Background: Unconventional secretion of both HIV-Tat and FGF2 depends on the phosphoinositide PI(4,5)P2.
Results: HIV-Tat forms membrane-inserted oligomers concomitant with PI(4,5)P2-dependent membrane pore formation.
Conclusion: HIV-Tat and FGF2 show similar properties with a tight correlation between membrane pore formation and unconventional secretion from cells.
Significance: Evidence is provided that suggests a common mechanism of unconventional secretion with potential relevance for a broad range of cargoes.
Keywords: FGF; membrane bilayer; membrane lipid; membrane reconstitution; plasma membrane; FGF2 and membrane translocation; HIV-Tat; membrane recruitment and pore formation; phosphoinositide PI(4,5)P2; unconventional protein secretion
Abstract
HIV-Tat has been demonstrated to be secreted from cells in a phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)-dependent manner. Here we show that HIV-Tat forms membrane-inserted oligomers, a process that is accompanied by changes in secondary structure with a strong increase in antiparallel β sheet content. Intriguingly, oligomerization of HIV-Tat on membrane surfaces leads to the formation of membrane pores, as demonstrated by physical membrane passage of small fluorescent tracer molecules. Although membrane binding of HIV-Tat did not strictly depend on PI(4,5)P2 but, rather, was mediated by a range of acidic membrane lipids, a functional interaction between PI(4,5)P2 and HIV-Tat was critically required for efficient membrane pore formation by HIV-Tat oligomers. These properties are strikingly similar to what has been reported previously for fibroblast growth factor 2 (FGF2), providing strong evidence of a common core mechanism of unconventional secretion shared by HIV-Tat and fibroblast growth factor 2.
Introduction
The HIV-1 transactivator of transcription (HIV-Tat)2 is a small protein of 86–101 amino acids that is synthesized in the very early steps of viral infection. HIV-Tat is a regulatory protein involved in viral gene expression by enhancing transcriptional rates and, therefore, is crucial for viral viability (1, 2). In addition to these classical functions, HIV-Tat is secreted from infected cells, followed by uptake by non-infected cells (3–6). An extracellular localization of HIV-Tat is also evident from studies demonstrating substantial levels of HIV-Tat in the sera of patients with AIDS (7, 8). Despite the lack of a signal peptide, secretion of HIV-Tat has been shown to occur independent of cell damage, and, therefore, HIV-Tat has been classified as a protein secreted by unconventional means (4, 9–14). Extracellular HIV-Tat is supposed to be essential for viral spread and plays an important role in AIDS pathogenesis by modulating the immune response (9, 12, 14). Recent studies indicate that vaccination with anti-Tat antibodies slows down AIDS progression and may restore immune functions (15–17).
The externalization of a subpopulation of HIV-Tat molecules has recently been shown to occur by direct translocation across plasma membranes (18). This process has been further demonstrated to depend on the phosphoinositide phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) (19). These features point to the possibility that HIV-Tat and FGF2 share a similar mechanism of unconventional secretion to gain access to the extracellular space (10, 20). As with HIV-Tat, FGF2 secretion is mediated by direct translocation across the plasma membrane (21, 22). This process is initiated by recruitment of FGF2 at the inner leaflet mediated by PI(4,5)P2 (23, 24) and an integral membrane protein, ATP1A1 (25). FGF2 translocation into the extracellular space involves membrane insertion of oligomeric forms of FGF2 and the formation of membrane pores (13, 26–29). This process is triggered by the interaction of FGF2 with PI(4,5)P2 (29, 30) and is regulated by Tec-kinase-mediated tyrosine phosphorylation of FGF2 (29–31). More recently, oligomerization of membrane-inserted forms of FGF2 has been shown to be driven by the formation of intermolecular disulfide bridges (26). Their formation depends on two conserved surface-exposed cysteines uniquely present in FGF2 but absent in signal peptide-containing FGF family members. In a late step of FGF2 membrane translocation, membrane proximal heparan sulfate proteoglycans act as an extracellular trap, resulting in the translocation of FGF2 to cell surfaces (32, 33).
On the basis of the observation that unconventional secretion of HIV-Tat from infected T cells depends on PI(4,5)P2 (19), we tested whether HIV-Tat shares biochemical and biophysical properties with FGF2 in terms of membrane-associated intermediates relevant for unconventional secretion. We found that HIV-Tat shares a number of key features known from FGF2 as being relevant for unconventional secretion. Most importantly, this includes the formation of membrane-inserted oligomers and the ability of HIV-Tat oligomers to form membrane pores in a PI(4,5)P2-dependent manner. Although HIV-Tat bound to a range of acidic membrane lipids, membrane pore formation was largely dependent on a functional interaction between HIV-Tat and PI(4,5)P2. Although the general ability of HIV-Tat to form membrane pores resembled the properties of FGF2, HIV-Tat oligomers formed pores with much higher efficiency and at significantly lower protein concentrations. Also, as opposed to FGF2, oligomerization and membrane pore formation by HIV-Tat was not dependent on the formation of intermolecular disulfide bridges. In conclusion, our combined findings suggest a common core mechanism for the secretion of HIV-Tat and FGF2 on the basis of PI(4,5)P2-dependent membrane pore formation. However, this study also reveals mechanistic differences between HIV-Tat and FGF2 with regard to the formation of membrane-inserted oligomers as intermediates during unconventional secretion.
Experimental Procedures
Recombinant Proteins and Antibodies
HIV-Tat variants (Tat wild-type, Tat W11Y, Tat R49Q/K50Q/K51Q, and Tat W11Y/R49Q/K50Q/K51Q) were expressed as recombinant fusion proteins in Escherichia coli strain W3110Z1 (37 °C for 6 h in LB medium (10 g/L tryptone, 10 g/L yeast extract, and 5 g/L NaCl)) using the expression vector pQE30 (Qiagen). HIV-Tat variants were affinity-purified through a N-terminal His tag using denaturing conditions (6 m guanidinhydrochloride and 10 mm tris(2-carboxyethyl)phosphine (34)), followed by refolding through buffer exchange using buffer A (25 mm HEPES and 150 mm KCl (pH 7.4)). The wild-type form of His-tagged HIV-Tat was used to generate polyclonal antibodies in rabbits employing standard procedures. Expression and purification of a phosphomimetic form of FGF2 (FGF2-Y81pCMF) was performed as described previously (26, 29).
Preparation of Liposomes
All lipids were from Avanti Polar Lipids: phosphatidylcholine (PC), phosphatidylinositol (PI), and phosphatidylethanolamine (PE) from bovine liver; phosphatidylserine (PS) and PI(4,5)P2 from porcine brain; cholesterol (Chol) from ovine wool; sphingomyelin (SM) from poultry eggs; and lissamine rhodamine B-labeled PE (16:0) (a synthetic product included to monitor liposome formation). All lipids were stored at −20 °C under argon. Liposomes were made as described previously (23, 24). Lipid mixtures were prepared in chloroform that was evaporated under a stream of nitrogen. The resulting lipid film was further dried under vacuum for 1.5 h and then resuspended in buffer B (25 mm HEPES, 150 mm KCl (pH 7.4), and 10% (w/v) sucrose) at 45 °C to form liposomes with a final lipid concentration of 4 mm for binding experiments. Unilamellar liposomes were made by 10 freeze/thaw cycles, followed by size extrusion through a 400-nm filter 21 times (Avanti Polar Lipids mini extruder). Analysis of liposome preparations using dynamic light scattering (Wyatt Technology, DynaPro NanoStar) showed a size distribution of 200–400 nm in diameter. The following lipid mixtures were used to generate liposomes: liposomes with a plasma membrane-like (PM-like) lipid composition (17.5 mol% PC, 9.0 mol% PE, 5.0 mol% PI, 5.0 mol% PS, 12.5 mol% SM, 50.0 mol% Chol, and 1.0 mol% Rhod-PE), PM-like without PI and PS (27.5 mol% PC, 9.0 mol% PE, 12.5 mol% SM, 50.0 mol% Chol, and 1.0 mol% Rhod-PE), PM-like with PI(4,5)P2 (15.5 mol% PC, 9.0 mol% PE, 5.0 mol% PI, 5.0 mol% PS, 12.5 mol% SM, 50.0 mol% Chol, 1.0 mol% Rhod-PE, and 2.0 mol% PI(4,5)P2), PM-like with Ni-NTA (15.5 mol% PC, 9.0 mol% PE, 5.0 mol% PI, 5.0 mol% PS, 12.5 mol% SM, 50.0 mol% Chol, 1.0 mol% Rhod-PE, and 2.0 mol% Ni-NTA), PC (99.0 mol% PC and 1.0 mol% Rhod-PE), PC with PI(4,5)P2 (89.0 mol% PC, 1.0 mol% Rhod-PE, and 10.0 mol% PI(4,5)P2), and PC with Ni-NTA (89.0 mol% PC, 1.0 mol% Rhod-PE, and 10.0 mol% Ni-NTA).
Analysis of Protein-Lipid Interactions Using Flotation Gradients
Binding of HIV-Tat variant forms and FGF2-Y81pCMF to liposomes of various lipid compositions was analyzed as follows. Proteins (5 μm) were first incubated with liposomes for 30 min at 25 °C in buffer A. Membranes were reisolated by flotation using Nycodenz density gradients (40:30:0% (w/v) in buffer A) as described in Ref. 35. Following ultracentrifugation, gradients were divided into four fractions, with the floated liposomes being contained in fraction 1. Samples were analyzed by SDS-PAGE under reducing conditions (2.25% of both the load and fractions 1–4), followed by Western blotting using anti-Tat (this study) and anti-FGF2 (36) antibodies, respectively. Signals were recorded using an Odyssey infrared imaging system and quantified using Image Studio software version 2.1.10 (LI-COR Bioscience). For carbonate extraction, a membrane binding assay was performed as described above, adding Na2CO3 (pH 11) at a final concentration of 100 mm prior to flotation. Buffer A was used as a control.
Conformational Analysis of HIV-Tat in the Absence and Presence of Membranes Using FTIR Spectroscopy
To detect conformational changes in HIV-Tat upon binding to membranes, FTIR spectra were recorded. Because of a technical limitation of this method, high protein concentrations are required (37). Therefore, measurements were conducted at a final concentration of HIV-Tat of 435 μm incubated with either PC, PM-like with PI(4,5)P2, or PC with PI(4,5)P2 liposomes. Control conditions included measurements with liposomes alone (PC, PM-like with PI(4,5)P2, and PC with PI(4,5)P2; 2 mm total lipid) and HIV-Tat wild-type (435 μm, 5 mg/ml) alone. All measurements were conducted in buffer A in a Bio-ATRII cell using a Tensor 27 instrument (Bruker, Ettenheim, Germany). Spectra were corrected for water vapor and vector-normalized.
Analysis of Membrane Pore Formation by Phosphomimetic FGF2 and HIV-Tat Variant Forms
Membrane pore formation by HIV-Tat variants and FGF2-Y81pCMF was analyzed as described previously (26, 29). In brief, liposomes with various lipid compositions were prepared in reconstitution buffer C (25 mm HEPES, 100 mm KCl (pH 7.4), and 10% (w/v) sucrose) supplemented with the membrane-impermeant fluorophore 5(6)-carboxyfluorescein (Sigma) at a final concentration of 100 μm and a total lipid concentration of 8 mm. To separate extraluminal carboxyfluorescein, liposomes were first diluted in buffer A (25 mm HEPES and 150 mm KCl (pH 7.4)) and collected by centrifugation at 15,000 × g for 10 min at 20 °C, followed by size exclusion chromatography using a PD10 column (GE Healthcare). The column was equilibrated beforehand in buffer D (25 mm HEPES, 150 mm KCl (pH 7.4), 10% (w/v) sucrose, and 2% (w/v) glucose) that was titrated with glucose to reach iso-osmolality with reconstitution buffer C. Afterward, HIV-Tat variants and FGF2-Y81pCMF (at the indicated protein concentrations) were incubated with liposomes (10 μm total lipid). Fluorescence dequenching was measured using a SpectraMax GeminiXS fluorescence plate reader (Molecular Devices). At the end of each experiment, Triton X-100 (0.2% (w/v) final concentration) was added to measure the maximal dequenching signal, which was used to normalize data. To investigate a potential role of cysteine residues in oligomerization-dependent membrane pore formation, the assay was performed in the presence and absence of the reducing agent DTT at a final concentration of 100 mm.
Results
HIV-Tat Oligomerizes on Membrane Surfaces
In the first set of experiments, we analyzed whether HIV-Tat can form oligomers in the presence of liposomes containing PI(4,5)P2, a known property of FGF2 (13, 26, 29). As shown in Fig. 1A, in the absence of liposomes, the vast majority of HIV-Tat remained a monomer for extended incubation times of up to 5 h. Under these conditions, only a minor dimeric form could be detected, which did not change in abundance over the time course shown (Fig. 1A). By contrast, in the presence of liposomes with a plasma-membrane-like lipid composition including PI(4,5)P2, oligomerization of HIV-Tat was observed (Fig. 1B). This process was also evident from the concomitant decrease in HIV-Tat monomers along the time course shown. HIV-Tat dimers were most abundant at 2 h of incubation, followed by a decline at later time points indicating conversion into higher oligomers. Therefore, similar to FGF2, HIV-Tat oligomerizes in the presence of liposomes with a plasma membrane-like lipid composition including PI(4,5)P2.
FIGURE 1.
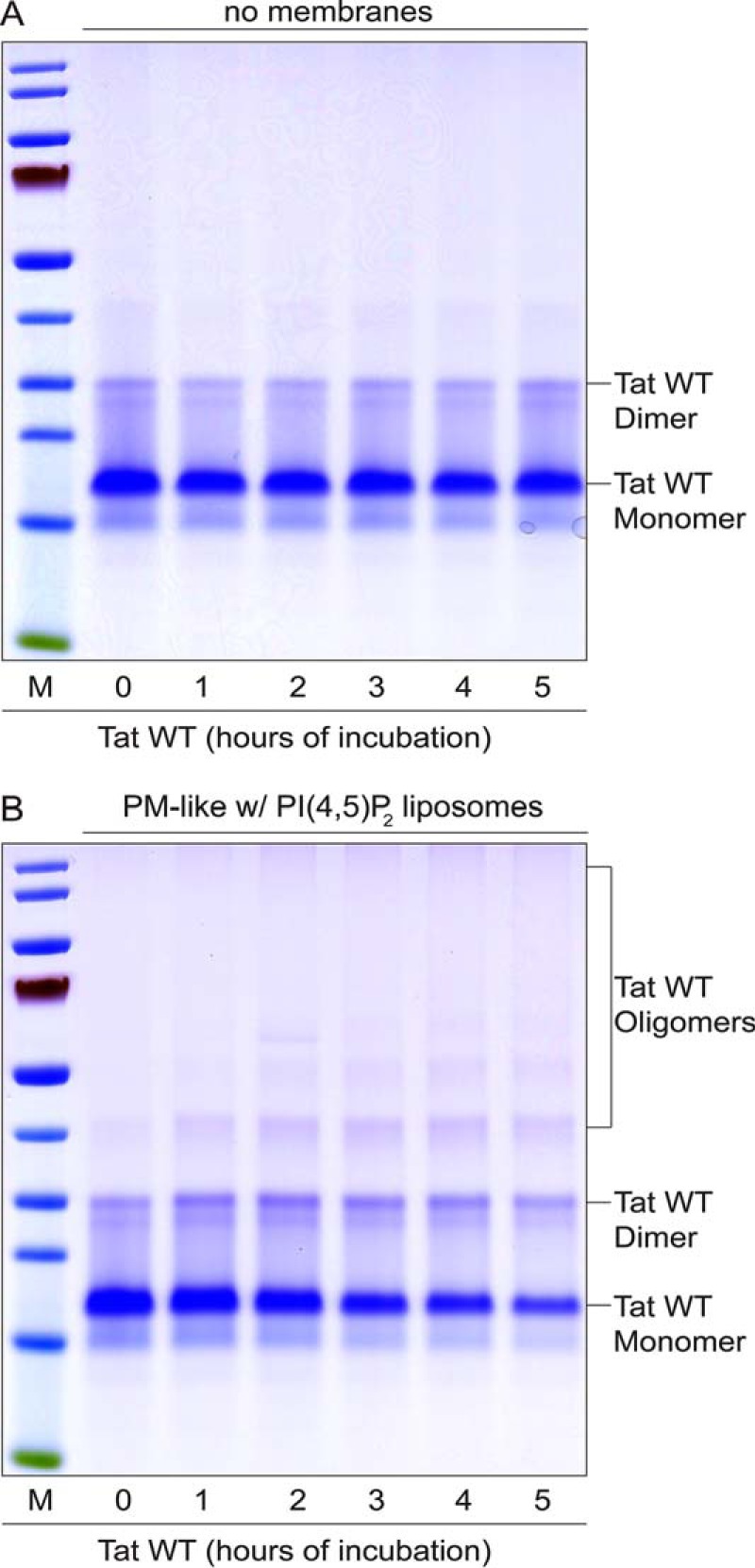
Analysis of HIV-Tat oligomerization in the absence and presence of PI(4,5)P2-containing liposomes. A and B, HIV-Tat wild-type (0.25 μg/μl, 21.7 μm) was incubated at 25 °C in buffer A for the indicated times. Experiments were conducted both in the absence (A) and presence (B) of liposomes with a plasma membrane-like composition containing PI(4,5)P2 (1.4 mm total membrane lipids). Samples were mixed with SDS sample buffer (non-reducing), followed by incubation for 5 min at 95 °C. Per lane, 2.5 μg of total protein was analyzed by non-reducing SDS-PAGE (4–12% gradient gels). Proteins were visualized by Coomassie staining. The results shown are representative of three independent experiments. The markers (M) shown indicate relative molecular masses of 10 (green), 15, 25, 30, 40, 50, 70 (red), 80, 115, and 140 kDa. w, with.
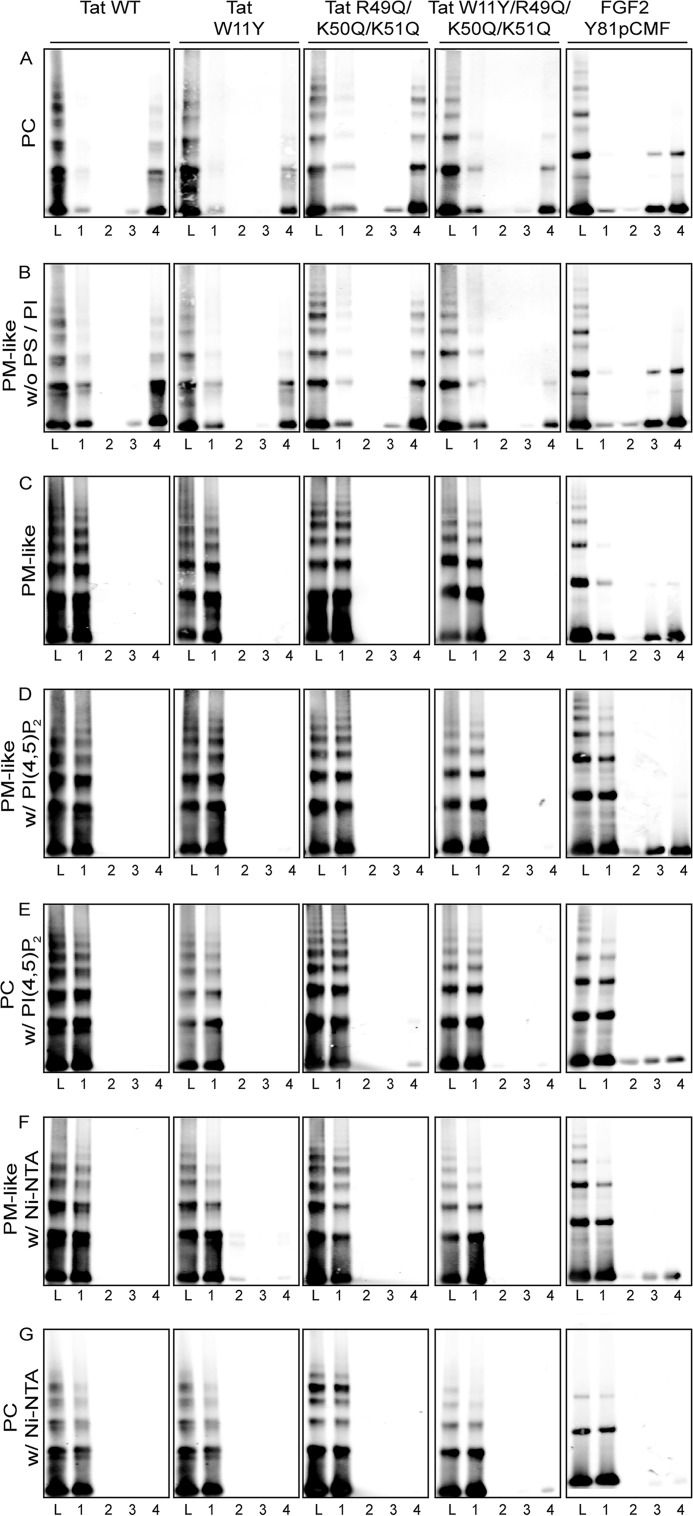
In a second series of experiments, we analyzed the lipid binding properties of HIV-Tat and compared them with FGF2 (Fig. 2). This analysis was made on the basis of flotation experiments and revealed that HIV-Tat binding to liposomes is not exclusively mediated by PI(4,5)P2. Rather, HIV-Tat did bind to liposomes with a plasma membrane-like lipid composition in the absence (Fig. 2C, fraction 1) or presence of PI(4,5)P2 (Fig. 2D, fraction 1). In addition, HIV-Tat did bind to PC liposomes containing PI(4,5)P2 (Fig. 2E, fraction 1). Binding of HIV-Tat to these types of liposomes was highly efficient, with the protein appearing in floated liposomes in a quantitative manner (Fig. 3). By contrast, removing PS, PI, and PI(4,5)P2 from complex plasma membrane-like lipid mixtures (Fig. 2B, fraction 1) or using liposomes containing PC only (Fig. 2A, fraction 1) resulted in largely reduced amounts of HIV-Tat associated with membranes (Fig. 3). These properties of HIV-Tat were distinct from those of FGF2, with membrane binding being highly efficient and, beyond phosphoinositide PI(4,5)P2, also mediated by other acidic membrane lipids (Fig. 3). As with FGF2 (Fig. 2, D and E, fraction 1) (13, 26, 29), and consistent with the results shown in Fig. 1, we found that HIV-Tat forms oligomers associated with floated liposomes. Under the experimental conditions described, various mutant forms of HIV-Tat that have been described previously to be impaired in binding to PI(4,5)P2 (19) were found to bind to PM-like liposomes (Fig. 2C, fraction 1), PM-like liposomes containing PI(4,5)P2 (Fig. 2D, fraction 1), and PC liposomes containing PI(4,5)P2 (Fig. 2E, fraction 1), with an efficiency comparable with the wild-type form of HIV-Tat (Fig. 3). Like the wild-type form of HIV-Tat, binding to PM-like without PS or PI (Fig. 2B, fraction 1) and PC liposomes (Fig. 2B, fraction 1) of these mutant forms was largely reduced (Fig. 3). Finally, efficient binding of all forms of HIV-Tat and phosphomimetic FGF2 to liposomes containing an Ni-NTA lipid either in a PM-like (Fig. 2F, fractions 1 and Fig. 3) or PC (Figs. 2G, fraction 1, and 3) lipid background was observed on the basis of the N-terminal His tags all of these proteins carried (Figs. 2, F and G, fraction 1, and 3). Interestingly, HIV-Tat oligomers were observed under these conditions, suggesting that this process does not require PI(4,5)P2.
FIGURE 2.
Analysis of HIV-Tat and FGF2 interactions with membrane lipids employing flotation gradients. Various kinds of unilamellar liposomes with the membrane lipid compositions were generated and incubated with variant forms of HIV-Tat and phosphomimetic FGF2 as indicated. Following the separation of liposomes from unbound proteins by flotation, gradients were divided into four fractions, with fraction 1 containing floated liposomes and fraction 4 containing unbound protein and potential aggregates. The four fractions as well as the load (L, a mixture of proteins and liposomes as indicated) of the gradient were analyzed by SDS-PAGE and Western blot using anti-HIV Tat antibodies (this study) and the LI-COR infrared imaging platform. A, PC liposomes. B, PM-like liposomes lacking PS and PI. C, PM-like liposomes. D, PM-like liposomes containing PI(4,5)P2. E, PC liposomes containing PI(4,5)P2. F, PM-like liposomes containing Ni-NTA lipid. G, PC liposomes containing Ni-NTA lipid. w/o, without; w, with.
FIGURE 3.
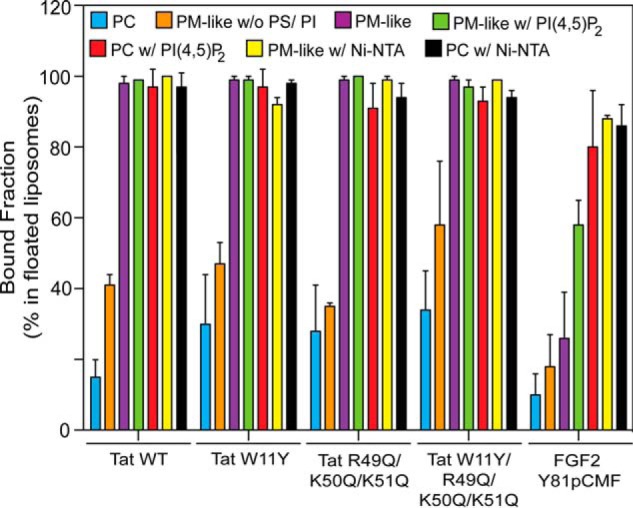
Quantification of membrane binding of HIV-Tat variant forms and phosphomimetic FGF2 to various kinds of liposomes. Using the LI-COR infrared imaging platform, the data in Fig. 2 were quantified. The amount of protein associated with floated liposomes is given as the percentage of the signals obtained from all four fractions of the gradient (n = 3). w/o, without; w, with.
Oligomers of HIV-Tat and FGF2 Are Inserted Tightly into Membranes
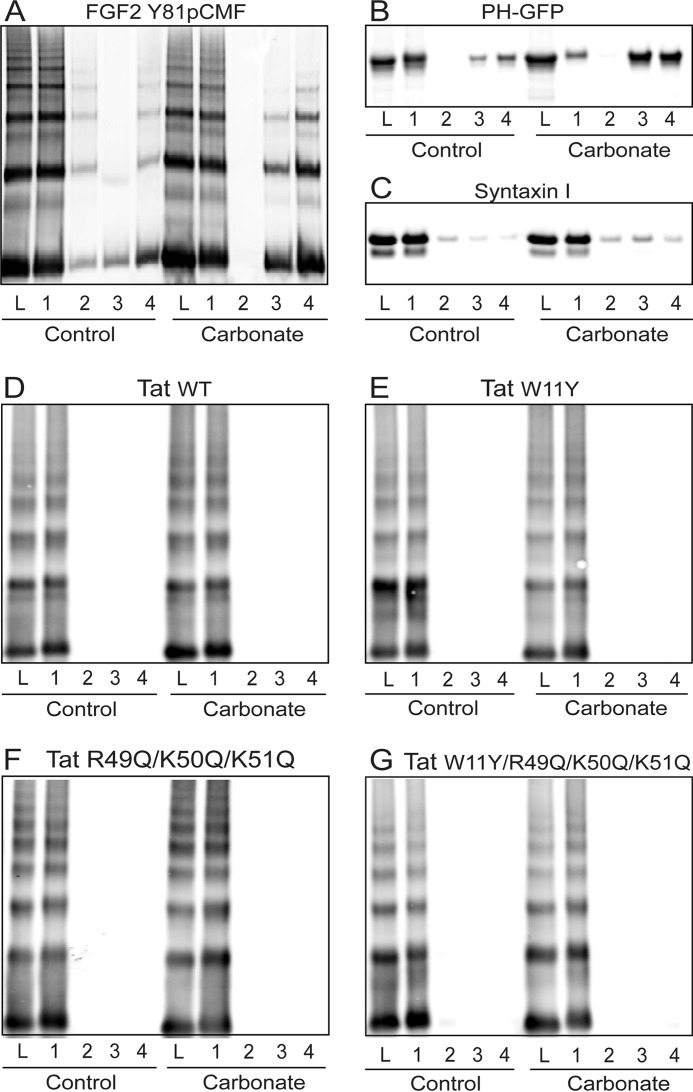
To characterize the type of membrane association beyond electrostatic interactions with the headgroups of acidic membrane lipids, we tested whether HIV-Tat and FGF2 can be extracted from liposomes with a plasma membrane-like lipid composition including PI(4,5)P2 using alkaline carbonate treatment (Figs. 4 and 5). As controls, a PI(4,5)P2 binding PH domain (PH-GFP) (38) and an integral membrane protein reconstituted in liposomes (syntaxin I) (39) were used. The majority of PH-GFP was readily extractable from membranes (Fig. 4B), with a fraction of only 30% remaining on floated liposomes in the presence of carbonate (Fig. 5). By contrast, syntaxin I membrane association (Fig. 4C) was only slightly reduced upon carbonate treatment (Fig. 5). These properties reflect the peripheral association of PH-GFP on the basis of electrostatic interactions with PI(4,5)P2 versus the integral embedding of syntaxin I into the hydrophobic core of the membrane. By comparison, HIV-Tat oligomers behaved like an integral membrane protein such as syntaxin I in that membrane association was fully resistant to carbonate treatment (Figs. 4D and 5). Variant forms of HIV-Tat shown in this study to bind to acidic membrane lipids (Figs. 2 and 3) were also found to associate with membranes in a carbonate-resistant manner (Figs. 4, E–G, and 5). Phosphomimetic FGF2 showed an intermediate phenotype, with about 65% of the membrane-associated fraction being resistant to carbonate (Figs. 4A and 5). These findings suggest that membrane-associated oligomers of HIV-Tat and FGF2 are stably anchored in membranes that, in addition to electrostatic interactions with PI(4,5)P2, are on the basis of hydrophobic interactions.
FIGURE 4.
HIV-Tat associates with lipid bilayers in a carbonate-resistant manner. Binding experiments with phosphomimetic FGF2 and various forms of HIV-Tat were conducted using liposomes with a plasma membrane-like lipid composition, including PI(4,5)P2 (PM-like with PI(4,5)P2) as described in Fig. 2. As protein controls with known properties, a PI(4,5)P2 binding PH domain (PH-GFP) and liposomes containing an integral membrane protein (syntaxin I) were used. Before samples were subjected to flotation in density gradients, they were either treated with buffer A (control) or with carbonate (100 mm (pH 11)). Both types of samples were loaded at the bottom of flotation gradients, followed by ultracentrifugation to separate floating liposomes from unbound proteins and aggregates. Gradients were divided into four fractions, with fraction 1 containing floated liposomes and fraction 4 containing unbound protein and potential aggregates. The four fractions as well as the load (L) of the gradient were analyzed by SDS-PAGE and Western blot using the LI-COR infrared imaging platform. A, FGF2-Y81pCMF (phosphomimetic FGF2). B, PH-GFP. C, syntaxin I. D, HIV-Tat wild-type. E, HIV-Tat W11Y. F, HIV-Tat R49Q/K50Q/K51Q. G, HIV-Tat W11Y/R49Q/K50Q/K51Q.
FIGURE 5.
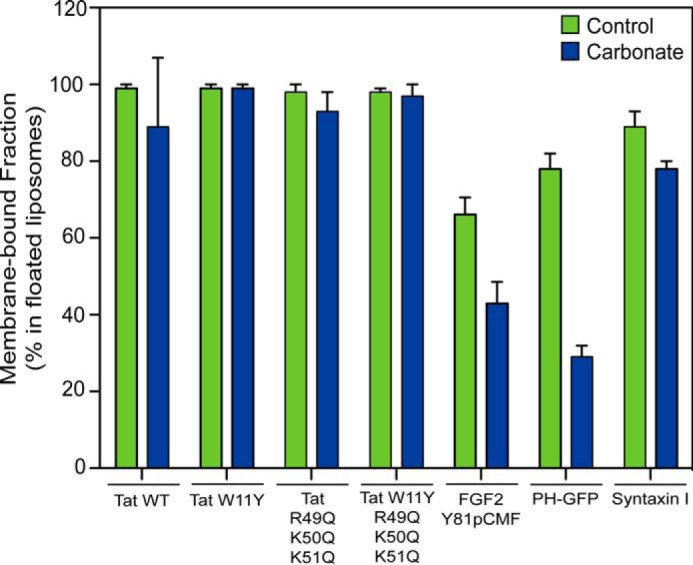
Quantification of HIV-Tat and FGF2 membrane binding in the absence and presence of carbonate treatment. Using the LI-COR infrared imaging platform, the data in Fig. 4 were quantified. The amount of protein associated with floated liposomes is given as the percentage of the signals obtained from all four fractions of the gradient (n = 3).
HIV-Tat Undergoes Conformational Changes in the Presence of Membranes
Because HIV-Tat is an intrinsically unfolded protein (40, 41), we tested whether membrane binding and oligomerization result in conformational changes employing FTIR spectroscopy (Fig. 6). Experiments were conducted with HIV-Tat wild-type at 5 mg/ml (435 μm) in the presence of either PC, PC with PI(4,5)P2, or PM-like with PI(4,5)P2 liposomes. As controls, FTIR spectra of both HIV-Tat alone and the various types of liposomes alone were recorded. The control spectra were subtracted from the spectra of HIV-Tat in the presence of lipids to expose membrane-induced changes. This approach revealed differences in secondary structures of HIV-Tat in the absence and presence of membranes as a deviation from zero (Fig. 6). We found that the relative amounts of both α helices (maximum at ∼1654 cm−1) and parallel β sheets (maximum at ∼1640 cm−1) are reduced in the presence of membranes. By contrast, the relative amounts of antiparallel β sheets (maxima at ∼1620 and ∼1695 cm−1) increased strongly in the presence of membranes. When different types of liposomes were compared, a direct dependence of the observed conformational changes of HIV-Tat on PI(4,5)P2 could not be observed. This was probably due to the high concentration of HIV-Tat (435 μm) that was required for FTIR experiments, resulting in some degree of PI(4,5)P2-independent membrane pore formation (see below; Fig. 7C). Therefore, the observed changes in HIV-Tat secondary structure were triggered in the presence of lipid bilayers and correlated with membrane pore formation.
FIGURE 6.
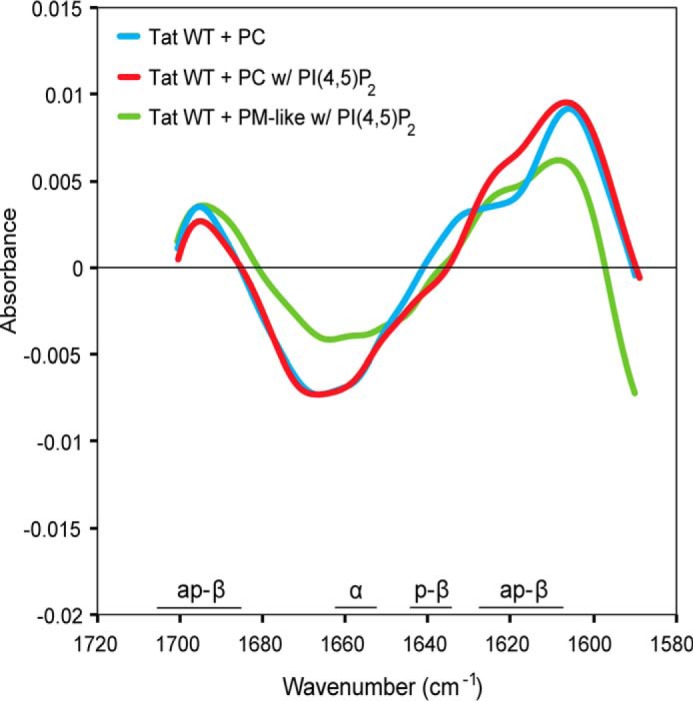
HIV-Tat undergoes conformational changes in the presence of lipid bilayers. The wild-type form of HIV-Tat was incubated in the absence or presence of liposomes with the indicated lipid compositions. Difference plots of vector normalized FTIR spectra (amide I band) of HIV-Tat in the presence of liposomes minus FTIR spectra of HIV-Tat alone minus FTIR spectra of liposomes alone are shown. Deviations from zero indicate liposome-induced changes in peptide backbone carbonyl stretching vibrations of the protein. Absorbance bands characteristic for secondary structure elements are indicated as antiparallel β-sheet (ap-β, maxima ∼1620 and ∼1695 cm−1), parallel β sheet (p-β, maxima ∼1640 cm−1), and α helices (α, maxima ∼1654 cm−1). The spectra shown are the average of at least three measurements. w, with.
FIGURE 7.
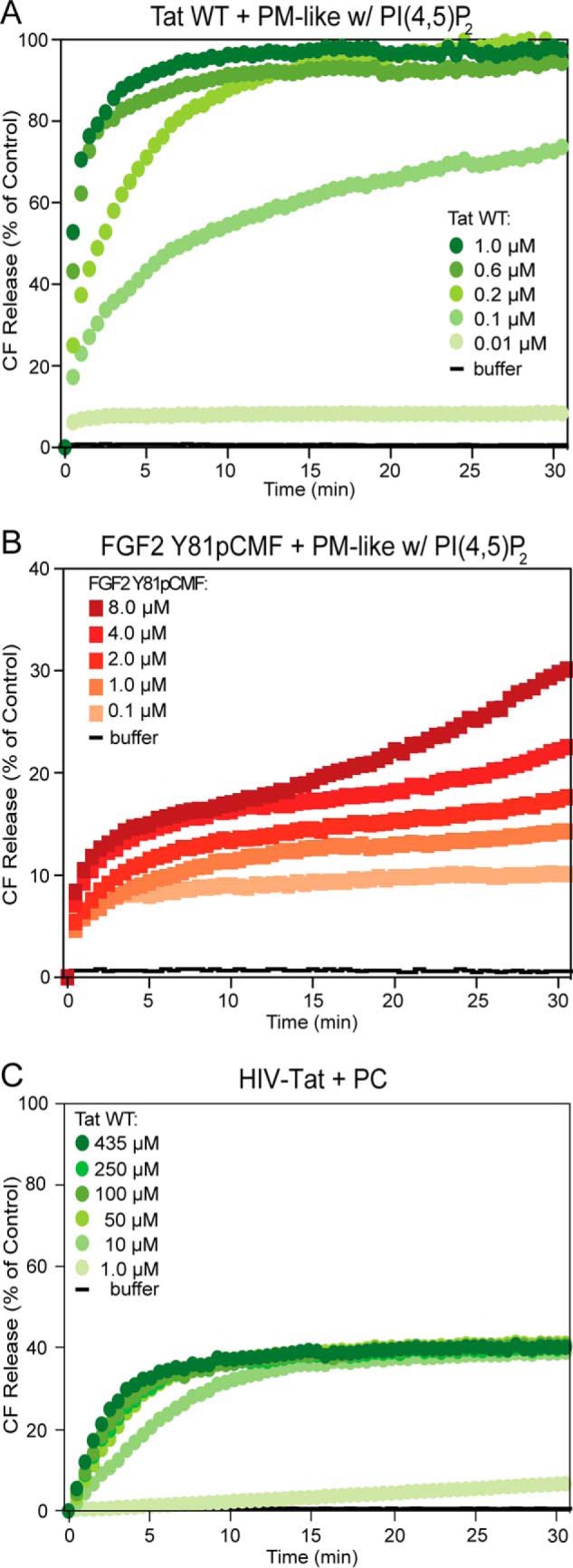
HIV-Tat forms membrane pores with high efficiency. Carboxyfluorescein (CF) was sequestered in unilamellar liposomes with a plasma membrane-like lipid composition, including PI(4,5)P2 (PM-like with PI(4,5)P2, 10 μm total lipid, see “Experimental Procedures” for details). A and B, following incubation with either HIV-Tat wild-type (A) or phosphomimetic FGF2 (B) at the indicated protein concentrations, membrane pore formation was measured by luminal release of carboxyfluorescein on the basis of kinetic dequenching experiments (26, 29). The buffer control is shown in black. The results shown are representative of three independent experiments. w, with. C, a titration of HIV-Tat wild-type is shown to analyze membrane pore formation with PC liposomes at concentrations of HIV-Tat (up to 435 μm) that were used in the FTIR experiments (Fig. 6).
HIV-Tat Efficiently Forms Membrane Pores in a PI(4,5)P2-dependent Manner
Because PI(4,5)P2-dependent oligomerization and membrane pore formation are key events in unconventional secretion of FGF2 (11, 13, 26, 29, 30), we tested whether HIV-Tat has similar properties. A well established dequenching assay was used to monitor membrane pore formation on the basis of physical membrane passage of a small fluorescent tracer (26, 29). These studies revealed that HIV-Tat is capable of forming membrane pores in a highly efficient manner, with substantial activity at protein concentrations as low as 0.1 μm (Fig. 7A). By comparison, appreciable amounts of membrane pore formation by phosphomimetic FGF2 required a protein concentration of at least 1 μm (Fig. 7B) (26, 29). When HIV-Tat was incubated with liposomes entirely formed from PC, membrane pore formation was only detectable at high concentrations of HIV-Tat, starting at 10 μm (Fig. 7C). At a concentration of HIV-Tat of 435 μm, a condition that revealed conformational changes in the presence of lipid bilayers (Fig. 6), substantial amounts of membrane pore formation were observed (Fig. 7C). However, in the presence of PI(4,5)P2, a similar activity was already observed at 0.1 μm HIV-Tat, establishing the strong stimulatory effect of PI(4,5)P2 on membrane pore formation mediated by HIV-Tat.
As opposed to the membrane binding properties of HIV-Tat (Figs. 2 and 3), membrane pore formation was critically dependent on PI(4,5)P2 (Fig. 8, A and B). First, replacing PI(4,5)P2 with an Ni-NTA lipid to recruit HIV-Tat to membranes via a His tag resulted in much lower (PM-like with Ni-NTA liposomes) or no activity (PC with Ni-NTA liposomes) of membrane pore formation (Fig. 8A), a phenomenon also known for FGF2 (13, 29). Second, variant forms of HIV-Tat with amino acid substitutions that have been implicated previously in PI(4,5)P2 binding and HIV-Tat secretion from cells (19) were found to cause a massive loss in membrane pore formation (Fig. 8B). In comparison to phosphomimetic FGF2 (26), however, oligomerization and membrane pore formation by HIV-Tat was not driven by intermolecular disulfide-dependent formation of HIV-Tat oligomers (Fig. 8C). While pore formation activity of phosphomimetic FGF2 was inhibited in the presence of DTT, the formation of membrane pores by HIV-Tat was even enhanced in the presence of reducing agents. With regard to phosphomimetic FGF2, these data are consistent with earlier findings demonstrating a direct role of two surface-exposed cysteines in both membrane pore formation and FGF2 secretion from cells (26). By contrast, stimulation of HIV-Tat membrane pore formation in the presence of DTT is likely to be due to preventing random oxidation of cysteine residues in HIV-Tat. This may, in turn, maintain the membrane activity of HIV-Tat. Therefore, although both phosphomimetic FGF2 and HIV-Tat form oligomers involved in membrane pore formation, only FGF2 depends on the formation of intermolecular disulfide bridges in terms of efficient oligomerization.
FIGURE 8.
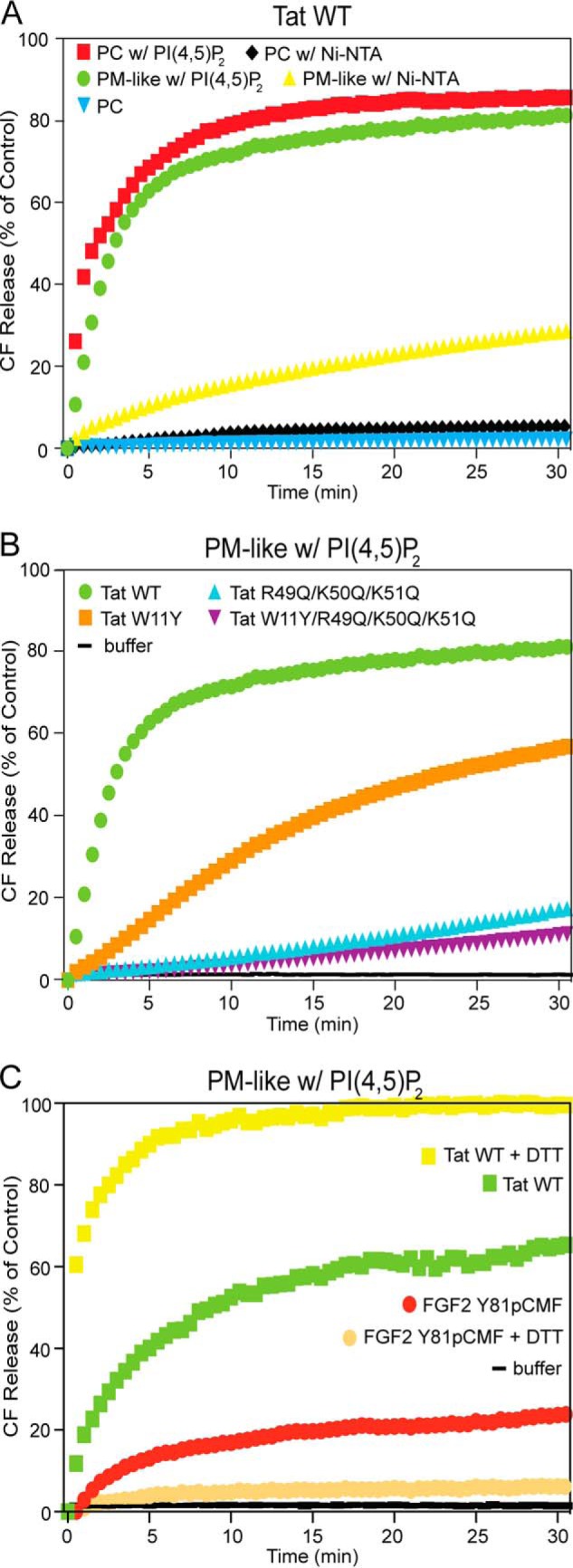
HIV-Tat forms membrane pores in a PI(4,5)P2-dependent manner. Membrane pore formation was measured by luminal release of carboxyfluorescein (CF) on the basis of kinetic dequenching experiments (26, 29) as described in Fig. 7 (see “Experimental Procedures” for details). A, experiments comparing various kinds of lipid compositions with regard to membrane pore formation by HIV-Tat wild-type. w, with. B, comparison of membrane pore formation mediated by HIV-Tat wild-type versus variant forms with amino acid substitutions that prevent binding to PI(4,5)P2. C, comparison of the effect of the reducing agent DTT (100 mm) on membrane pore formation mediated by either HIV-Tat wild-type or phosphomimetic FGF2. In all experiments, HIV-Tat wild-type and its variant forms were used at a final concentration of 0.2 μm. Phosphomimetic FGF2 was used at a final concentration of 4 μm. The buffer control is shown in black. The results shown are representative of three independent experiments.
Discussion
This study aimed to establish a biochemical basis for the previously observed dependence of HIV-Tat secretion from infected T cells on the phosphoinositide PI(4,5)P2 (18, 19). In addition, we analyzed whether HIV-Tat and FGF2 share key properties that point to a common core mechanism by which these proteins are secreted from cells. The central result from these studies is the demonstration of a common ability of HIV-Tat and FGF2 to form oligomers in the presence of lipid bilayers concomitant with the formation of PI(4,5)P2-dependent membrane pores. Similar to previous studies on FGF2, membrane pores formed by HIV-Tat were interpreted as intermediates in HIV-Tat membrane translocation. Full membrane translocation as it occurs in cells is likely to depend on additional factors that are not yet included in our reconstitution system. For example, HIV-Tat has been shown to bind to heparan sulfate proteoglycans on cell surfaces (3), a property known to be required for full membrane translocation of FGF2 (32, 33).
Although HIV-Tat and FGF2 differed in terms of specificity regarding binding to acidic membrane lipids, both proteins formed membrane-inserted oligomeric structures that allowed for physical passage of a fluorescent tracer. For both proteins, this process was dependent on the phosphoinositide PI(4,5)P2. However, both membrane binding and pore formation by HIV-Tat were much more efficient than what was observed for FGF2. At protein concentrations as low as 0.1 μm, HIV-Tat displayed substantial membrane activity, whereas appreciable amounts of membrane pore formation mediated by FGF2 required protein concentrations of at least 1 μm. These observations may at least in part explain why HIV-Tat secretion from cells is more efficient compared with FGF2 (19, 33). For HIV-Tat, the phenomenon of membrane pore formation could be separated from membrane binding because acidic membrane lipids such as PI and PS allowed for HIV-Tat membrane recruitment. However, in the absence of PI(4,5)P2, membrane-bound HIV-Tat did not promote pore formation. This finding was consistent with other experimental conditions recruiting HIV-Tat to membranes by an artificial membrane anchor, an Ni-NTA membrane lipid recruiting His-tagged proteins. Similar to previous observations with FGF2 (29), His-tagged HIV-Tat recruited through the Ni-NTA lipid did not promote membrane pore formation in an efficient manner. Intriguingly, residues in HIV-Tat (tryptophan 11, arginine 49, and lysines 50 and 51) that have been shown previously to be critical for unconventional secretion of HIV-Tat from infected T cells (19) were also essential for membrane pore formation induced by recombinant HIV-Tat. Our combined findings suggest that these residues are not essential for membrane binding. However, they are critical for orienting HIV-Tat in the PI(4,5)P2-dependent manner required for proper oligomerization and membrane pore formation. This study further provides evidence that this process involves conformational changes converting HIV-Tat from an intrinsically unfolded protein (40, 41) into membrane-inserted oligomers. In the course of this, hydrophobic contacts with the core of the membrane are established, as indicated by membrane insertion of HIV-Tat oligomers in a carbonate-resistant manner. These hydrophobic interactions within the membrane core are likely to be mediated by the central hydrophobic domain of HIV-Tat (42). A similar phenomenon was observed for FGF2, with a significant fraction of oligomers being inserted into the membrane in a carbonate-resistant manner. Despite these similarities, we also observed differences in the molecular requirements for HIV-Tat- versus FGF2-mediated membrane pore formation. Most strikingly, FGF2 oligomerization and membrane insertion are known to be driven by the formation of intermolecular disulfide bridges (26). By contrast, we found that membrane pore formation by HIV-Tat occurs with increased efficiency in the presence of reducing agents, indicating that random oxidation of HIV-Tat is prevented under these conditions. In conclusion, despite differences in binding specificity to acidic membrane lipids and the mechanism by which HIV-Tat and FGF2 oligomerize in the presence of lipid bilayers, this study identifies a common core mechanism of unconventional secretion of FGF2 and HIV-Tat: the ability to oligomerize and to form membrane pores in a phosphoinositide-dependent manner. Beyond FGF2 and HIV-Tat, this mechanism of unconventional secretion is likely to be relevant for a much broader group of unconventionally secreted proteins.
Author Contributions
M. Z., J. P. S., H. M. M., and M. P. M. performed and analyzed the experiments. M. Z., J. P. S., and M. P. M. contributed to the preparation of figures. W. N. conceived and coordinated the study, designed and analyzed the experiments, and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Jörg Malsam and Thomas Söllner (Heidelberg University Biochemistry Center) for liposomes containing the integral membrane protein syntaxin I as well as antibodies directed against syntaxin I.
This work was supported by German Research Council Grants DFG-SFB 638, DFG-SFB/TRR 83, and DFG-GRK1188; the AID-NET program of the Federal Ministry for Education and Research of Germany, and the cluster of excellence CellNetworks. The authors declare that they have no conflicts of interest with the contents of this article.
- HIV-Tat
- HIV-1 transactivator of transcription
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- PC
- phosphatidylcholine
- PI
- phosphatidylinositol
- PE
- phosphatidylethanolamine
- PS
- phosphatidylserine
- Chol
- cholesterol
- SM
- sphingomyelin
- PM
- plasma membrane
- Ni-NTA
- nickel-nitrilotriacetic acid
- FGF2
- fibroblast growth factor 2.
References
- 1. Campbell G. R., Loret E. P. (2009) What does the structure-function relationship of the HIV-1 Tat protein teach us about developing an AIDS vaccine? Retrovirology 6, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruben S., Perkins A., Purcell R., Joung K., Sia R., Burghoff R., Haseltine W. A., Rosen C. A. (1989) Structural and functional characterization of human immunodeficiency virus Tat protein. J. Virol. 63, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang H. C., Samaniego F., Nair B. C., Buonaguro L., Ensoli B. (1997) HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11, 1421–1431 [DOI] [PubMed] [Google Scholar]
- 4. Debaisieux S., Rayne F., Yezid H., Beaumelle B. (2012) The ins and outs of HIV-1 Tat. Traffic 13, 355–363 [DOI] [PubMed] [Google Scholar]
- 5. Frankel A. D., Pabo C. O. (1988) Cellular uptake of the Tat protein from human immunodeficiency virus. Cell 55, 1189–1193 [DOI] [PubMed] [Google Scholar]
- 6. Tyagi M., Rusnati M., Presta M., Giacca M. (2001) Internalization of HIV-1 Tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 276, 3254–3261 [DOI] [PubMed] [Google Scholar]
- 7. Shi B., Raina J., Lorenzo A., Busciglio J., Gabuzda D. (1998) Neuronal apoptosis induced by HIV-1 Tat protein and TNF-α: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. J. Neurovirol. 4, 281–290 [DOI] [PubMed] [Google Scholar]
- 8. Xiao H., Neuveut C., Tiffany H. L., Benkirane M., Rich E. A., Murphy P. M., Jeang K. T. (2000) Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc. Natl. Acad. Sci. U.S.A. 97, 11466–11471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. (1990) Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345, 84–86 [DOI] [PubMed] [Google Scholar]
- 10. Nickel W., Rabouille C. (2009) Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 10, 148–155 [DOI] [PubMed] [Google Scholar]
- 11. Rabouille C., Malhotra V., Nickel W. (2012) Diversity in unconventional protein secretion. J. Cell Sci. 125, 5251–5255 [DOI] [PubMed] [Google Scholar]
- 12. Ensoli B., Buonaguro L., Barillari G., Fiorelli V., Gendelman R., Morgan R. A., Wingfield P., Gallo R. C. (1993) Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67, 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steringer J. P., Müller H. M., Nickel W. (2015) Unconventional secretion of fibroblast growth factor 2: a novel type of protein translocation across membranes? J. Mol. Biol. 427, 1202–1210 [DOI] [PubMed] [Google Scholar]
- 14. Rubartelli A., Poggi A., Sitia R., Zocchi M. R. (1998) HIV-I Tat: a polypeptide for all seasons. Immunol. Today 19, 543–545 [DOI] [PubMed] [Google Scholar]
- 15. Bellino S., Tripiciano A., Picconi O., Francavilla V., Longo O., Sgadari C., Paniccia G., Arancio A., Angarano G., Ladisa N., Lazzarin A., Tambussi G., Nozza S., Torti C., Focà E., Palamara G., Latini A., Sighinolfi L., Mazzotta F., Di Pietro M., Di Perri G., Bonora S., Mercurio V. S., Mussini C., Gori A., Galli M., Monini P., Cafaro A., Ensoli F., Ensoli B. (2014) The presence of anti-Tat antibodies in HIV-infected individuals is associated with containment of CD4+ T-cell decay and viral load, and with delay of disease progression: results of a 3-year cohort study. Retrovirology 11, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ensoli B., Fiorelli V., Ensoli F., Cafaro A., Titti F., Buttò S., Monini P., Magnani M., Caputo A., Garaci E. (2006) Candidate HIV-1 Tat vaccine development: from basic science to clinical trials. AIDS 20, 2245–2261 [DOI] [PubMed] [Google Scholar]
- 17. Ensoli F., Cafaro A., Casabianca A., Tripiciano A., Bellino S., Longo O., Francavilla V., Picconi O., Sgadari C., Moretti S., Cossut M. R., Arancio A., Orlandi C., Sernicola L., Maggiorella M. T., Paniccia G., Mussini C., Lazzarin A., Sighinolfi L., Palamara G., Gori A., Angarano G., Di Pietro M., Galli M., Mercurio V. S., Castelli F., Di Perri G., Monini P., Magnani M., Garaci E., Ensoli B. (2015) HIV-1 Tat immunization restores immune homeostasis and attacks the HAART-resistant blood HIV DNA: results of a randomized phase II exploratory clinical trial. Retrovirology 12, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rayne F., Debaisieux S., Bonhoure A., Beaumelle B. (2010) HIV-1 Tat is unconventionally secreted through the plasma membrane. Cell Biol. Int. 34, 409–413 [DOI] [PubMed] [Google Scholar]
- 19. Rayne F., Debaisieux S., Yezid H., Lin Y. L., Mettling C., Konate K., Chazal N., Arold S. T., Pugnière M., Sanchez F., Bonhoure A., Briant L., Loret E., Roy C., Beaumelle B. (2010) Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J. 29, 1348–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nickel W., Seedorf M. (2008) Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu. Rev. Cell Dev. Biol. 24, 287–308 [DOI] [PubMed] [Google Scholar]
- 21. Nickel W. (2005) Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic 6, 607–614 [DOI] [PubMed] [Google Scholar]
- 22. Schäfer T., Zentgraf H., Zehe C., Brügger B., Bernhagen J., Nickel W. (2004) Unconventional secretion of fibroblast growth factor 2 is mediated by direct translocation across the plasma membrane of mammalian cells. J. Biol. Chem. 279, 6244–6251 [DOI] [PubMed] [Google Scholar]
- 23. Temmerman K., Ebert A. D., Müller H. M., Sinning I., Tews I., Nickel W. (2008) A direct role for phosphatidylinositol-4,5-bisphosphate in unconventional secretion of fibroblast growth factor 2. Traffic 9, 1204–1217 [DOI] [PubMed] [Google Scholar]
- 24. Temmerman K., Nickel W. (2009) A novel flow cytometric assay to quantify interactions between proteins and membrane lipids. J. Lipid Res. 50, 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zacherl S., La Venuta G., Müller H. M., Wegehingel S., Dimou E., Sehr P., Lewis J. D., Erfle H., Pepperkok R., Nickel W. (2015) A direct role for ATP1A1 in unconventional secretion of fibroblast growth factor 2. J. Biol. Chem. 290, 3654–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Müller H. M., Steringer J. P., Wegehingel S., Bleicken S., Münster M., Dimou E., Unger S., Weidmann G., Andreas H., García-Sáez A. J., Wild K., Sinning I., Nickel W. (2015) Formation of disulfide bridges drives oligomerization, membrane pore formation and translocation of fibroblast growth factor 2 to cell surfaces. J. Biol. Chem. 290, 8925–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Backhaus R., Zehe C., Wegehingel S., Kehlenbach A., Schwappach B., Nickel W. (2004) Unconventional protein secretion: membrane translocation of FGF-2 does not require protein unfolding. J. Cell Sci. 117, 1727–1736 [DOI] [PubMed] [Google Scholar]
- 28. Torrado L. C., Temmerman K., Müller H. M., Mayer M. P., Seelenmeyer C., Backhaus R., Nickel W. (2009) An intrinsic quality-control mechanism ensures unconventional secretion of fibroblast growth factor 2 in a folded conformation. J. Cell Sci. 122, 3322–3329 [DOI] [PubMed] [Google Scholar]
- 29. Steringer J. P., Bleicken S., Andreas H., Zacherl S., Laussmann M., Temmerman K., Contreras F. X., Bharat T. A., Lechner J., Müller H. M., Briggs J. A., García-Sáez A. J., Nickel W. (2012) Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)-dependent oligomerization of fibroblast growth factor 2 (FGF2) triggers the formation of a lipidic membrane pore implicated in unconventional secretion. J. Biol. Chem. 287, 27659–27669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nickel W. (2011) The unconventional secretory machinery of fibroblast growth factor 2. Traffic 12, 799–805 [DOI] [PubMed] [Google Scholar]
- 31. Ebert A. D., Laussmann M., Wegehingel S., Kaderali L., Erfle H., Reichert J., Lechner J., Beer H. D., Pepperkok R., Nickel W. (2010) Tec-kinase-mediated phosphorylation of fibroblast growth factor 2 is essential for unconventional secretion. Traffic 11, 813–826 [DOI] [PubMed] [Google Scholar]
- 32. Nickel W. (2007) Unconventional secretion: an extracellular trap for export of fibroblast growth factor 2. J. Cell Sci. 120, 2295–2299 [DOI] [PubMed] [Google Scholar]
- 33. Zehe C., Engling A., Wegehingel S., Schäfer T., Nickel W. (2006) Cell-surface heparan sulfate proteoglycans are essential components of the unconventional export machinery of FGF-2. Proc. Natl. Acad. Sci. U.S.A. 103, 15479–15484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shojania S., Henry G. D., Chen V. C., Vo T. N., Perreault H., O'Neil J. D. (2010) High yield expression and purification of HIV-1 Tat1–72 for structural studies. J. Virol. Methods 164, 35–42 [DOI] [PubMed] [Google Scholar]
- 35. Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Söllner T. H., Rothman J. E. (1998) SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 [DOI] [PubMed] [Google Scholar]
- 36. Engling A., Backhaus R., Stegmayer C., Zehe C., Seelenmeyer C., Kehlenbach A., Schwappach B., Wegehingel S., Nickel W. (2002) Biosynthetic FGF-2 is targeted to non-lipid raft microdomains following translocation to the extracellular surface of CHO cells. J. Cell Sci. 115, 3619–3631 [DOI] [PubMed] [Google Scholar]
- 37. Barth A. (2007) Infrared spectroscopy of proteins. Biochim. Biophys. Acta 1767, 1073–1101 [DOI] [PubMed] [Google Scholar]
- 38. Milosevic I., Sørensen J. B., Lang T., Krauss M., Nagy G., Haucke V., Jahn R., Neher E. (2005) Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J. Neurosci. 25, 2557–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Malsam J., Seiler F., Schollmeier Y., Rusu P., Krause J. M., Söllner T. H. (2009) The carboxy-terminal domain of complexin I stimulates liposome fusion. Proc. Natl. Acad. Sci. U.S.A. 106, 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peloponese J. M. Jr., Gregoire C., Opi S., Esquieu D., Sturgis J., Lebrun E., Meurs E., Collette Y., Olive D., Aubertin A. M., Witvrow M., Pannecouque C., De Clercq E., Bailly C., Lebreton J., Loret E. P. (2000) 1H-13C nuclear magnetic resonance assignment and structural characterization of HIV-1 Tat protein. Comptes rendus de l'Academie des sciences. Serie III, Sciences de la vie 323, 883–894 [DOI] [PubMed] [Google Scholar]
- 41. Shojania S., O'Neil J. D. (2006) HIV-1 Tat is a natively unfolded protein: the solution conformation and dynamics of reduced HIV-1 Tat-(1–72) by NMR spectroscopy. J. Biol. Chem. 281, 8347–8356 [DOI] [PubMed] [Google Scholar]
- 42. Bayer P., Kraft M., Ejchart A., Westendorp M., Frank R., Rösch P. (1995) Structural studies of HIV-1 Tat protein. J. Mol. Biol. 247, 529–535 [DOI] [PubMed] [Google Scholar]