Background: A complex containing an innate immunity protein Tag7, and Hsp70 kills various cancer cells.
Results: Tag7 and its complex with Hsp70 bind to the TNFR1 receptor, but only the Tag7-Hsp70 complex induces a cytotoxic effect via apoptosis and necroptosis.
Conclusion: A new ligand has been found for the TNFR1 death receptor.
Significance: Tag7 may be used as an inhibitor of the TNF-α-induced cytotoxicity.
Keywords: apoptosis, cell death, heat shock protein (HSP), RNA interference (RNAi), tumor necrosis factor (TNF), TNFR1, Tag7, Tag7-Hsp70 complex, necroptosis, Hsp70, TNFR1, cytotoxicity
Abstract
Tag7 (also known as peptidoglycan recognition protein PGRP-S, PGLYRP1), an innate immunity protein, interacts with Hsp70 to form a stable Tag7-Hsp70 complex with cytotoxic activity against some tumor cell lines. In this study, we have analyzed the programmed cell death mechanisms that are induced when cells interact with the Tag7-Hsp70 complex, which was previously shown to be released by human lymphocytes and is cytotoxic to cancer cells. We show that this complex induces both apoptotic and necroptotic processes in the cells. Apoptosis follows the classic caspase-8 and caspase-3 activation pathway. Inhibition of apoptosis leads to a switch to the RIP1-dependent necroptosis. Both of these cytotoxic processes are initiated by the involvement of TNFR1, a receptor for TNF-α. Our results suggest that the Tag7-Hsp70 complex is a novel ligand for this receptor. One of its components, the innate immunity protein Tag7, can bind to the TNFR1 receptor, thereby inhibiting the cytotoxic actions of the Tag7-Hsp70 complex and TNF-α, an acquired immunity cytokine.
Introduction
The innate immunity protein Tag7, which is also known as a peptidoglycan recognition protein PGRP-S or PGLYRP1, is involved in the antimicrobial and antitumor defense systems. Its gene was identified in our laboratory (1–4). This protein is found in insects and mammals and has a conserved structure (2, 5). Its role in the antibacterial defense mechanisms in insects has been studied in detail (6, 7). In particular, it has been shown that Tag7 interacts with the Toll-like receptors, with the subsequent activation of the NFκB transcription factor and production of the antibacterial peptides (8).
By analyzing the role of mammalian Tag7 in antitumor defense in vitro, we found that it forms a specific 1:1 complex with the human 70-kDa heat shock protein 1A (Hsp70).3 This complex had a cytotoxic effect on some tumor cells, whereas neither Tag7 nor Hsp70 alone showed any cytotoxic activity (9). The same Tag7-Hsp70 complex is secreted by cytotoxic lymphocytes upon their interaction with tumor cells and inhibits tumor growth in mice (9, 10). However, its mechanism of action on tumor cells remains obscure, and insights into the cell death mechanisms induced by this complex are necessary to develop new tumor-specific immunotherapeutic strategies.
It was considered for some time that apoptosis was the only programmed cell death pathway, with activation of the caspase cascade playing the central role in apoptotic processes (11–13), but this opinion has subsequently been disproved. As has been shown during the past few decades, necrosis is not merely a form of passive cell death, but it is a regulated process that is mediated by specific molecular mechanisms. This has provided a basis for the concept of necroptosis as an alternative form of programmed cell death (14, 15).
It is noteworthy that necroptosis and apoptosis can be induced in the same cells by the same ligands and through the same receptors, referred to as the cell death receptors (TNF-α receptor (TNFR1), Fas, TNF-related apoptosis-inducing ligand (TRAILR1)) (15, 16). The mechanism of the ligand-cell death receptor interaction is fairly well known, particularly for the TNFR1-TNF-α interaction. The complex that controls cell death has been described. It contains both the adaptor proteins that transmit the signal from the receptor and the effectors of cytolysis, namely caspase-8 and receptor-interacting protein kinase RIP1, which activate apoptosis and necroptosis, respectively (17–19). It has been shown that activation of apoptosis prevents necroptosis because the RIP1 kinase that initiates the latter process is cleaved by caspase-8 (20, 21). When caspase-8 activity is absent or inhibited, the cell death follows the necroptotic pathway (22, 23).
Hence, the question arises as to whether the cell death receptor is involved in the cytotoxic signal transduction from the Tag7-Hsp70 complex. The purpose of this study was to analyze the pathways of cell death induced by the Tag7-Hsp70 complex and to identify the receptor involved in the cytotoxic signal transduction pathway. Here, we show that the Tag7-Hsp70 complex induces apoptosis and necroptosis in cancer cells via the TNFR1 receptor. Tag7 alone is able to inhibit the cytotoxic effects of both the Tag7-Hsp70 complex and TNF-α.
Experimental Procedures
Cells and Cell Subpopulations
The L-929 murine fibroblast cell line was cultured in 24-well plastic plates (Corning) filled with DMEM (Gibco) containing 10% fetal calf serum (Invitrogen), 2 mm l-glutamine, 10 mm HEPES (pH 7.4), and antibiotics in a 5% CO2 atmosphere at 37 °C. The HEK293 human embryonic kidney cell line was cultured in DMEM with 10% fetal calf serum, penicillin (100 units/ml), and streptomycin (100 mg/ml) in a humidified 5% CO2 atmosphere at 37 °C.
Clones of the L-929 cells were isolated by a limiting dilution method. An aliquot of the culture containing 100 cells was repeatedly diluted with the same volume of the medium in a 96-well plate. After 3 days, the plate was examined to detect wells containing a single cell clone. Such clones were cultured in the same wells until a confluent cell monolayer was formed, and then these cells were used in the experiments.
Proteins and Antibodies
The cDNAs for the recombinant human Tag7, Hsp70, and human TNF-α (GenBank accession numbers: NM_005091, NM_005345, and NM_000594, respectively) were subcloned into pQE-31 and expressed in Escherichia coli M15 (pREP4) (Qiagen). Hsp70 and TNF-α were purified on a Ni2-nitrilotriacetic acid agarose column (Qiagen) according to the manufacturer's instructions. Tag7 was purified as described previously (24).
The rabbit polyclonal antibodies to recombinant Tag7 were affinity-purified on a CNBr-activated Sepharose 4B column (GE Healthcare) coupled with recombinant Tag7 from yeast (9), as recommended by the manufacturer. The sheep polyclonal antibodies against TNFR1 were prepared as described (25). The rabbit polyclonal antibodies to the murine TNFR1, TNF-α, and soluble sTNFR1 were from Sigma-Aldrich. The Tag7-Hsp70 complex was obtained by incubating Tag7 with Hsp70 at 37 °C for 30 min.
Affinity Chromatography, Immunoadsorption, and Immunoblotting
The rabbit anti-Tag7 and sheep anti-TNFR1 antibodies were conjugated with CNBr-activated Sepharose 4B (GE Healthcare) according to the manufacturer's protocol. The Tag7-Hsp70 complex was adsorbed onto the anti-Tag7 Sepharose 4B column, which was then loaded with the solubilized L-929 cell membrane proteins or sTNFR1. The column was thoroughly washed with PBS/0.5 m NaCl and PBS alone, and then eluted with 0.25 m triethylamine, pH 12. The eluted material was resolved by SDS-PAGE as described (9) and blotted onto a nitrocellulose membrane. The biotinylated products were visualized by incubating the membrane with streptavidin-conjugated HRP and then with an ECL Plus kit (GE Healthcare). To detect sTNFR1, the blot was incubated with the rabbit anti-TNFR1 antibodies (1:10,000) and a secondary HRP-conjugated anti-rabbit antibody (GE Healthcare, 1:40,000) and then developed with an ECL Plus kit.
Biotinylation and Cross-linking
Tag7 or Hsp70 were incubated with N-hydroxysuccinimide biotin ester (Thermo Fisher Scientific) at a 1:100 molar ratio for 2 h at 20 °C and dialyzed against PBS for 18 h at 4 °C. The biotinylated Tag7 was incubated with the non-biotinylated Hsp70, or vice versa, as described above to form the Tag7-Hsp70 complex. The L-929 cells were treated with the Tag7-Hsp70 (10−9 m) for 2 h at 4 °C, washed, and then incubated with 0.2 mm BS3 (Thermo Fisher Scientific) for 40 min at 4 °C. After cross-linking, the cells were washed twice with PBS, and their membrane proteins were isolated and solubilized as described.
Cytotoxicity and Enzyme Inhibition Assays
Cytotoxicity was evaluated using Trypan blue staining, as described previously (9). The live and dead cells were counted under a microscope (no less than 100 cells per assay). In some experiments, a CytoTox 96 assay kit (Promega) was used. In the enzyme inhibition assays, the cells were treated for 1 h with the caspase inhibitors DEVD-CHO, IETD-CHO, and LEHD-FMK (50 μm each), the RIP1 kinase inhibitor necrostatin 1 (5 mm) (all from Sigma-Aldrich), Tag7 and Hsp70 (1 nm), or anti-TNFR1 antibodies (100 nm); then, the Tag7-Hsp70 complex or TNF-α was added.
Caspase Activity Measurements
Caspase activity was evaluated in L-929 cells using a caspase 3 assay kit (Sigma-Aldrich) as recommended by the manufacturer. The fluorescence of 7-amino-4-methylcoumarin was measured using a Shimadzu RF-1501 spectrofluorometer (λexc = 360 nm, λemission = 460 nm). One unit of caspase activity corresponded to 1 nm of the substrate hydrolyzed per minute.
RNAi Knockdown, Transfection, and Stable shTNFR1 HEK293 Cell Line Generation
The pSuper RNAi system (OligoEngine) was used to down-regulate mTNFR1 and hTNFR1 expression in the L-929 and HEK293 cells. A 19-nucleotide fragment of mTnfr1 (5′-CAAAGGAACCTACTTGGTG) for L-929 and a 21-nucleotide fragment of hTnfr1 (5′-CAAAGGAACCTACTTGTACAA) for HEK293 were cloned as hairpins in pSuper and used for the transfection with polyethylenimine (Polysciences Inc.; catalog number 23966). The control cells were transfected with empty pSuper. A heterogeneous stable HEK293 cell line was generated with puromycin (1 unit/ml), and then the individual clones with the most dramatic decrease in the TNFR1 mRNA were selected. Down-regulation of the TNFR1 mRNA was tested with the primer pairs 5′-CTTTTATCCCTCCTCTTCATTGG-3′ and 5′-GTAGTAGTTCCTTCAAGCTCCCC-3′; 5′-TCACCGCTTCAGAAAACCACC-3′ and 5′-CGGTCCACTGTGCAAGAAGAG-3′. The level of the ENY2 mRNA was used as a reference (primers 5′-GCAGATGAGAGCAGCGATTAACC-3′ and 5′-GGAGTGATTTCAGCCACCAAGTC-3′).
Statistical Analysis
An unpaired two-tailed Student's t test was used to determine statistical significance. p values of less than 0.05 were considered significant (*, p < 0.05; **, p < 0.005). Data were analyzed using MathCad.
Results
The Tag7-Hsp70 Complex Induces Cytotoxic Processes Occurring at Different Rates
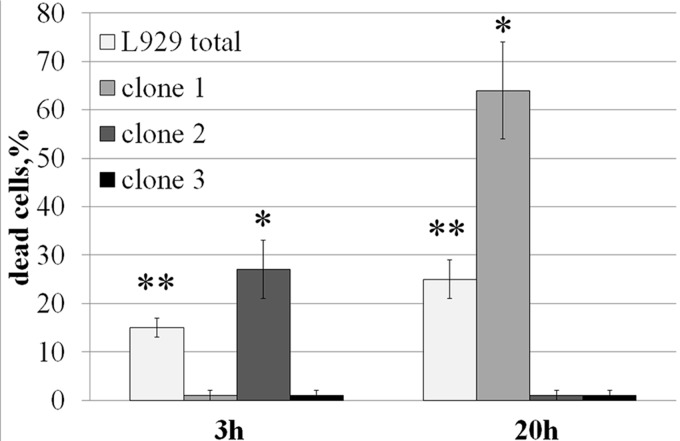
As was found in our previous experiments (9) with the L-929 cells, the Tag7-Hsp70 cytotoxicity curve as a function of the incubation time was non-linear and did not reach saturation but had several peaks. The highest peaks were recorded upon 3-h (∼15% of the dead cells) and 20-h (∼27%) incubations (supplemental Fig. 1).
The distinct pattern of cytotoxicity and the marked difference in the rate of cytolysis suggested that the test culture contained groups of cells in which the Tag7-Hsp70 complex induced different cytotoxic signal transduction pathways that consequently resulted in the different times of cell death. Indeed, using a method of limited dilutions, we obtained L-929 cell clones that died either after 3 h or after 20 h of exposure to the Tag7-Hsp70 complex; we also obtained several resistant clones (Fig. 1). However, the cytotoxic response of the sensitive clones was unstable. At first, their cytotoxicity was higher than the heterogeneous culture but then decreased, returning to the level of the mixed population or even disappearing in the 7-day cultures. Hence, we then analyzed the cytotoxic mechanisms induced by the Tag7-Hsp70 complex after a 3-h or 20-h exposure using a stable heterogeneous L-929 cell culture. In addition, we performed the key experiments on the sensitive L-929 clones as well.
FIGURE 1.
Tag7-Hsp70 has a cytotoxic effect on the L-929 cell clones. The clones were isolated by a limiting dilution method and were incubated with Tag7-Hsp70 (0.1 nm). The proportion of dead cells (identified by trypan blue staining) was determined after 3 and 20 h. All data are presented as the means ± S.E. from at least five independent assays. p values of less than 0.05 were considered significant (*, p < 0.05; **, p < 0.005).
Exposure to Tag7-Hsp70 for 3 h Induces Caspase-dependent Cell Death
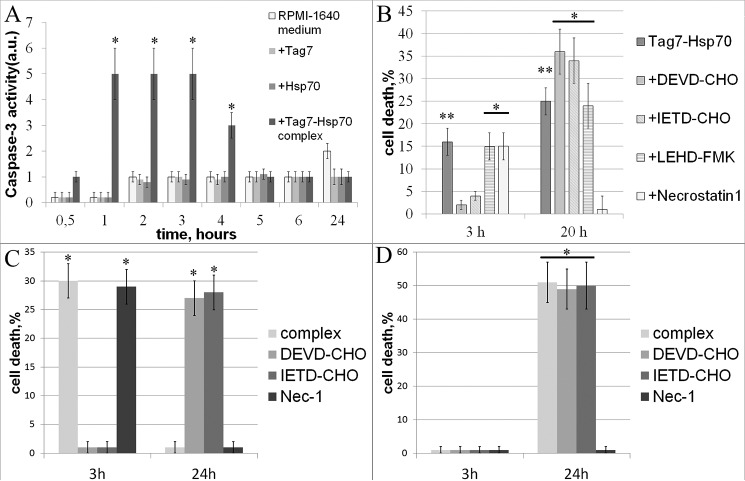
To determine whether Tag7-Hsp70 activated an apoptotic process in the L-929 cells, we analyzed the role of caspases in the rapid and slow cytotoxic processes. As early as 1 h after the exposure to this complex, the caspase-3 activity in the exposed cells was significantly increased as compared with the control cells, indicating that caspase-dependent apoptosis was induced (Fig. 2A). This activity dropped to the initial level after 5 h, which correlated with the decrease in the cytotoxicity (supplemental Fig. 1). The caspase activity was not stimulated further by a 20-h exposure to Tag7-Hsp70 (we used Tag7 alone and Hsp70 alone as the controls).
FIGURE 2.
Tag7-Hsp70 interacts with L-929 cells to induce apoptosis after 3 h and necroptosis after 20 h. A, Tag7-Hsp70 (0.1 nm) induces an increase in caspase-3 activity (see “Experimental Procedures”). Neither Tag7 alone nor Hsp70 alone induces caspase-3 activation. B, effects of a 1-h preincubation with the caspase-3 (5 mm DEVD-CHO), caspase-8 (5 mm IEDT-CHO), caspase-9 (5 mm LEHD-FMK), and RIP1 kinase (5 mm necrostatin 1) inhibitors on the cytotoxicity of Tag7-Hsp70 (0.1 nm) after 3 and 20 h of exposure to the L-929 heterogeneous cultures. C, effects of the 1-h preincubation with the caspase-3 (5 mm DEVD-CHO), caspase-8 (5 mm IEDT-CHO), and RIP1 kinase (5 mm necrostatin 1) inhibitors on the cytotoxicity of Tag7-Hsp70 (0.1 nm) after 3 and 20 h of incubation with the clones that were sensitive to the 3-h death and the 20-h death. D, all data are presented as the means ± S.E. from at least five independent assays. p values of less than 0.05 were considered significant (*, p < 0.05; **, p < 0.005).
These results were verified by an inhibitor study. Preincubation with the caspase-3 (Ac-DEVD-CHO) and caspase-8 (Ac-IETD-CHO) inhibitors prevented the cytotoxic effects of the 3-h exposure to Tag7-Hsp70, whereas treatment with caspase-9 (Z-LEHD-FMK) inhibitor, which is activated in the cell upon a mitochondrial membrane damage, failed to suppress this effect (Fig. 2B).
Thus, at 3 h, the Tag7-Hsp70 complex induces the classic apoptotic cascade, with the initial activation of caspase-8, followed by caspase-3 activation. Apoptosis is induced without the involvement of the mitochondria or the formation of apoptosomes.
Exposure to Tag7-Hsp70 for 20 h Induces Necroptosis
We then studied the effect of the caspase inhibitors on cell death after a 20-h exposure to Tag7-Hsp70 (Fig. 2B). As in the previous case, the caspase-9 inhibitor had no effect on the slow cytotoxicity, whereas preincubation with the caspase-8 and caspase-3 inhibitors enhanced the long-term cytotoxic effect of Tag7-Hsp70, rather than inhibiting it. The increase in the proportion of dead cells after the 20-h incubation was commensurate with the decrease in their proportion following the 3-h incubation as compared with the variant without the inhibitors. Therefore, the cells that survived the 3-h incubation with the cytotoxic complex in the presence of the caspase inhibitors to inhibit apoptosis died after 20 h.
To validate these results, we performed inhibition experiments using L-929 clones. The clones that were sensitive to the 3-h exposure to the Tag7-Hsp70 complex did not display cytotoxicity in the presence of either the caspase-3 or the caspase-8 inhibitors after 3 h, but the cytotoxic activity was gained after 20 h and was comparable with the 3-h cytotoxicity. The clones that were sensitive to the 20-h exposure to the Tag7-Hsp70 complex did not gain any additional activity upon treatment with the caspase inhibitors. These results are in complete agreement with the data obtained using the heterogeneous populations of the L-929 cells. (Fig. 2, C and D, supplemental Table 1).
An alternative character of cell death is described for the death receptors (14). This fact suggests that both the rapid and the slow cytotoxic responses were induced via the same receptor, with the apoptosis inhibition upon a 3-h exposure leading to a switch to the induction of a different cell death mechanism.
As shown in the studies on the cell death receptors, caspase and apoptosis inhibition stimulate phosphorylation of the RIP1 kinase and initiate necroptosis (26). Preincubation with necrostatin-1, a RIP1 kinase inhibitor, prevented the cytotoxic effects of a 20-h exposure to Tag7-Hsp70 (Fig. 2B). These results suggested that the cells that were incubated with the cytotoxic complex for 20 h died by RIP1-dependent necroptosis. In contrast, the cytotoxic activity observed after 3 h was not inhibited by necrostatin-1, indicating that the cell death pathway in this case was not necroptosis. Similarly, necrostatin did not inhibit the cytotoxic activity of the clones sensitive to the 3-h Tag7-Hsp70 complex exposure but completely blocked the activity of the clones sensitive to the 20-h exposure (Fig. 2, C and D).
The Tag7-Hsp70 Complex Interacts with TNFR1
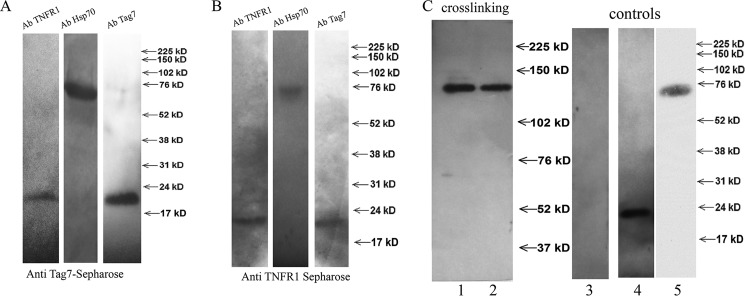
Our experimental data on the mechanisms of L-929 cell death in response to the Tag7-Hsp70 complex and the switch in the cell death signaling pathways suggested that the cytotoxic activity of this complex may be mediated by one of the cell death receptors. To test this hypothesis, we prepared a specific CNBr-Sepharose column coupled to anti-Tag7 antibodies (supplemental Fig. 2 A) on which the Tag7-Hsp70 complex was immunoadsorbed. The solubilized L-929 cell membrane proteins were applied onto this column, and the material that was specifically retained was resolved by SDS-PAGE. One of the resulting bands had an apparent molecular mass of 52 kDa, which corresponded to that of the murine TNFR1. A mass spectrometry analysis indicated a more than 95% probability that this protein was identical to TNFR1. The MALDI analysis was performed using the MASCOT database.
To demonstrate the interaction between Tag7-Hsp70 and TNFR1, the column with the adsorbed Tag7-Hsp70 complex was loaded with sTNFR1, a recombinant soluble fragment of TNFR1 located on the outer cell membrane; all three proteins, namely Tag7, Hsp70, and sTNFR1, were retained on this column (Fig. 3A). This interaction was confirmed by a complementary experiment. A specific anti-TNFR1 column (supplemental Fig. 2B) with adsorbed sTNFR1 was loaded with the Tag7-Hsp70 complex, and all three proteins were also retained on this column (Fig. 3B). Thus, the solubilized full-length TNFR1 and its soluble fragment interacted with the Tag7-Hsp70 complex. The Tag7 and TNFR1 proteins were not bound to the control column, where CNBr-Sepharose was coupled with the preimmune IgG.
FIGURE 3.
Tag7-Hsp70 interacts with TNFR1. A, sTNFR1 was applied to the anti-Tag7-conjugated Sepharose 4B, with pre-adsorbed Tag7-Hsp70 complex. The retained proteins were eluted with triethanolamine, fractioned by SDS-PAGE (12%), and analyzed by Western blotting with rabbit antibodies to TNFR1 (1:10,000), Tag7 (1:10,000), or Hsp70 (1:10,000). B, the Tag7-Hsp70 complex was applied to anti-TNFR1-conjugated Sepharose 4B, with pre-adsorbed sTNFR1. The retained proteins were eluted with triethanolamine, fractionated by SDS-PAGE (12%), and analyzed by Western blotting with rabbit antibodies to TNFR1 (1:10,000), Tag7 (1:10,000), or Hsp70 (1:10,000). C, the Tag7-Hsp70 complex containing biotinylated Tag7 (10 nm) was added to L-929 cells and incubated for 2 h at 4 °C, and then 0.2 mm BS3 was added. After 30 min of incubation, the cells were washed and the membrane fraction was solubilized and applied to the anti-TNFR1 column. Lane 1, the retained proteins were analyzed by Western blotting with streptavidin-peroxidase. Lane 2, the same experiment was performed with the Tag7-Hsp70 complex containing biotinylated Hsp70. Lanes 3–5, the membrane fraction of the L-929 cells (lane 3), the Tag7-Hsp70 complex with biotinylated Tag7 (lane 4), and the Tag7-Hsp70 complex with biotinylated Hsp70 (lane 5) were analyzed by Western blotting with streptavidin-peroxidase and are shown as the controls.
Our next step was to determine whether the ternary Tag7-Hsp70-TNFR1 molecular complex is formed on the L-929 cells. To this end, we produced two variants of the Tag7-Hsp70 complex in which only one component (either Tag7 or Hsp70) was biotinylated (Fig. 3C) and used the water-soluble cross-linking agent BS3 to fix the contacts between Tag7-Hsp70 and TNFR1. The cells were incubated with the complex containing the biotinylated Tag7 in the presence of BS3 under conditions that prevented cell lysis, and the solubilized membrane proteins were applied onto a specific anti-TNFR1 Sepharose column. The material retained on the column contained a biotin-labeled product with an apparent molecular mass of ∼140 kDa (Fig. 3C). The same product was detected after the cells were incubated under the same conditions with the complex containing biotinylated Hsp70 (Fig. 3C). No biotinylated material was bound to the control column, where CNBr-Sepharose was coupled with the preimmune IgG.
These results provide evidence that the Tag7-Hsp70 complex coming in the contact with the cell is in close proximity to the TNFR1 receptor. The molecular mass of the ternary product (140 kDa) is equal to the sum of the molecular masses of its components (50, 20, and 70 kDa for TNFR1, Tag7, and Hsp70, respectively), suggesting that a single Tag7-Hsp70 complex binds to a single TNFR1 molecule; therefore, the ligand-receptor binding ratio under the given experimental conditions is 1:1.
Tag7-Hsp70 Complex Induces Cytotoxicity with the Involvement of TNFR1 Receptor
The next question was whether the observed interaction between Tag7-Hsp70 and TNFR1 triggers cell death signal transduction. To answer this question, we analyzed the cytotoxic activity of Tag7-Hsp70 in the presence of agents that prevent its interaction with TNFR1. In the control experiments, TNF-α was used instead of Tag7-Hsp70.
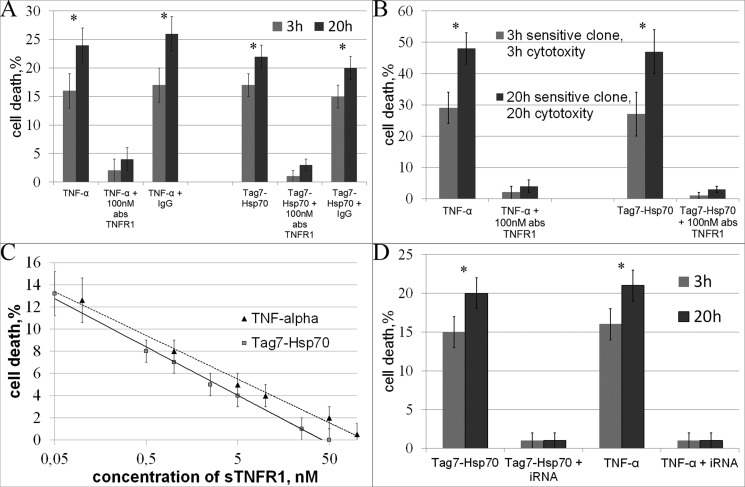
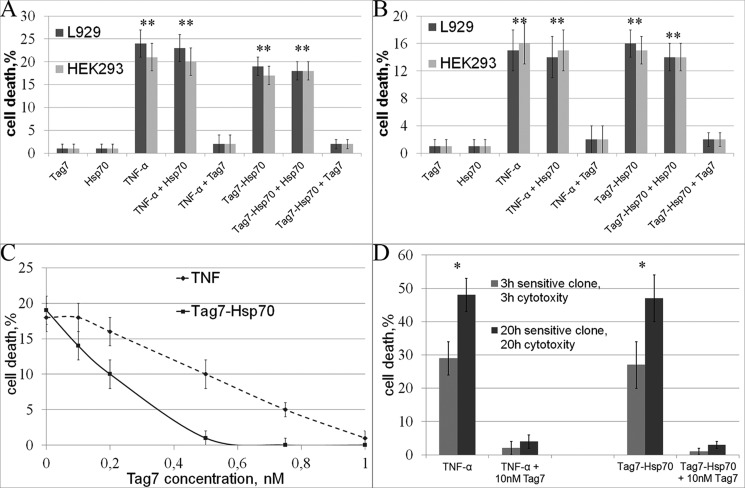
Preincubation of L-929 cells with the anti-TNFR1 antibodies completely blocked the cytotoxicity of either TNF-α or Tag7-Hsp70, after both 3 h and 20 h of exposure to the Tag7-Hsp70 complex. The preimmune IgG had no such effect (Fig. 4A). The IC50 of the antibodies after the 20-h exposure to the Tag7-Hsp70 complex was 40 nm (supplemental Fig. 3). The same results were obtained using another cell line, HEK293 cells (the IC50 of the antibodies for Tag7-Hsp70 was 40 nm, supplemental Fig. 3). The activity of the L-929 clones was also inhibited by the anti-TNFR1 antibodies (Fig. 4B).
FIGURE 4.
The interaction with TNFR1 is necessary for TNF-α- and Tag7-Hsp70-induced cell death. A, the cytotoxic effects of TNF-α and Tag7-Hsp70 (1 nm each) on both apoptotic (3 h) and necroptotic (20 h) cell death were inhibited after the L-929 cells were preincubated with anti-TNFR1 antibodies (100 nm). B, the same experiment was performed on the 3-h-sensitive clones for the apoptotic (3 h) cell death and on the 20-h-sensitive clones for the necroptotic (20 h) cell death. C, preincubation of TNF-α (0.05 nm) and Tag7-Hsp70 (0.1 nm) with increasing concentrations of sTNFR1 inhibits their cytotoxic activity in L-929 cells. D, TNFR1 knockdown blocks the cytotoxic effect of TNF-α and Tag7-Hsp70 (see “Experimental Procedures”). All data are presented as the means ± S.E. from at least five independent assays. p values of less than 0.05 were considered significant (*, p < 0.05).
Taking into account that the Tag7-Hsp70 complex can bind to sTNFR1 in solution (Fig. 3A), we decided to abolish the cytotoxic effect of this complex by adding excess sTNFR1. We preincubated either the Tag7-Hsp70 complex (0.1 nm) or TNF-α (0.05 nm) (as a control) with increasing concentrations of sTNFR1 and added them to the L-929 cells. The cytotoxicity was inhibited in a dose-dependent manner (the IC50 values for sTNFR1 were 3 and 1.5 nm, respectively; see Fig. 4C). These data show that the Tag7-Hsp70 complex can bind to sTNFR1 in solution and that this binding prevented the interaction of this complex with the TNFR1 receptor on the target cell surface. The inhibition of the TNF-α cytotoxicity by sTNFR1 addition was described earlier (27).
To verify these results, we knocked down TNFR1 expression in the HEK293 cells and subsequently treated the cells with Tag7-Hsp70 and TNF-α. We produced a stable knockdown line and selected clones in which the level of the unprocessed TNFR1 transcript was only 10% of the control cells (quantitative PCR data). This mix of clones was not susceptible to the treatment with either TNF-α or Tag7-Hsp70 after both 3 h and 20 h of incubation. Therefore, TNFR1 knockdown completely blocked the cytotoxic effect of these proteins (Fig. 4D). This fact confirms that the TNFR1 receptor is necessary to induce cell death through either its specific ligand, TNF-α, or the Tag7-Hsp70 complex.
Tag7 Blocks the Cytotoxic Effects of the Tag7-Hsp70 Complex and TNF-α
Next, we tested whether the individual protein components of the cytotoxic complex, Tag7 and Hsp70, can interact with the receptor and thus protect the cell against the cytotoxic actions of Tag7-Hsp70 or TNF-α. Prior to adding the cytotoxic proteins, the L-929 cells were incubated with 1 nm Tag7 or Hsp70 alone, which did not have an effect on cell viability. Subsequent exposure to Tag7-Hsp70 or TNF-α for 3 or 20 h (0.1 nm each) showed that the cytotoxic effect on the Hsp70-treated cells remained unchanged but was completely suppressed in the Tag7-treated cells, suggesting that Tag7 interacted with TNFR1 (Fig. 5, A and B). The same results were obtained in the experiments with the HEK293 cells; preincubation with Tag7 (but not with Hsp70) inhibited the cytotoxic effects of TNF-α and Tag7-Hsp70 (Fig. 5, A and B).
FIGURE 5.
The Tag7 protein inhibits the cytotoxic effects of both the Tag7-Hsp70 complex and TNF-α. A, the 3-h apoptotic effects of TNF-α (0.05 nm) and Tag7-Hsp70 (0.1 nm) are inhibited after preincubating the L-929 and HEK293 cells with Tag7 (1 nm). B, the 20-h necroptotic effects of TNF-α (0.05 nm) and Tag7-Hsp70 (0.1 nm) are inhibited after preincubating the L-929 and HEK293 cells with Tag7 (1 nm). C, the 20-h necroptotic effects of TNF-α (0.05 nm) and Tag7-Hsp70 (0.1 nm) are inhibited after preincubating the L-929 cells with increasing concentrations of Tag7. D, the cytotoxic effects of TNF-α (0.05 nm) and Tag7-Hsp70 (0.1 nm) are inhibited after preincubating the L-929 clones with Tag7 (1 nm). The apoptotic death of the 3-h-sensitive clones and necroptotic death of the 20-h-sensitive clones are shown. All data are presented as the means ± S.E. from at least five independent assays. p values of less than 0.05 were considered significant (*, p < 0.05; **, p < 0.005).
By analyzing the decrease in the TNF-α- and Tag7-Hsp70-mediated cytotoxicity that depended on the concentration of free Tag7, we found that the amount of Tag7 required for blocking the cytotoxic effect was twice as great for TNF-α as for Tag7-Hsp70, IC50 = 5.2 versus 2.2 nm, respectively (Fig. 5C). In other words, the affinity to TNFR1 is approximately twice higher for TNF-α than for the Tag7-Hsp70 complex. The cytotoxic effect of TNF-α and Tag7-Hsp70 on the L-929 clones was also inhibited after preincubation with Tag7 protein (Fig. 5D). Thus, we found that Tag7 inhibits the cytotoxic action of TNF-α and the Tag7-Hsp70 complex. Tag7 also likely interacts with the TNFR1 receptor.
Discussion
The results presented above show that the Tag7-Hsp70 complex induces both apoptosis and necroptosis in the L-929 cells, with either cell death pathway being activated upon the interaction of this complex with the TNFR1 receptor. It has been repeatedly reported that the L-929 cell line is heterogeneous and contains both TNF-α-sensitive and TNF-α-resistant clones (28, 29). We managed to isolate L-929 cell clones that died after different periods of exposure to Tag7-Hsp70 (3 and 20 h), which was due to the activation of different cell death mechanisms, namely apoptosis or necroptosis, in different clones.
After a 3-h incubation with the complex, ∼15% of the heterogeneous L-929 cells died through an apoptotic pathway involving the activation of the caspase-8 and caspase-3 cascade. Caspase-9 inhibition had no effect on the Tag7-Hsp70-induced cytotoxicity, suggesting that apoptosomes were not formed, and therefore, the mitochondria were not involved in the cytotoxic signal transduction. The classic apoptotic signal transduction pathway was apparently disrupted in the 27% of the cells, and they died after 20 h of exposure to the complex via the RIP1-dependent necroptotic pathway. Caspase inhibition in the 15% subpopulation of cells that were subject to apoptosis resulted in an activation of the necroptosis mechanisms in these cells. This was evident when we used the L-929 clones. Inhibiting caspase-3 or caspase-8 in the cells that were dying after only 3 h of incubation with Tag7-Hsp70 complex led to their death after 20 h.
Based on recent publications, caspase-8-dependent cleavage of the RIP1 kinase is considered to play the main role in inhibiting necroptosis (30). It has been shown that caspase-3 cleaves RIP1 at multiple sites in vitro but is incapable of cleaving it in vivo (21). According to our observations, both caspases are essential for switching between the two alternative pathways of the Tag7-Hsp70-induced L-929 cell death. This was obvious when we performed experiments L-929 clones. Activation of RIP1-dependent necroptosis was recorded when the caspase-8 and caspase-3 inhibitors were both added to the culture. It is unlikely that caspase-8 is directly involved in the RIP1 kinase cleavage because the inhibition of caspase-3 alone results in cell death by necroptosis, although caspase-8 remains active. It may be speculated that the role of caspase-8 is not to cleave RIP1 kinase but rather to activate caspase-3, which, in turn, cleaves either RIP1 kinase itself or the protein responsible for the regulation of its activity.
Induction of both apoptosis and necroptosis upon a cell's exposure to the Tag7-Hsp70 complex suggested that its cytotoxic action was mediated by a cell death receptor. Indeed, the preponderance of data (the interaction of Tag7-Hsp70 with TNFR1 either in solution or on the surface of live cells, the protective effect of anti-TNFR1, sTNFR1 protein and Tag7 protein Tag7-Hsp70 cytotoxic activity, and elimination of this activity upon TNFR1 knockdown) provide evidence that the cytotoxic effect of Tag7-Hsp70 is mediated by the TNFR1 receptor.
It is worth noting that Tag7 is the only component of the Tag7-Hsp70 complex that can bind to TNFR1 on the cell surface, although this does not cause a cytotoxic effect. Its primary and ternary structures differ from that of TNF-α, and it may well be that Tag7 binds to only a small fragment of TNFR1 that is insufficient for causing the conformational changes in the receptor that could induce cytolysis. However, Tag7 partially occupies the TNF-α and Tag7-Hsp70 binding sites on TNFR1 and thus interferes with the protein-receptor interaction by acting as a substrate-like inhibitor.
Regardless of the structural differences between TNF-α and Tag7, both TNF-α and Tag7-Hsp70 exert their cytotoxic effects at similar concentrations. The cytotoxicity curve as a function of the TNF-α protein concentration reaches saturation at a concentration that is half that of Tag7-Hsp70 complex (supplemental Fig. 4). Likewise, the concentration of Tag7 that is necessary to prevent the interaction of TNFR1 with the ligand is ∼2-fold higher for TNF-α than the Tag7-Hsp70 complex. The results of this study provide evidence for the existence of a new ligand for the TNFR1 receptor that is structurally different from TNF-α. Because the Tag7-Hsp70 complex is secreted by the immune system cells, similar to TNF-α, it may well be that this complex can perform some functions of TNF-α, with Tag7 acting as an inhibitor of these functions by binding to the TNFR1 receptor.
The results of our previous studies (9, 29) show that the innate immunity protein Tag7 may be involved in some functions of acquired immunity. It is expressed on the surface of the highly specialized cytotoxic lymphocytes and enables them to recognize the HLA-negative tumor cells. Moreover, these lymphocytes secrete Tag7 in a complex with the Hsp70 protein, and this complex has a direct cytotoxic effect on different tumor cells. Here, we have shown that Tag7 can bind to the cell receptor that is specific for the TNF-α cytokine, which is secreted by lymphocytes upon activation of acquired immunity.
The discovery of the mechanistic peculiarities of the receptor-ligand interactions can provide a detailed understanding of the fundamentals of the molecular processes tightly linked with cell biology/immunology and molecular oncology to allow the development of novel therapeutics (31). TNF-α is regarded as the key player in cancer cell death via the cytotoxic pathway (32). It is also evident that this pro-inflammatory cytokine is highly involved in the activation and proliferation of B and T lymphocytes (33, 34). It was also shown that TNFR1 has no unique specific ligand and may interact specifically with LT-α, the closest homolog of TNF-α. This cytokine may act differently with the receptor in the membrane-bound and the soluble form and highly affects IgA production (35). In this study, we are also proposing a “multifunctional” regulatory role for TNFR1 by demonstrating its specific response to proteins from the innate immunity cascade. We are speculating that the identification of the Tag7-Hsp70-TNFR1 interaction machinery may demonstrate an additional link between adaptive and innate immunity and may provide a basis for therapeutic applications.
Author Contributions
D. V. Y. and L. P. S. designed the study, performed the experiments, and wrote the paper. O. K. I., N. V. S., A. A. S., E. A. R., E. A. D., and A. G. T. performed and analyzed the experiments. N. V. G., A. G. G., and G. P. G. analyzed the experiments and contributed to the preparation of the paper. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
This work was supported by the Russian Foundation for Basic Research, RAS program for Molecular and Cellular Biology, International Centre for Genetic Engineering and Biotechnology (ICGEB). The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Figs. 1–4 and supplemental Table 1.
- Hsp70
- heat shock protein 70
- TNFR1
- TNF-α receptor
- sTNFR1
- soluble TNFR1
- Z
- benzyloxycarbonyl
- FMK
- fluoromethylketone
- BS3
- bis(sulfosuccinimidyl)suberate.
References
- 1. Kustikova O. S., Kiselev S. L., Borodulina O. R., Senin V. M., Afanas'eva A. V., Kabishev A. A. (1996) Cloning of the tag7 gene expressed in metastatic mouse tumors. Genetika 32, 621–628 [PubMed] [Google Scholar]
- 2. Kiselev S. L., Kustikova O. S., Korobko E. V., Prokhortchouk E. B., Kabishev A. A., Lukanidin E. M., Georgiev G. P. (1998) Molecular cloning and characterization of the mouse tag7 gene encoding a novel cytokine. J. Biol. Chem. 273, 18633–18639 [DOI] [PubMed] [Google Scholar]
- 3. Dziarski R. (2004) Peptidoglycan recognition proteins (PGRPs). Mol. Immunol. 40, 877–886 [DOI] [PubMed] [Google Scholar]
- 4. Larin S. S., Korobko E. V., Kustikova O. S., Borodulina O. R., Raikhlin N. T., Brisgalov I. P., Georgiev G. P., Kiselev S. L. (2004) Immunotherapy with autologous tumor cells engineered to secrete Tag7/PGRP, an innate immunity recognition molecule. J. Gene Med. 6, 798–808 [DOI] [PubMed] [Google Scholar]
- 5. Kang D., Liu G., Lundström A., Gelius E., Steiner H. (1998) A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc. Natl. Acad. Sci. U.S.A. 95, 10078–10082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lemaitre B., Nicolas E., Michaut L., Reichhart J. M., Hoffmann J. A. (1996) The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 [DOI] [PubMed] [Google Scholar]
- 7. Hoffmann J. A. (2003) The immune response of Drosophila. Nature 426, 33–38 [DOI] [PubMed] [Google Scholar]
- 8. Michel T., Reichhart J. M., Hoffmann J. A., Royet J. (2001) Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414, 756–759 [DOI] [PubMed] [Google Scholar]
- 9. Sashchenko L. P., Dukhanina E. A., Yashin D. V., Shatalov Y. V., Romanova E. A., Korobko E. V., Demin A. V., Lukyanova T. I., Kabanova O. D., Khaidukov S. V., Kiselev S. L., Gabibov A. G., Gnuchev N. V., Georgiev G. P. (2004) Peptidoglycan recognition protein Tag7 forms a cytotoxic complex with heat shock protein 70 in solution and in lymphocytes. J. Biol. Chem. 279, 2117–2124 [DOI] [PubMed] [Google Scholar]
- 10. Dukhanina E. A., Yashin D. V., Lukjanova T. I., Romanova E. A., Kabanova O. D., Shatalov Y. V., Sashchenko L. P., Gnuchev N. V. (2007) Administration of the cytotoxic complex Tag7-Hsp70 to mice with transplanted tumors inhibits tumor growth. Dokl. Biol. Sci. 414, 246–248 [DOI] [PubMed] [Google Scholar]
- 11. Hengartner M. O. (2000) The biochemistry of apoptosis. Nature 407, 770–776 [DOI] [PubMed] [Google Scholar]
- 12. Degterev A., Boyce M., Yuan J. (2003) A decade of caspases. Oncogene 22, 8543–8567 [DOI] [PubMed] [Google Scholar]
- 13. Lettre G., Hengartner M. O. (2006) Developmental apoptosis in C. elegans: a complex CEDnario. Nat. Rev. Mol. Cell Biol. 7, 97–108 [DOI] [PubMed] [Google Scholar]
- 14. Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G. D., Mitchison T. J., Moskowitz M. A., Yuan J. (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 [DOI] [PubMed] [Google Scholar]
- 15. Christofferson D. E., Yuan J. (2010) Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 22, 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holler N., Zaru R., Micheau O., Thome M., Attinger A., Valitutti S., Bodmer J. L., Schneider P., Seed B., Tschopp J. (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1, 489–495 [DOI] [PubMed] [Google Scholar]
- 17. Vandenabeele P., Galluzzi L., Vanden Berghe T., Kroemer G. (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 11, 700–714 [DOI] [PubMed] [Google Scholar]
- 18. Cho Y. S., Challa S., Moquin D., Genga R., Ray T. D., Guildford M., Chan F. K. (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vanden Berghe T., Linkermann A., Jouan-Lanhouet S., Walczak H., Vandenabeele P. (2014) Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 15, 135–147 [DOI] [PubMed] [Google Scholar]
- 20. Oberst A., Dillon C. P., Weinlich R., McCormick L. L., Fitzgerald P., Pop C., Hakem R., Salvesen G. S., Green D. R. (2011) Catalytic activity of the caspase-8-FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin Y., Devin A., Rodriguez Y., Liu Z. G. (1999) Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13, 2514–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galluzzi L., Vanden Berghe T., Vanlangenakker N., Buettner S., Eisenberg T., Vandenabeele P., Madeo F., Kroemer G. (2011) Programmed necrosis from molecules to health and disease. Int. Rev. Cell Mol. Biol. 289, 1–35 [DOI] [PubMed] [Google Scholar]
- 23. Vanlangenakker N., Vanden Berghe T., Bogaert P., Laukens B., Zobel K., Deshayes K., Vucic D., Fulda S., Vandenabeele P., Bertrand M. J. (2011) cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 18, 656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guan R., Wang Q., Sundberg E. J., Mariuzza R. A. (2005) Crystal structure of human peptidoglycan recognition protein S (PGRP-S) at 1.70 Å resolution. J. Mol. Biol. 347, 683–691 [DOI] [PubMed] [Google Scholar]
- 25. Kohler M., Püschel K., Sakharov D., Tonevitskiy A., Schänzer W., Thevis M. (2008) Detection of recombinant growth hormone in human plasma by a 2-D PAGE method. Electrophoresis 29, 4495–4502 [DOI] [PubMed] [Google Scholar]
- 26. Vandenabeele P., Declercq W., Van Herreweghe F., Vanden Berghe T. (2010) The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci. Signal. 3, re4. [DOI] [PubMed] [Google Scholar]
- 27. Marsters S. A., Frutkin A. D., Simpson N. J., Fendly B. M., Ashkenazi A. (1992) Identification of cysteine-rich domains of the type 1 tumor necrosis factor receptor involved in ligand binding. J. Biol. Chem. 267, 5747–5750 [PubMed] [Google Scholar]
- 28. Vanhaesebroeck B., Van Bladel S., Lenaerts A., Suffys P., Beyaert R., Lucas R., Van Roy F., Fiers W. (1991) Two discrete types of tumor necrosis factor-resistant cells derived from the same cell line. Cancer Res. 51, 2469–2477 [PubMed] [Google Scholar]
- 29. Sashchenko L. P., Dukhanina E. A., Shatalov Y. V., Yashin D. V., Lukyanova T. I., Kabanova O. D., Romanova E. A., Khaidukov S. V., Galkin A. V., Gnuchev N. V., Georgiev G. P. (2007) Cytotoxic T lymphocytes carrying a pattern recognition protein Tag7 can detect evasive, HLA-negative but Hsp70-exposing tumor cells, thereby ensuring FasL/Fas-mediated contact killing. Blood 110, 1997–2004 [DOI] [PubMed] [Google Scholar]
- 30. Vanlangenakker N., Bertrand M. J., Bogaert P., Vandenabeele P., Vanden Berghe T. (2011) TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2, e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. (1989) EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell 57, 1101–1107 [DOI] [PubMed] [Google Scholar]
- 32. Lejeune F. J., Liénard D., Matter M., Rüegg C. (2006) Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 6, 6. [PubMed] [Google Scholar]
- 33. Guan Y. J, Zhang Z., Yu C., Ma L., Hu W., Xu L., Gao J. S., Chung C. S., Wang L., Yang Z. F., Fast L. D., Chung A. S., Kim M., Ayala A., Zhuang S., Zheng S., Chin Y. E. (2011) Phospho-SXXE/D motif mediated TNF receptor 1-TRADD death domain complex formation for T cell activation and migration. J. Immunol. 187, 1289–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corcione A., Ottonello L., Tortolina G., Tasso P., Ghiotto F., Airoldi I., Taborelli G., Malavasi F., Dallegri F., Pistoia V. (1997) Recombinant tumor necrosis factor enhances the locomotion of memory and naive B lymphocytes from human tonsils through the selective engagement of the type II receptor. Blood 90, 4493–4501 [PubMed] [Google Scholar]
- 35. Kruglov A. A., Grivennikov S. I., Kuprash D. V., Winsauer C., Prepens S., Seleznik G. M., Eberl G., Littman D. R., Heikenwalder M., Tumanov A. V., Nedospasov S. A. (2013) Nonredundant function of soluble LTα3 produced by innate lymphoid cells in intestinal homeostasis. Science 342, 1243–1246 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.