered levels of pCREB and pCaMKII in mock and α4β2-nAChR-transfected cells treated with Aβ for different time-points. GAPDH was used as a loading control. B and C show the quantification of the blots for pCREB and pCaMKII respectively. Data are expressed as % (±S.E.) of untreated, mock-transfected controls (n = 3 experiments). **, p < 0.01 compared with controls.
Discussion
Acute-short-term application of Aβ, or often a toxic C-terminal fragment of Aβ (Aβ25–35) has been found to activate several signaling pathways (2), including the MAPK cascade (5, 6, 16, 36). Here, we examined the impact of prolonged exposure to Aβ on intracellular signaling in a model cultured nerve cell system (differentiated clonal neuroblastoma cells) selectively expressing α4β2-nAChRs, as defined high-affinity targets for Aβ (22–24). The advantage of this model neuronal system is that the timeframe for the basic sequence of events in Aβ neurotoxicity, steps previously identified for a wide range of other neural systems, has been clearly delineated (Fig. 8; Ref. 22). In addition, the presence of α4β2-nAChRs was found to substantially sensitize the cells to Aβ-induced oxidative stress and ultimately apoptosis (22), essentially as a shift in the dose-response such that nanomolar Aβ was now toxic, whereas in the absence of nAChRs micromolar Aβ was required to induce toxicity. How this sensitization engages intracellular signaling pathways was the objective of the present study.
FIGURE 8.
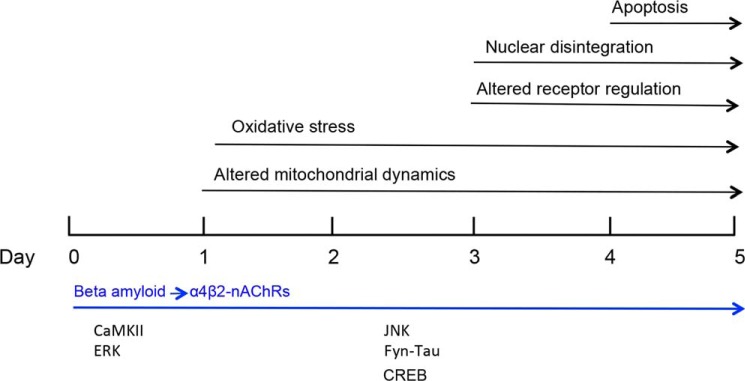
Schematic timeline for sensitization of Aβ-induced neurotoxicity in the presence of high-affinity nAChRs: Steps leading to apoptosis (22) and the key signaling molecules involved.
We found substantial ERK activation following nanomolar Aβ treatment of cells expressing α4β2-nAChRs, as one of the earliest sensitization events connected with oxidative stress leading to frank neurotoxicity (Fig. 8). This is in contrast to the nominal ERK activation on treatment with nanomolar Aβ of cells without nAChRs or in other primary systems (e.g. Ref. 7). CaMKII activation was also an early event, but it did not follow a pronounced, sustained timeline, unlike ERK activation which continued to increase substantially over several days. (There may remain maintained, Ca2+-independent CaMKII activity under these conditions, but the change in the level of pCaMKII was modest.) Activation of the JNK pathway by prolonged Aβ exposure in nerve cells expressing α4β2-nAChRs, on the other hand, followed ERK activation, correlating with later phases in neurotoxicity. This finding confirms the central role of the JNK pathway in Aβ neurotoxicity, here using a defined, reconstituted neuronal system.
The subsequent oxidative stress induced by chronic Aβ was blocked by co-incubation with selective inhibitors of either the upstream MEK (for ERK) or JNK. As ERK and JNK are known to play important roles in hippocampus-based synaptic plasticity (10), learning (11, 13) and certain types of memory formation (12–14), progressive activation of MAPKs following prolonged Aβ exposure suggests a molecular basis for the disruption of neuronal function in AD. For JNK, this extends to synaptic dysfunction (38). Furthermore, this selective toxicity in the presence of α4β2-nAChRs may contribute to the cholinergic deficit that occurs in Alzheimer disease.
The sensitization by α4β2-nAChRs appears to be specific. In a preliminary study, it was found that the presence of α7-nAChRs did not lead to sensitization to Aβ toxicity.3 Moreover, improvement in cognition has been found with activation of α7-nAChRs (e.g. 39), which have separately been shown to play a neuroprotective role against Aβ toxicity (40, 41), possibly involving the PI3K/Akt anti-apoptotic pathway (42). On the other hand, the up-regulation of α4β2-nAChRs on chronic Aβ treatment may or may not play a role in the sensitization process. Certainly, ERK activation on chronic Aβ treatment well precedes the nAChR up-regulation, which was not significant until day 3 (see Ref. 22). However, to address the role of up-regulation, it would require a means to prevent up-regulation in a controlled and defined way.
While investigating the possible mechanisms underlying the observed changes, it should be noted that MAP kinase pathways are implicated in hyperphosphorylation of tau protein. Specifically, both ERK1 and ERK2 are capable of phosphorylating tau at several of the same sites as those found for PHF-tau (43–45). By way of example, it has been demonstrated that microinjection of rat hippocampal primary neurons with purified ERK1 resulted in PHF-like tau hyperphosphorylation associated with compromised microtubule assembly (46). Our findings showing that ERK activation precedes PHF-tau indicate that up-regulation of phospho-ERK might be one of the earliest signals in response to Aβ exposure, followed by formation of PHF-tau.
Besides Aβ and tau, another molecule that has been suggested to be the third member of this toxic triad is Fyn (21, 47). Several lines of evidence suggest that Fyn kinase is involved in the synaptic and behavioral deficits associated with Aβ (48–50). Indeed, mature hippocampal cells from Fyn null (−/−) mice are protected against Aβ-mediated toxicity (51). Similarly, reducing or eliminating tau in a mouse model of AD significantly diminished behavioral deficits and improved survival, again strongly implicating tau in the mediation of the toxic effects of Aβ (52). However, we found that Fyn activation was increased with Aβ exposure, but not until 2–3 days, and the activation was entirely nAChR-independent. These results are in accordance with receptor-independent cross-talk between Fyn and tau during axonal myelination (53). Thus, while Fyn activation parallels the appearance of PHF-tau, it is likely not downstream of the ERK activation and represents an independent, converging pathway for Aβ toxicity. This raises the unique possibility that the signaling sensitized by nAChRs acts in conjunction with a MAP kinase-independent pathway activated by Aβ. Recently, it was found that Fyn is linked to Aβ signaling through cellular prion (37), which, in turn, regulates surface expression of NMDA receptors. It would be of interest to consider the role of prion, as a separate target for Aβ, in this scheme.
Based on our findings, we propose a link between receptor targets for Aβ and Aβ-mediated neurotoxicity. Taken together, our results provide an insight into how different pathways might converge at a very early stage of Alzheimer disease, particularly in the presence of high-affinity nAChRs.
Author Contributions
K. A. and R. A. N. designed the study and co-wrote the paper. K. A. performed all of the experiments, with assistance by J. C. for several experiments, notably the immunocytochemistry. K. A. and R. A. N. analyzed all of the experiments. All authors analyzed the results and approved the final version of the manuscript.
Acknowledgments
We thank Dr. Jerry Stitzel of the University of Colorado for kindly providing the mouse α4, α7, and β2 nAChR subunit sequences. We thank Drs. Cedomir Todorvic and Tessi Sherrin for providing anti-JNK and MKP antibodies for preliminary experiments. We thank Gene Yoshikawa for sharing the results of his preliminary experiments examining α7-nAChRs. We thank Drs. Berry, Hoffmann, and Todorovic for insightful comments on the manuscript.
This work was supported by grants (to R. A. N.) from the Hawaii Community Foundation (09ADVC-45413), the National Institute on Aging (AG21586) and the National Institute for General Medical Sciences (P20 GM103466). Additional support for the microscopy core at the University of Hawaii was provided by a Research Center for Minority Institutions grant from the National Institute on Minority Health and Health Disparities (G12MD007601). The authors declare that they have no conflicts of interest with the contents of this article.
G. Yoshikawa and R. A. Nichols, unpublished results.
- AD
- Alzheimer disease
- Aβ
- β-amyloid
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- CREB
- cyclic AMP-regulatory element-binding protein
- ERK
- extracellular signal-regulated kinase
- JNK
- c-Jun N-terminal kinase
- MAPK
- MAP kinases
- MKP
- MAPK phosphatase
- nAChR
- nicotinic acetylcholine receptor
- ROS
- reactive oxygen species.
References
- 1. Holtzman D. M., Morris J. C., Goate A. M. (2011) Alzheimer's disease: the challenge of the second century. Sci. Transl. Med. 3, 77sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balleza-Tapia H., Peña F. (2009) Pharmacology of the intracellular pathways activated by Amyloid beta protein. Mini-Rev. Med. Chem. 9, 724–740 [DOI] [PubMed] [Google Scholar]
- 3. Pei J. J., Braak E., Braak H., Grundke-Iqbal I., Iqbal K., Winblad B., Cowburn R. F. (2001) Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer's disease brains at different stages of neurofibrillary degeneration. J. Alzheimers Dis. 3, 41–48 [DOI] [PubMed] [Google Scholar]
- 4. Ferrer I., Blanco R., Carmona M., Ribera R., Goutan E., Puig B., Rey M. J., Cardozo A., Viñals R., Ribalta T. (2001) Phosphorylated MAP kinase (ERK1, ERK2) expression is associated with early tau deposition in neurons and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Brain Pathol. 11, 144–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dineley K. T., Westerman M., Bui D., Bell K., Ashe K. H., Sweatt J. D. (2001) Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer's disease. J. Neurosci. 21, 4125–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bell K. A., O'Riordan K. J., Sweatt J. D., Dineley K. T. (2004) MAPK recruitment by beta-amyloid in organotypic hippocampal slice cultures depends on physical state and exposure time. J. Neurochem. 91, 349–361 [DOI] [PubMed] [Google Scholar]
- 7. Chong Y. H., Shin Y. J., Lee E. O., Kayed R., Glabe C. G., Tenner A. J. (2006) ERK1/2 activation mediates Aβ oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J. Biol. Chem. 281, 20315–20325 [DOI] [PubMed] [Google Scholar]
- 8. Tu S., Okamoto S., Lipton S. A., Xu H. (2014) Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease. Mol. Neurodegener. 9, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lisman J. (2003) Long-term potentiation: outstanding questions and attempted synthesis. Phil. Trans. Roy. Soc. Lond. B: Biol. Sci. 358, 829–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smolen P., Baxter D. A., Byrne J. H. (2006) A model of the roles of essential kinases in the induction and expression of late long-term potentiation. Biophys. J. 90, 2760–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atkins C. M., Selcher J. C., Petraitis J. J., Trzaskos J. M., Sweatt J. D. (1998) The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1, 602–609 [DOI] [PubMed] [Google Scholar]
- 12. Blum S., Moore A. N., Adams F., Dash P. K. (1999) A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J. Neurosci. 19, 3535–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schafe G. E., Nadel N. V., Sullivan G. M., Harris A., LeDoux J. E. (1999) Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn. Mem. 6, 97–110 [PMC free article] [PubMed] [Google Scholar]
- 14. Selcher J. C., Atkins C. M., Trzaskos J. M., Paylor R., Sweatt J. D. (1999) A necessity for MAP kinase activation in mammalian spatial learning. Learn. Mem. 6, 478–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mazzitelli S., Xu P., Ferrer I., Davis R. J., Tournier C. (2011) The loss of c-Jun N-terminal protein kinase activity prevents the amyloidogenic cleavage of amyloid precursor protein and the formation of amyloid plaques in vivo. J. Neurosci. 31, 16969–16976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morishima Y., Gotoh Y., Zieg J., Barrett T., Takano H., Flavell R., Davis R. J., Shirasaki Y., Greenberg M. E. (2001) Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. 21, 7551–7560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bogoyevitch M. A., Boehm I., Oakley A., Ketterman A. J., Barr R. K. (2004) Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim. Biophys. Acta 1697, 89–101 [DOI] [PubMed] [Google Scholar]
- 18. Rapoport M., Ferreira A. (2000) PD98059 prevents neurite degeneration induced by fibrillar beta-amyloid in mature hippocampal neurons. J. Neurochem. 74, 125–133 [DOI] [PubMed] [Google Scholar]
- 19. Keyse S. M. (2000) Protein phosphatases and the regulation of mitogen-activated protein kinase signaling. Curr. Opin. Cell Biol. 12, 186–192 [DOI] [PubMed] [Google Scholar]
- 20. Owens D. M., Keyse S. M. (2007) Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26, 3203–3213 [DOI] [PubMed] [Google Scholar]
- 21. Yang K., Belrose J., Trepanier C. H., Lei G., Jackson M. F., MacDonald J. F. (2011) Fyn, a potential target for Alzheimer's disease. J. Alzheimers Dis. 27, 243–252 [DOI] [PubMed] [Google Scholar]
- 22. Arora K., Alfulaij N., Higa J. K., Panee J., Nichols R. A. (2013) Impact of sustained exposure to β-amyloid on calcium homeostasis and neuronal integrity in model nerve cell system expressing α4β2 nicotinic acetylcholine receptors. J. Biol. Chem. 288, 11175–11190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khan G. M., Tong M., Jhun M., Arora K., Nichols R. A. (2010) β-amyloid activates presynaptic α7 nicotinic acetylcholine receptors reconstituted into a model nerve cell system: involvement of lipid rafts. Eur. J. Neurosci. 31, 788–796 [DOI] [PubMed] [Google Scholar]
- 24. Tong M., Arora K., White M. M., Nichols R. A. (2011) Role of key aromatic residues in the ligand-binding domain of α7 nicotinic receptors in the agonist action of beta-amyloid. J. Biol. Chem. 286, 34373–34381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sherrin T., Blank T., Hippel C., Rayner M., Davis R. J., Todorovic C. (2010) Hippocampal c-Jun-N-terminal kinases serve as negative regulators of associative learning. J. Neurosci. 30, 13348–13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Braithwaite S. P., Schmid R. S., He D. N., Sung M. L., Cho S., Resnick L., Monaghan M. M., Hirst W. D., Essrich C., Reinhart P. H., Lo D. C. (2010) Inhibition of c-Jun kinase provides neuroprotection in a model of Alzheimer's disease. Neurobiol. Dis. 39, 311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramin M., Azizi P., Motamedi F., Haghparast A., Khodagholi F. (2011) Inhibition of JNK phosphorylation reverses memory deficit induced by β-amyloid (1–42) associated with decrease of apoptotic factors. Behav. Brain Res. 217, 424–431 [DOI] [PubMed] [Google Scholar]
- 28. Zempel H., Thies E., Mandelkow E., Mandelkow EM. (2010) Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous tau into dendrites, tau phosphorylation, and destruction of microtubules and spines. J. Neurosci. 30, 11938–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ittner L. M., Ke Y. D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B. C., Christie M. J., Napier I. A., Eckert A., Staufenbiel M., Hardeman E., Götz J. (2010) Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell 142, 387–397 [DOI] [PubMed] [Google Scholar]
- 30. Liao D., Miller E. C., Teravskis P. J. (2014) Tau acts as a mediator for Alzheimer's disease-related synaptic deficits. Eur. J. Neurosci. 39, 1202–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dajas-Bailador F. A., Soliakov L., Wonnacott S. (2002) Nicotine activates the extracellular signal-regulated kinase 1/2 via the α7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J. Neurochem. 80, 520–530 [DOI] [PubMed] [Google Scholar]
- 32. Nakayama H., Numakawa T., Ikeuchi T., Hatanaka H. (2001) Nicotine induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J. Neurochem. 79, 489–498 [DOI] [PubMed] [Google Scholar]
- 33. Hu M., Liu Q. S., Chang K. T., Berg D. K. (2002) Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol. Cell. Neurosci. 21, 616–625 [DOI] [PubMed] [Google Scholar]
- 34. Gubbins E. J., Gopalakrishnan M., Li J. (2010) α7 nAChR-mediated activation of MAP kinase pathways in PC12 cells. Brain Res. 1328, 1–11 [DOI] [PubMed] [Google Scholar]
- 35. Steiner R. C., Heath C. J., Picciotto M. R. (2007) Nicotine-induced phosphorylation of ERK in mouse primary cortical neurons: evidence for involvement of glutamatergic signaling and CaMKII. J. Neurochem. 103, 666–678 [DOI] [PubMed] [Google Scholar]
- 36. Yao M., Nguyen T. V., Pike C. J. (2005) β-amyloid-induced neuronal apoptosis involves c-jun N-terminal kinase-dependent downregulation of Bcl-w. J. Neurosci. 25, 1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Um J. W., Nygaard H. B., Heiss J. K., Kostylev M. A., Stagi M., Vortmeyer A., Wisniewski T., Gunther E. C., Strittmatter S. M. (2012) Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci. 15, 1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sclip A., Tozzi A., Abaza A., Cardinetti D., Colombo I., Calabresi P., Salmona M., Welker E., Borsello T. (2014) c-Jun N-terminal kinase has a key role in Alzheimer disease synaptic dysfunction in vivo. Cell Death Dis. 5, e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thomsen M. S., Hansen H. H., Timmerman D. B., Mikkelsen J. D. (2010) Cognitive improvement by activation of α7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr. Pharm. Design 16, 323–343 [DOI] [PubMed] [Google Scholar]
- 40. Kihara T., Shimohama S., Sawada H., Kimura J., Kume T., Kochiyama H., Maeda T., Akaike A. (1997) Nicotinic receptor stimulation protects neurons against beta-amyloid toxicity. Ann. Neurol. 42, 159–163 [DOI] [PubMed] [Google Scholar]
- 41. Hernandez C. M., Kayed R., Zheng H., Sweatt J. D., Dineley K. T. (2010) Loss of alpha7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer's disease. J. Neurosci. 30, 2442–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kihara T., Shimohama S., Sawada H., Honda K., Nakamizo T., Shibasaki H., Kume T., Akaike A. (2001) α7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J. Biol. Chem. 276, 13541–13546 [DOI] [PubMed] [Google Scholar]
- 43. Goedert M., Cohen E. S., Jakes R., Cohen P. (1992) p42 MAP kinase phosphorylation sites in microtubule-associated protein tau are dephosphorylated by protein phosphatase 2A. FEBS Lett. 312, 95–99 [DOI] [PubMed] [Google Scholar]
- 44. Drewes G., Lichtenberg-Kraag B., Döring F., Mandelkow E. M., Biernat J., Goris J., Dorée M., Mandelkow E. (1992) Mitogen-activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 11, 2131–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Illenberger S., Zheng-Fischhöfer Q., Preuss U., Stamer K., Baumann K., Trinczek B., Biernat J., Godemann R., Mandelkow E. M., Mandelkow E. (1998) The endogenous and cell cycle-dependent phosphorylation of tau protein in living cells: implications for Alzheimer's disease. Mol. Biol. Cell 9, 1495–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lu Q., Soria J. P., Wood J. G. (1993) p44 MAP kinase induces Alzheimer type alterations in tau function and in primary hippocampal neurons. J. Neurosci. 35, 439–444 [DOI] [PubMed] [Google Scholar]
- 47. Haass C., Mandelkow E. (2010) Fyn-tau-amyloid: a toxic triad. Cell 142, 356–358 [DOI] [PubMed] [Google Scholar]
- 48. Chin J., Palop J. J., Yu G. Q., Kojima N., Masliah E., Mucke L. (2004) Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice. J. Neurosci. 24, 4692–4697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chin J., Palop J. J., Puoliväli J., Massaro C., Bien-Ly N., Gerstein H., Scearce-Levie K., Masliah E., Mucke L. (2005) Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 25, 9694–9703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peña F., Ordaz B., Balleza-Tapia H., Bernal-Pedraza R., Márquez-Ramos A., Carmona-Aparicio L., Giordano M. (2010) Beta-amyloid protein (25–35) disrupts hippocampal network activity: Role of Fyn-kinase. Hippocampus 20, 78–96 [DOI] [PubMed] [Google Scholar]
- 51. Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., Morgan T. E., Rozovsky I., Trommer B., Viola K. L., Wals P., Zhang C., Finch C. E., Krafft G. A., Klein W. L. (1998) Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U.S.A. 95, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roberson E. D., Scearce-Levie K., Palop J. J., Yan F., Cheng I. H., Wu T., Gerstein H., Yu G. Q., Mucke L. (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science 316, 750–754 [DOI] [PubMed] [Google Scholar]
- 53. Klein C., Kramer E. M., Cardine A. M., Schraven B., Brandt R., Trotter J. (2002) Process outgrowth of oligodendrocytes is promoted by interaction of Fyn kinase with the cytoskeletal protein tau. J. Neurosci. 22, 698–707 [DOI] [PMC free article] [PubMed] [Google Scholar]