Abstract
Objective
The aim of this retrospective study was to investigate the relationship between thyroid transcription factor-1 (TTF-1) expression and epidermal growth factor receptor (EGFR) gene mutations in lung adenocarcinomas of Chinese patients.
Methods
There were 200 lung adenocarcinoma patients who were enrolled in this study. Tumor specimens of these patients were investigated for TTF-1 expression and mutations in EGFR using immunohistochemistry and a liquid chip platform for DNA analysis of slides with sections of formalin-fixed, paraffin-embedded specimens.
Results
The rates of TTF-1 expression and EGFR mutations were 81.5% and 45.5%, respectively, in the lung adenocarcinoma specimens of the recruited patients. Among female nonsmokers (n=72), 93.1% of specimens were positive for TTF-1 expression, and 63.9% had EGFR mutations. Of 89 patients with EGFR mutations, 83 (50.9%) specimens were simultaneously positive for TTF-1 expression. Kaplan–Meier analysis of all patient specimens found that postoperative survival time was not significantly associated with TTF-1 expression and the presence of EGFR mutations. However, patients with disease stages III–IV whose tumors were positive for TTF-1 expression and EGFR mutations had better postoperative survival than similar patients whose tumors were negative for TTF-1 expression and EGFR mutations.
Conclusion
Our study showed a significant association between TTF-1 positivity and the presence of EGFR mutations (exon 21) in the Chinese lung adenocarcinoma patients. We further identify that patients with disease stages III–IV who were positive for TTF-1 expression and EGFR mutations had a better postoperative survival than those patients who were negative for TTF-1 expression and EGFR mutations. Therefore, TTF-1 might be a potential prognostic biomarker for stages III–IV lung adenocarcinoma patients. In clinical practice, TTF-1 expression may be a marker for planning therapy for certain patients with lung adenocarcinoma, especially for selection of EGFR tyrosine kinase inhibitors.
Keywords: EGFR, lung adenocarcinoma, survival, TTF-1
Introduction
Lung cancer is a worldwide oncology-related disease, with an annually increasing rate of morbidity and mortality.1,2 Nonsmall-cell lung cancers (NSCLCs) make up 85% of lung cancers, with a 5-year survival rate of only 15%–17%.3 Adenocarcinoma of the lung is one of the main subtypes of NSCLC; recently, its morbidity has increased in both male and female patients. With the identification of epidermal growth factor receptor (EGFR) gene mutations in NSCLCs and the development of the EGFR tyrosine kinase inhibitors (TKIs), gefitinib and erlotinib, the survival and quality of life of adenocarcinoma patients have improved greatly. The NEJ 002 clinical trial found that NSCLC patients with EGFR mutations treated with EGFR TKIs as first-line treatments had a median progression-free survival of 10.8 months and a median overall survival of 30.5 months.4 The current National Comprehensive Cancer Network (NCCN) guidelines indicate that genetic testing to evaluate EGFR mutation status is essential for patients with lung adenocarcinoma. However, for some patients, EGFR mutation status cannot be easily determined because of the expense or inadequate tumor specimen, leading to lack of supporting evidence for using EGFR TKI treatment. Therefore, identifying other markers that predict EGFR mutation status is necessary.
Along with EGFR mutations, thyroid transcription factor-1 (TTF-1), a biomarker for lung adenocarcinoma, was reported to have a much higher rate of expression in the lung adenocarcinoma specimens of Asian females and nonsmoking lung cancer patients. The NEJ 002 clinical trial also found that the rate of EGFR mutations was significantly higher in lung adenocarcinoma specimens that were positive for TTF-1 expression than in specimens that were TTF-1 negative.4 Therefore, clarifying whether there is a relationship between EGFR mutations and TTF-1 positivity in lung adenocarcinomas and whether TTF-1 can be a biomarker of EGFR mutation status is essential, especially for some patients with advanced lung cancer having inadequate specimen for evaluating the EGFR status.
Materials and methods
Materials and patients
This retrospective study enrolled 200 patients with histologically confirmed primary lung adenocarcinoma who underwent lung cancer surgery at Tianjin Medical University General Hospital between January 2008 and May 2013. All evaluated samples were obtained from resected lung cancer tissue. Surgical procedures included partial lobectomy, lobectomy, pneumonectomy, and partial resection of the superior vena cava with artificial blood vessel replacement. Neither chemotherapy nor radiotherapy was administered prior to surgery. Basically, the NSCLC patients with EGFR mutations (exon 19 or exon 21 mutations) were given four or six cycles of chemotherapy after surgery with a rigorous follow-up every 3 months. EGFR TKIs were administered upon disease progression of the patients. If EGFR TKIs did not work, other treatment alternatives were adopted according to the individual’s condition, including surgery, radiotherapy, and chemotherapy. The treatment flowchart is depicted in Figure 1.
Figure 1.
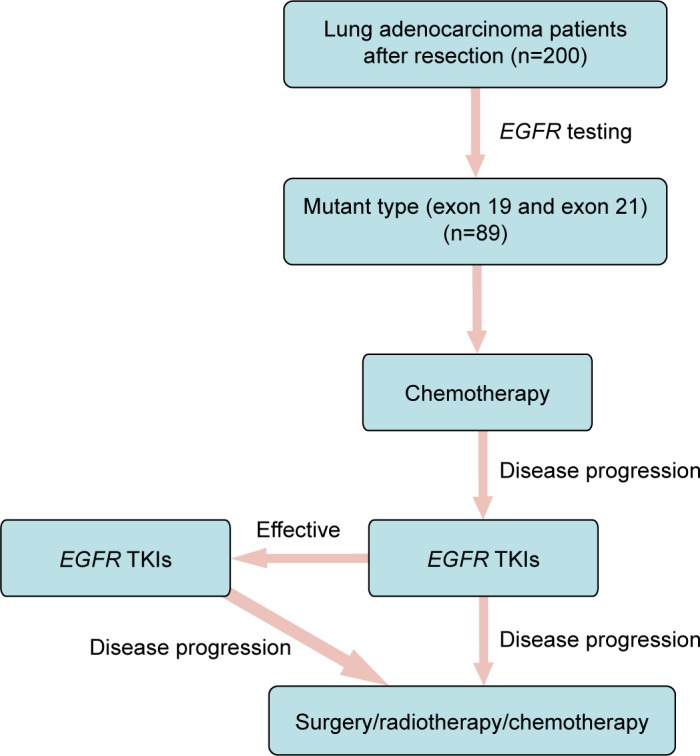
Treatment flowchart of lung adenocarcinoma patients with EGFR mutations in this study.
Abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Clinical information on each patient was obtained from the hospital medical records database. The clinical characteristics of participants are shown in Table 1. There were 76 male and 124 female patients, with a median age of 61 years, ranging from 32 years to 78 years. There were 101 nonsmokers and 99 current or former smokers (>400 cigarettes/year), 75 patients with tumors located in the left lung, and 125 with tumors in the right. The seventh lung cancer TNM classification and staging system released by the Union for International Cancer Control (UICC) in 2009 was used for pathological staging.5 The pathological stages of all patients were evaluated, and included 87 patients with stages I–II and 113 with stages III–IV of the disease.
Table 1.
Patient demographics
| Category | Patients, n (%) |
|---|---|
| Age, median (range) | 61 (32–78) |
| >61 years | 94 (47.0) |
| ≤61 years | 106 (53.0) |
| Sex | |
| Male | 76 (38.0) |
| Female | 124 (62.0) |
| Smoking | |
| Ever | 99 (50.5) |
| Never | 101 (49.5) |
| Tumor location | |
| Left | 75 (37.5) |
| Right | 125 (62.5) |
| Pathological stage | |
| I–II | 87 (43.5) |
| III–IV | 113 (56.5) |
Follow-up was performed by telephone. The postoperative survival time was defined from the date of operation to the date of follow-up or the date of death. Up to July 25, 2014, there were 119 survivors, 55 deaths, and 26 patients lost to follow-up. The mean survival time was 935.9 days (range, 16–2,243 days).
All patients signed an informed consent before enrollment. The Institutional Ethics Committee of Tianjin Medical University General Hospital approved the study.
Methods of detection
Immunohistochemistry was used to assess TTF-1 expression and a liquid chip platform was used to identify EGFR mutations on separate slides of formalin-fixed, paraffin-embedded patient specimens.6
TTF-1 detection
The tissue specimens were fixed using 10% formaldehyde. After standard processing, the paraffin-embedded specimens were cut into a 4 μm thick section and serial sections were generally used for the following staining. The sections were stained using hematoxylin–eosin stain and immunohistochemical staining employing mouse-anti TTF-1 monoclonal antibody (diluted at 1:100) from Fuzhou Maixin Biotechnology Company, according to the instructions.
Histopathologic diagnosis was performed by two experienced pathologists who used the World Health Organization tumor histological analysis method to classify cell types.7 Nuclei staining tan or brown after staining for TTF-1 expression were considered positive for TTF-1 expression, as shown in Figure 2 (arrows). A tumor was considered positive or negative for TTF-1 based on the percentage of positive cells. As described by Shanzhi et al a sample was considered negative (−) for TTF-1 expression, if 0%–10% of tumor cells were positive, partially positive (±) if 10%–50% of tumor cells were positive, and positive (+) if >50% of tumor cells were positive. To facilitate statistical comparisons, tumors classified as strongly positive or partially positive for TTF-1 expression were uniformly classified positive.8
Figure 2.
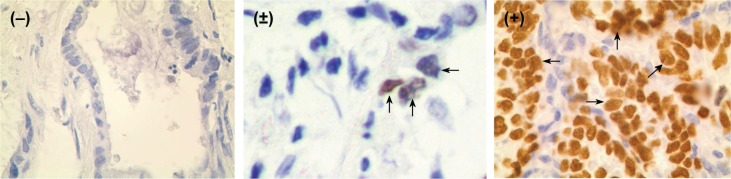
Thyroid transcription factor-1 (TTF-1) expression in positive (+), partially positive (±), and negative (−) adenocarcinoma cells; ×400).
Note: The arrows indicate the cells that are positive for TTF1.
Detection of EGFR mutations
All fixed samples with slides were sent to the Guangzhou Yishan Company for mutation analysis using the SurPlex-xTAG70plex-EGFR liquid chip.6 The procedure includes five major steps in sequence: 1) multiplex PCR was used to amplify the regions of target genes; 2) excess nucleotides and primers of PCR mixture were removed by exonuclease and shrimp alkaline phosphatase; 3) the left product was subjected to the process of allele specific primer extension, in which 70 universal tags were, respectively, linked to a specific primer sequence complementary to a specific gene of interest. Primers that only matched the templates were extended by the Tsp DNA; 4) the allele specific primer extension products were made to hybridize to specific anti-tag probes precoated on the polystyrene microspheres; and 5) the products of hybridization were subjected to the Luminex 200 and median fluorescence intensity (MFI) was read and analyzed. The sensitivity and specificity of EGFR liquid chip that we exploited in this study is very high compared to the real mutation status. When the real EGFR mutation rate is as low as 1%, the sensitivity and specificity of EGFR mutation by liquid chip is almost 100%. And the assay is under rigorous quality control.
Statistical analysis
The χ2 or the Fisher’s exact tests were used to analyze the relationships between EGFR mutations, TTF-1 expression, and clinical factors. The Kaplan–Meier method and Cox regression analysis were used to assess the relationship between postoperative survival, TTF-1 expression, and EGFR mutation status. All significant levels were two-sided. A P-value <0.05 was considered statistically significant. SPSS software (Version 17.0) was used for analysis.
Results
General characteristics of patients with tumors positive for EGFR mutations and TTF-1 expression
As shown in Table 2, 89 of 200 adenocarcinoma specimens (44.5%) had EGFR mutations, which included 44 specimens with exon 19 deletion mutations (22%) and 45 with exon 21 mutations (22.5%). EGFR mutations occurred in specimens from 40 patients older than 61 years (42.6%) and 49 patients 61 years of age or younger (46.2%). In 124 female participants, EGFR mutations were detected in specimens from 66 of 124 (53.2%) women and 23 of 76 men (30.3%, P<0.001). EGFR mutations were detected in specimens from 56 of 99 (56.6%) nonsmokers and 33 of 101 (32.7%, P=0.001) smokers. EGFR mutations were detected in 32 of 75 (42.7%) left lung specimens and 57 of 125 (45.6%, P=0.686) right lung specimens. EGFR mutations were detected in specimens from 36 of 87 (41.4%) patients with disease stages I–II and 53 of 113 (39.8%, P=0.436) patients with disease stages III–IV. EGFR mutations were detected in specimens from 46 of 72 (63.9%) female nonsmokers, of whom 24 (33.3%) had mutations in exon 19 and 22 (30.6%) had mutations in exon 21. The EGFR mutation rate in female nonsmokers was significantly higher than in the rest of the patients.
Table 2.
TTF-1 expression and EGFR gene mutation status in lung adenocarcinomas of 200 patients
| Category | TTF-1
|
EGFR
|
||||
|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | P-value | Positive (%) | Negative (%) | P-value | |
| Age | ||||||
| >61 years | 75 (79.8) | 19 (20.2) | 0.557 | 40 (42.6) | 54 (57.4) | 0.602 |
| ≤61 years | 88 (83.0) | 18 (17.0) | 49 (46.2) | 57 (53.8) | ||
| Sex | ||||||
| Male | 54 (71.1) | 22 (28.9) | 0.004 | 23 (30.3) | 53 (69.7) | 0.000 |
| Female | 109 (88.0) | 15 (22.0) | 66 (53.2) | 58 (46.8) | ||
| Smoking | ||||||
| Ever | 74 (73.3) | 27 (26.7) | 0.002 | 33 (32.7) | 68 (67.3) | 0.001 |
| Never | 89 (89.9) | 10 (10.1) | 56 (56.6) | 43 (43.4) | ||
| Tumor location | ||||||
| Left | 63 (84.0) | 12 (16.0) | 0.481 | 32 (42.7) | 43 (57.3) | 0.686 |
| Right | 100 (80.0) | 25 (20.0) | 57 (45.6) | 68 (54.4) | ||
| Pathological stage | ||||||
| I–II | 69 (79.3) | 18 (20.7) | 0.484 | 36 (41.4) | 51 (58.6) | 0.436 |
| III–IV | 94 (83.2) | 19 (16.8) | 53 (39.8) | 60 (60.2) | ||
Abbreviations: EGFR, epidermal growth factor receptor; TTF-1, thyroid transcription factor-1.
TTF-1 positive expression was found in 163 of 200 (81.5%) specimens. TTF-1 positive expression was found in specimens from 75 (79.8%) patients older than 61 years and 88 patients of age (83.0%) 61 years or younger. TTF-1 positive expression was found in specimens from 109 of 124 (88.0%) women and 54 of 76 men (71.1%, P=0.004). TTF-1 positive expression was found in specimens from 89 of 99 (89.9%) nonsmokers and 74 of 101 (73.3%, P=0.002) smokers. TTF-1 positive expression was found in 63 of 75 (84.0%) left lung specimens and 100 of 125 (80.0%, P=0.481) right lung specimens. TTF-1 positive expression was found in specimens from 69 of 87 (79.3%) patients with disease stages I–II and 94 of 113 (83.2%, P=0.484) patients with disease stages III–IV. TTF-1 positive expression was found in specimens from 67 of 72 (93.1%) female nonsmokers. The TTF-1 positive expression rate in female nonsmokers was also significantly higher than in the rest of the patients.
TTF-1 expression and EGFR mutations
EGFR mutations were detected in 83 of 163 (50.9%) TTF-1-positive specimens and six of 37 (16.2%, P<0.001) TTF-1-negative specimens (Table 3). EGFR mutations were detected in 46 of 67 (63.9%, n=72) TTF-1-positive specimens from female nonsmokers. Rates of TTF-1 expression positivity and EGFR mutations were higher in specimens from female nonsmokers than in the rest of the patients.
Table 3.
Relationship between TTF-1 expression and EGFR gene mutation status in tumors of different categories of patients
| Category | Patient, n | TTF-1 expression | Status of TTF-1 expression and EGFR mutation
|
|||
|---|---|---|---|---|---|---|
| EGFR(+) | EGFR(−) | Total | P-value | |||
| Overall status | ||||||
| 200 | TTF-1(+) | 83 | 80 | 163 | 0.000 | |
| TTF-1(−) | 6 | 31 | 37 | |||
| Total | 89 | 111 | 200 | |||
| Age | ||||||
| >61 years | 94 | TTF-1(+) | 38 | 37 | 75 | 0.002 |
| TTF-1(−) | 2 | 17 | 19 | |||
| Total | 40 | 54 | 94 | |||
| ≤61 years | 106 | TTF-1(+) | 45 | 43 | 88 | 0.025 |
| TTF-1(−) | 4 | 14 | 18 | |||
| Total | 49 | 57 | 106 | |||
| Sex | ||||||
| Male | 76 | TTF-1(+) | 19 | 35 | 54 | 0.080 |
| TTF-1(−) | 4 | 18 | 22 | |||
| Total | 23 | 53 | 76 | |||
| Female | 124 | TTF-1(+) | 64 | 45 | 109 | 0.001 |
| TTF-1(−) | 2 | 13 | 15 | |||
| Total | 66 | 58 | 124 | |||
| Smoking | ||||||
| Ever | 101 | TTF-1(+) | 29 | 45 | 74 | 0.021 |
| TTF-1(−) | 4 | 23 | 27 | |||
| Total | 33 | 68 | 101 | |||
| Never | 99 | TTF-1(+) | 54 | 35 | 89 | 0.034 |
| TTF-1(−) | 2 | 8 | 10 | |||
| Total | 56 | 43 | 99 | |||
| Tumor location | ||||||
| Left | 75 | TTF-1(+) | 32 | 31 | 63 | 0.001 |
| TTF-1(−) | 0 | 12 | 12 | |||
| Total | 32 | 43 | 75 | |||
| Right | 125 | TTF-1(+) | 51 | 49 | 100 | 0.015 |
| TTF-1(−) | 6 | 19 | 25 | |||
| Total | 57 | 68 | 125 | |||
| Clinical stage | ||||||
| I–II | 87 | TTF-1(+) | 34 | 35 | 69 | 0.003 |
| TTF-1(−) | 2 | 16 | 18 | |||
| Total | 36 | 51 | 87 | |||
| III–IV | 113 | TTF-1(+) | 49 | 45 | 94 | 0.013 |
| TTF-1(−) | 4 | 15 | 19 | |||
| Total | 53 | 60 | 113 | |||
| Sex, smoking history | ||||||
| Female nonsmokers | 87 | TTF-1(+) | 45 | 22 | 67 | 0.054 |
| TTF-1(−) | 1 | 4 | 5 | |||
| Total | 46 | 26 | 72 | |||
| Others | 113 | TTF-1(+) | 49 | 45 | 94 | 0.013 |
| TTF-1(−) | 4 | 15 | 19 | |||
| Total | 53 | 60 | 113 | |||
Abbreviations: EGFR, epidermal growth factor receptor; TTF-1, thyroid transcription factor-1.
There was a significant association of TTF-1 expression with EGFR mutations by the χ2 test (P<0.001). Table 3 shows the relationship between TTF-1 expression and EGFR mutations according to different groups of patients. There was a significant association between TTF-1 expression and EGFR mutations in specimens from female patients, but not from male patients. In addition, of 72 female nonsmokers, 45 (62.5%) cases were found to have dual expression of TTF-1 positivity and EGFR mutation, which was higher than the dual negative expression rate (5.6%, 4/72).
Association between TTF-1 expression and EGFR mutation subtypes
The rate of mutations in exons 19 and 21 were 22.0% (44/200) and 22.5% (45/200), respectively. No mutations were detected in exons 18 and 20. As shown in Table 4, of 163 specimens positive for TTF-1 expression, exon 21 mutations were detected in 44 (27.0%), which was significantly higher than the rate (2.7%, P=0.001) detected in TTF-1-negative specimens. There was a significant association between exon 21 mutations and TTF-1 positivity by the Fisher’s exact test (P=0.001). The rate of exon 19 mutations in TTF-1-positive specimens was 23.9% (39/163), which was higher than the rate detected in TTF-1-negative specimens (13.5%, P=0.167).
Table 4.
Relationship between TTF-1 expression and EGFR gene mutation status subtypes
| TTF-1 | Exon 19
|
Exon 21
|
||||
|---|---|---|---|---|---|---|
| Mut (+), n (%) | Mut (−), n (%) | P-value | Mut (+), n (%) | Mut (−), n (%) | P-value | |
| Positive (+) | 39 (23.9) | 124 (76.1) | 0.167 | 44 (27.0) | 119 (73.0) | 0.001 |
| Negative (−) | 5 (13.5) | 32 (86.5) | 1 (2.7) | 36 (97.3) | ||
| Total | 44 (22.0) | 156 (78.0) | 45 (22.5) | 155 (77.5) | ||
Abbreviations: EGFR, epidermal growth factor receptor; Mut (+), with mutation; Mut (−), without mutation; TTF-1, thyroid transcription factor-1.
Relationship between postoperative survival time, EGFR mutations, and TTF-1 expression
This study was followed up for each patient by phone. The postoperative survival time was defined as from the date of operation to the day of follow-up or the date of patients’ death. Until July 25, 2014, 119 patients survived, 55 patients died, and 26 patients were lost for the follow-up. The mean survival time was 935.9 days (range, 16–2,243 days). Kaplan–Meier analysis showed that there was no association between postoperative survival time and TTF-1 expression or EGFR mutation status (P=0.353 and P=0.106), respectively (Figure 3).
Figure 3.
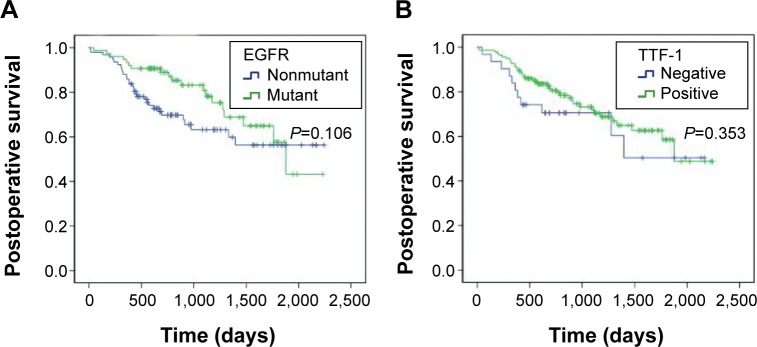
Kaplan–Meier estimates for postoperative survival according to EGFR gene mutation status and TTF-1 expression.
Notes: (A) Survival function in EGFR; (B) survival function in TTF-1.
Abbreviations: EGFR, epidermal growth factor receptor; TTF-1, thyroid transcription factor-1.
Table 5 shows the association between postoperative survival time, EGFR mutations, and TTF-1 expression among different patient subgroups, and the Kaplan–Meier method was used to analyze postoperative survival in relation to TTF-1 expression and EGFR mutation status among the different subgroups. The survival curves showed significant association between postoperative survival and TTF-1-positive specimens of patients with disease stages III–IV (P=0.032, Figure 4).
Table 5.
Postoperative survival and TTF-1 expression and EGFR gene mutation status in different categories of patients
| Category | TTF-1
|
EGFR
|
||
|---|---|---|---|---|
| χ2 | P-value | χ2 | P-value | |
| Age | ||||
| >61 years | 0.096 | 0.757 | 2.330 | 0.127 |
| ≤61 years | 3.729 | 0.053 | 0.763 | 0.382 |
| Sex | ||||
| Male | 0.381 | 0.537 | 1.062 | 0.303 |
| Female | 0.281 | 0.596 | 2.134 | 0.144 |
| Smoking | ||||
| Ever | 0.068 | 0.795 | 2.011 | 0.156 |
| Never | 1.224 | 0.269 | 0.427 | 0.514 |
| Tumor location | ||||
| Left | 0.000 | 0.999 | 2.889 | 0.089 |
| Right | 1.562 | 0.211 | 0.487 | 0.485 |
| Pathological stage | ||||
| I–II | 0.889 | 0.346 | 1.638 | 0.201 |
| III–IV | 4.578 | 0.032 | 2.075 | 0.150 |
Abbreviations: EGFR, epidermal growth factor receptor; TTF-1, thyroid transcription factor-1.
Figure 4.
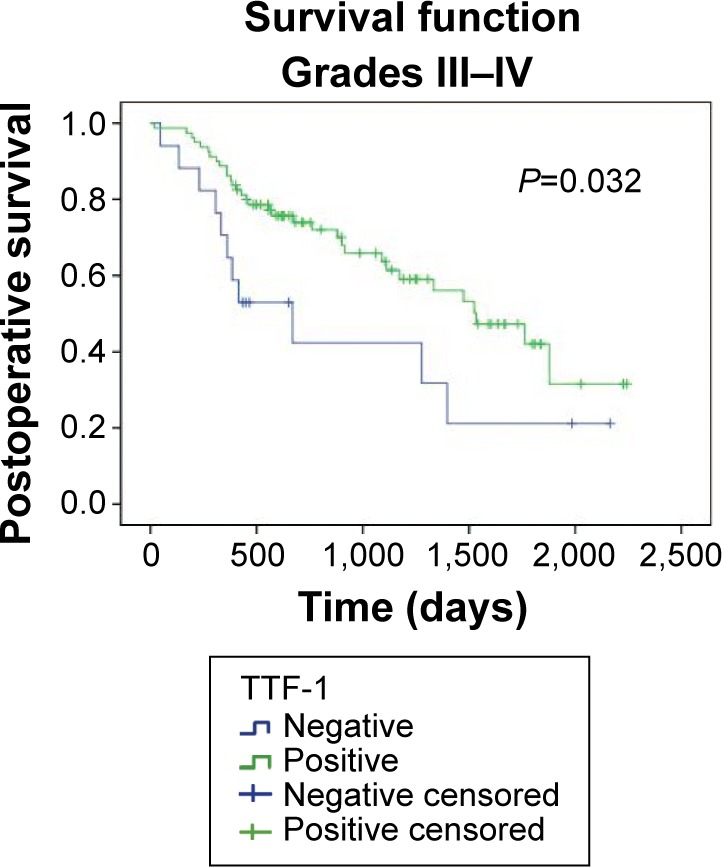
Kaplan–Meier estimates for postoperative survival according to TTF-1 expression in pathological stage III–IV patients.
Abbreviation: TTF-1, thyroid transcription factor-1.
Cox regression analysis was used to evaluate the association of postoperative survival and the following four subgroups of specimens and specimens of patients with disease stages III–IV: 1) TTF-1(+)/EGFR(+); 2) TTF-1(+)/EGFR(−); 3) TTF-1(−)/EGFR(+); and 4) TTF-1(−)/EGFR(−). There were no significant associations found when subgroups of all specimens were compared (Table 6). There was no significant difference between the risk of death for TTF-1(+)/EGFR(+) specimens and TTF-1(−)/EGFR(−) specimens (hazard ratio (HR) 1.706, 95% confidence interval (CI) 0.752–3.869; P=0.201; Table 6).
Table 6.
Postoperative survival analysis of patients with different EGFR and TTF-1 expression status
| Groups | Total
|
Stages III–IV
|
||||
|---|---|---|---|---|---|---|
| Hazard ratio | Confidence interval (95%) | P-value | Hazard ratio | Confidence interval (95%) | P-value | |
| EGFR | 0.636 | 0.369–1.097 | 0.104 | 0.644 | 0.352–1.178 | 0.153 |
| TTF-1 | 0.743 | 0.391–1.413 | 0.365 | 0.479 | 0.241–0.954 | 0.036 |
| EGFR(+)/TTF-1(+) vs others | 0.574 | 0.326–1.009 | 0.054 | 0.571 | 0.305–1.067 | 0.079 |
| EGFR(+)/TTF-1(−) vs others | 1.644 | 0.512–5.282 | 0.404 | 1.642 | 0.503–5.365 | 0.412 |
| EGFR(−)/TTF-1(+) vs others | 1.421 | 0.835–2.417 | 0.195 | 1.098 | 0.601–2.004 | 0.762 |
| EGFR(−)/TTF-1(−) vs others | 1.223 | 0.597–2.505 | 0.583 | 2.116 | 0.975–4.590 | 0.058 |
| EGFR(+)/TTF-1(+) vs EGFR(−)/TTF-1(−) | 1.706 | 0.752–3.869 | 0.201 | 2.616 | 1.085–6.306 | 0.027 |
Abbreviations: EGFR, epidermal growth factor receptor; TTF-1, thyroid transcription factor-1.
However, patients with disease stages III–IV and TTF-1(+)/EGFR(+) tumors had better survival than patients with disease stages III–IV and TTF-1(−)/EGFR(−) tumors (HR 2.616, 95% CI 1.085–6.306; P=0.027; Figure 5 and Table 6).
Figure 5.

Cox regression analysis for postoperative survival according to TTF-1 expression and EGFR mutation status in stage III–IV patients.
Notes: Patients with TTF-1(+)/EGFR(+) tumors showed better survival than those with TTF-1(−)/EGFR(−) tumors. (A) Survival function; (B) cumulative hazard function.
Abbreviations: EGFR, epidermal growth factor receptor; TTF-1, thyroid transcription factor-1.
Discussion
TTF-1 is an important member of the NKX2-1 family, and is mainly expressed in the thyroid, brain, lung, and other organs.9 A previous study found that TTF-1 was a significant biomarker for lung adenocarcinoma, with 100% specificity and 94.6% sensitivity.10 Therefore, TTF-1 is an important clinical indicator for differentiating lung adenocarcinoma from squamous cell carcinoma. TTF-1 can also be useful for differentiating primary from metastatic lung cancer, because TTF-1 is expressed predominantly in primary lung adenocarcinoma, but minimally in other metastatic tumors.11 Central and peripheral lung adenocarcinomas have been found to have different origins. Peripheral lung adenocarcinomas mainly originate from the terminal bronchioles, Clara cells, and type II alveolar epithelial cells; whereas central airway adenocarcinomas mainly originate from bronchial basal cells. Research has found that TTF-1 expression was always positive in peripheral adenocarcinomas and negative in central adenocarcinomas. A previous study has found that TTF-1 expression was associated with regulation of gene expression of surfactant protein-A (SPA), surfactant protein-B (SPB), and Clara cell antigen.12 TTF-1 was confirmed to have a strong correlation with the pulmonary surfactant protein, and was highly expressed in adenocarcinomas of female nonsmokers, with less frequent Rb loss, and negative p53 expression.13 Berghmans et al14 and Tang et al15 showed that TTF-1 was an independent prognostic factor for patients with lung cancer, especially those with lung adenocarcinoma.14,15 A recent study showed that TTF-1 expression (HR =0.340, 95% CI 0.143–0.811; P=0.015) was an independent predictor of survival in lung adenocarcinoma patients.10 Zamecnik and Kodet,16 Sakuma et al,17 and Zhao et al18 reported TTF-1 positive expression rates of 75%, 84.6%, and 80%, respectively, in lung adenocarcinoma patients. Our study found a TTF-1 expression rate of 81.5% (163/200), which is comparable to the results from the other investigators, confirming that TTF-1 is a specific marker for lung adenocarcinoma.
EGFR mutations play an important role in the pathogenesis of lung adenocarcinoma and are markers for patients with lung adenocarcinoma who take EGFR TKIs as the first-line treatment.19 EGFR gene mutations account for ~10% of NSCLC in western patients and 50% in Asian patients.20 Previous studies have reported that mutations mainly occur in exons 19 and 21 of EGFR in lung adenocarcinomas, especially among Asian female patients who are never smokers.21,22 According to retrospective reports, ~90% of patients who had a good response from TKIs were found to have EGFR mutations, and patients that did not respond to TKIs did not have EGFR mutations.21,23 Moreover, a study found that patients with EGFR mutations had a better response rate to erlotinib than to chemotherapy.24 The 2010 NCCN recommended EGFR mutation testing if first-line therapy for NSCLC was EGFR TKI, with Category 2A or 2B level of evidence.25 The 2011 NCCN guidelines were somewhat different. Testing for EGFR mutation status was strongly recommended for lung adenocarcinoma patients with Category 1 level of evidence.26 The proportion of tumor cells and normal cells in tissue samples is very important for detection of EGFR mutations, but currently there is no established requirement for the number of cells. The percentage of tumor cells needed for DNA sequencing has been >50%. Therefore, many investigators recommend as many tumor cells as possible for EGFR mutation testing. However, at times it has been difficult to obtain sufficient tissue, especially from fine needle and bronchial biopsies. Therefore, other biomarkers are needed that can predict which patients have tumors with EGFR mutations.
Two studies have recently demonstrated that TTF-1 expression and EGFR mutations in lung adenocarcinomas were associated with sex (P=0.003, P=0.002, respectively) and smoking history (P=0.002, P=0.001, respectively), and were significantly more frequent in nonsmoking female patients.8,27 Further analysis showed that the rate of TTF-1 expression in tumors of female patients was 88.0% (109/124) and 77.1% in male patients (54/76). Similarly, the EGFR mutation rate in tumors of female patients was 53.2% (66/124) and 30.3% in male patients (23/76). In addition, the rates of TTF-1 expression and EGFR mutations in nonsmokers were higher than in smoking patients (89.9% vs 73.3% and 56.6% vs 32.7%, respectively). More importantly, consistent with the results of Sun et al27 and Shanzi et al, we found that 83 tumors with EGFR mutations were also positive for TTF-1 expression (93.3%, 83/89; P<0.001; Table 3).8 We further analyzed the association between TTF-1 expression and EGFR mutation status in different subgroups of tumors, and found that TTF-1 had a significant association with EGFR mutation in all subgroups, but not in tumors of male patients (P=0.080, Table 3). In addition, we found mutation rates in exons 19 and 21 of 22% (44/200) and 22.5% (45/200), respectively (Table 4). Further analysis showed that there was a significant association between exon 21 mutations and TTF-1 expression (P=0.001, Table 4). We also observed that more tumors positive for TTF-1 expression had exon 19 mutation than tumors negative for TTF-1 expression, but the difference was not significant (P=0.167; Table 4).
As mentioned previously, EGFR mutation testing is very important for the diagnosis, treatment, and prognosis of patients with NSCLC, especially for those with lung adenocarcinoma. Investigators recently found that patients with tumors positive for EGFR mutations had better response to EGFR TKIs and chemotherapy than patients with tumors with wild-type EGFR.20 The findings suggest that testing patients with NSCLC for EGFR mutations status is crucial. However, the relationship between postoperative survival and EGFR mutation and/or TTF-1 expression has not been clearly elucidated. We found that postoperative survival time was not significantly associated with EGFR mutations or TTF-1 expression (P=0.106 and P=0.353, Figure 3). Further analysis showed significant association between postoperative survival time and TTF-1 expression in patients with disease stages III–IV disease (P=0.032, Figure 4). The postoperative survival of patients with disease stages III–IV and TTF-1(+)/EGFR(+) specimens was better than the survival of those with TTF-1(−)/EGFR(−) specimens (P=0.027, Figure 5), which may be a reflection of the fact that patients with TTF-1(+)/EGFR(+) tumors may be deriving benefit from targeted therapy. The associations between survival time and EGFR mutations and/or TTF-1 expression need further investigation.
Sometimes the status of EGFR mutations cannot be detected easily because of the difficulty in obtaining adequate tumor samples. This will result in the uncertain application of EGFR TKI treatment for adenocarcinoma patients and affect patient outcome. We found that the rate of EGFR mutations was higher in tumors of female patients (58.7%, 64/109) that were also positive for TTF-1 expression, and even higher in tumors of nonsmoking female patients with positive TTF-1 expression (67.2%, 45/67). These results may be accounted for by the high rate of EGFR mutations in lung cancers of Chinese patients. Therefore, both TTF-1 expression and EGFR mutations can be used as diagnostic markers and predictors of response to treatment for patients with lung adenocarcinoma. If the EGFR mutation status cannot be easily determined, testing for TTF-1 may be used to guide EGFR TKI therapy. For example, chemotherapy may be preferable for those lung adenocarcinoma patients with TTF-1-negative tumors, and unknown EGFR mutation status.
Conclusion
In summary, our study has demonstrated that TTF-1 expression in lung adenocarcinomas is significantly associated with the presence of EGFR mutation. Further investigation into the mechanism for this relationship may contribute to greater understanding of the oncogenic role of TTF-1 in lung adenocarcinomas, and to studies on EGFR TKIs and the mechanisms of drug resistance. In clinical practice, TTF-1 expression may be a marker used for planning therapy for patients with lung adenocarcinomas and unknown EGFR mutation status, especially guiding therapy with EGFR TKIs.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81172233, 81372306, and 81301812), the Specialized Research Fund for the Doctoral Program of Higher Education (20131202120004), the Tianjin key project of Natural Science Foundation (12JCZDJC24400), the Tianjin Natural Science Foundation (13JCYBJC22600), the Tianjin Science and Technology Support Program (12ZCDZSY16100), and the Tianjin Educational Committee Foundation (20120117). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger DS, Akerley W, Borghaei H, et al. National Comprehensive Cancer Network Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw. 2013;11:645–653. doi: 10.6004/jnccn.2013.0084. quiz 653. [DOI] [PubMed] [Google Scholar]
- 4.Maemondo M, Inoue A, Kobayashi K, et al. North-East Japan Study Group Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 5.Fisseler-Eckhoff A. Neue TNM-Klassifikation maligner Lungentumoren 2009 aus Sicht der Pathologie [New TNM classification of malignant lung tumors 2009 from a pathology perspective] Pathologe. 2009;30(suppl 2):193–199. doi: 10.1007/s00292-009-1195-3. German. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Luo X, He J, et al. A novel liquidchip platform for simultaneous detection of 70 alleles of DNA somatic mutations on EGFR, KRAS, BRAF and PIK3CA from formalin-fixed and paraffin-embedded slides containing tumor tissue. Clin Chem Lab Med. 2011;49:191–195. doi: 10.1515/CCLM.2011.040. [DOI] [PubMed] [Google Scholar]
- 7.Berghmans T, Mascaux C, Martin B, Ninane V, Sculier JP. Prognostic role of thyroid transcription factor-1 in stage III non-small cell lung cancer. Lung Cancer. 2006;52:219–224. doi: 10.1016/j.lungcan.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Shanzhi W, Yiping H, Ling H, Jianming Z, Qiang L. The relationship between TTF-1 expression and EGFR mutations in lung adenocarcinomas. PLoS One. 2014;9:e95479. doi: 10.1371/journal.pone.0095479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boggaram V. Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin Sci (Lond) 2009;116:27–35. doi: 10.1042/CS20080068. [DOI] [PubMed] [Google Scholar]
- 10.Xu XY, Yang GY, Yang JH, Li J. Analysis of clinical characteristics and differential diagnosis of the lung biopsy specimens in 99 adenocarcinoma cases and 111 squamous cell carcinoma cases: utility of an immunohistochemical panel containing CK5/6, CK34betaE12, p63, CK7 and TTF-1. Pathol Res Pract. 2014;210:680–685. doi: 10.1016/j.prp.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Chang YL, Lee YC, Liao WY, Wu CT. The utility and limitation of thyroid transcription factor-1 protein in primary and metastatic pulmonary neoplasms. Lung Cancer. 2004;44:149–157. doi: 10.1016/j.lungcan.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Yi M, Tong GX, Murry B, Mendelson CR. Role of CBP/p300 and SRC-1 in transcriptional regulation of the pulmonary surfactant protein-A (SP-A) gene by thyroid transcription factor-1 (TTF-1) J Biol Chem. 2002;277:2997–3005. doi: 10.1074/jbc.M109793200. [DOI] [PubMed] [Google Scholar]
- 13.Yatabe Y, Mitsudomi T, Takahashi T. TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol. 2002;26:767–773. doi: 10.1097/00000478-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Berghmans T, Paesmans M, Mascaux C, et al. Thyroid transcription factor 1 – a new prognostic factor in lung cancer: a meta-analysis. Ann Oncol. 2006;17:1673–1676. doi: 10.1093/annonc/mdl287. [DOI] [PubMed] [Google Scholar]
- 15.Tang X, Kadara H, Behrens C, et al. Abnormalities of the TITF-1 lineage-specific oncogene in NSCLC: implications in lung cancer pathogenesis and prognosis. Clin Cancer Res. 2011;17:2434–2443. doi: 10.1158/1078-0432.CCR-10-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zamecnik J, Kodet R. Value of thyroid transcription factor-1 and surfactant apoprotein A in the differential diagnosis of pulmonary carcinomas: a study of 109 cases. Virchows Arch. 2002;440:353–361. doi: 10.1007/s00428-001-0552-2. [DOI] [PubMed] [Google Scholar]
- 17.Sakuma Y, Matsukuma S, Yoshihara M, et al. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations. Am J Clin Pathol. 2007;128:100–108. doi: 10.1309/WVXFGAFLAUX48DU6. [DOI] [PubMed] [Google Scholar]
- 18.Zhao W, Wang H, Peng Y, Tian B, Peng L, Zhang DC. DeltaNp63, CK5/6, TTF-1 and napsin A, a reliable panel to subtype non-small cell lung cancer in biopsy specimens. Int J Clin Exp Pathol. 2014;7:4247–4253. [PMC free article] [PubMed] [Google Scholar]
- 19.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch FR, Bunn PA., Jr EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009;10:432–433. doi: 10.1016/S1470-2045(09)70110-X. [DOI] [PubMed] [Google Scholar]
- 21.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 22.Giaccone G, Gallegos Ruiz M, Le Chevalier T, et al. Erlotinib for frontline treatment of advanced non-small cell lung cancer: a phase II study. Clin Cancer Res. 2006;12:6049–6055. doi: 10.1158/1078-0432.CCR-06-0260. [DOI] [PubMed] [Google Scholar]
- 23.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 24.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 25.Ettinger DS, Akerley W, Bepler G, et al. NCCN Non-Small Cell Lung Cancer Panel Members. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 26.Xue C, Hu Z, Jiang W, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer. 2012;77:371–375. doi: 10.1016/j.lungcan.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Sun PL, Seol H, Lee HJ, et al. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features. J Thorac Oncol. 2012;7:323–330. doi: 10.1097/JTO.0b013e3182381515. [DOI] [PubMed] [Google Scholar]


