Abstract
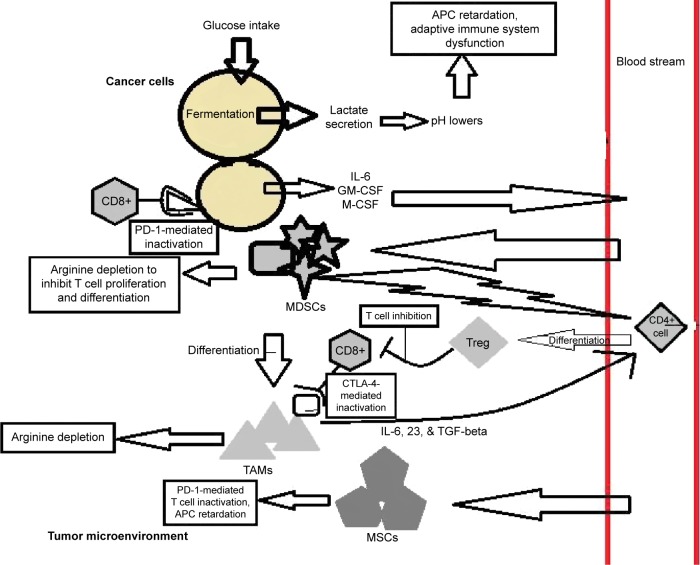
This review combines the recent research on the subject of tumor immunology and methods of correcting the immune system’s reaction to the tumor microenvironment while impeding the survival and growth of tumor cells, with a focus on breast cancer. Induction of hypoxia-inducible genes in the microenvironment leads to lowering of its pH. This impedes the adaptive immune response and acts to recruit cells of the immune system, which suppress the immune response. Regulatory T-cells (Tregs), myeloid-derived suppressor cells (MDSCs), and their derivatives coordinate an anti-autoimmunity response and a healing response in concert with tumor-secreted cytokines, enzymes, and antigens. Together, they suppress a proper immune reaction to tumor cells and promote cellular reproduction (Fig. 1). In addition, the hypoxia-inducible response and components of the tumor microenvironment such as cancer-associated fibroblasts (CAFs) also create an ideal environment for tumor growth and metastasis via neoangiogenesis and increased motility. Broad-spectrum chemotherapy drugs are problematic as breast cancer cells develop resistance through selective loss of a novel target and downregulation of apoptotic factors. A better understanding of the tumor microenvironment offers new therapeutic opportunities to rescue the immune response, inhibit cancer cell growth pathways, and subvert the tumor microenvironment with little toxicity and side effects.
Keywords: cytokines, tumor microenvironment, immune system, immunotherapy, breast cancer
Introduction
The innate and adaptive immune responses are essential for combating pathogen infection, rebuilding damaged tissue, and maintaining homeostasis. The immune system is composed largely of macrophages and lymphocytes, including B-cells, CD4+ T-cells, CD8+ cells, and natural killer cells.1,2 The innate immune response is a nonspecific general response to infection used mainly by macrophages and natural killer cells, while the adaptive immune system is a more advanced system in which certain lymphocytes “remember” specific pathogen anti-genic patterns and alert the immune system when triggered.
The macrophage is a constant player in the innate immune system and in giving aid to the adaptive immune system. Macrophages typically serve a sentinel function in various tissues throughout the body as a form of nominal security. For example, macrophages are present in the lung alveoli to prevent pathogens from gaining swift access to the blood. These macrophages phagocytize apoptotic cells and debris and digest them in lysosomes.2 They avoid an autoimmune response by having a system for recognizing cells that are native to the host body. A major example of this is the binding of antigens presented by major histocompatibility complex (MHC-I/II) to antigen-presenting cells’ (APCs’) toll-like receptors. The APCs then express the MHC/antigen complex and a costimulatory molecule to the naïve T-cells to suppress their activation against the normal tissue cells, preventing autoimmune damage.3
If an infection is detected by macrophages, they release cytokines (eg, IL-2) to recruit more leukocytes to the site of infection. Macrophages, differentiated from bone marrow-derived monocytes, fall in two main categories: the proinflammatory M1 macrophages and the anti-inflammatory M2 macrophages. The M1 group is recruited first in the innate immune response by cytokines. Once in the infection site, they release proinflammatory cytokines (eg, IL-23) that increase local blood flow and increase vascular permeability, allowing more leukocyte infiltration. Macrophages and dendritic cells also present antigens for the lymphocytes of the adaptive immune response to read.2 Natural killer cells are recruited to kill pathogens without the need of APCs and are inhibited by adequate concentrations of MHC-I on target cell surfaces in order to separate self from nonself.4 Once the pathogen is no longer detected, M2 macrophages are recruited to suppress the immune response (with cytokines like IL-10) and rebuild the damaged tissue with the help of transforming growth factor beta (TGF-beta) to induce cell proliferation and differentiation.5
A key factor in the adaptive immune system is the recognition of antigens. All microbes, cells, cancer cells, and other pathogens possess antigens. As explained earlier, MHC complexes present cell antigens for APCs to copy and express themselves. The APCs then present this MHC/antigen complex with a costimulatory molecule to activate or suppress naïve T-cells, depending on the nature of the antigens.3 Although derived from normal cells, cancer cells have enough mutations to significantly alter their antigenic peptide sequences and become immunogenic.6 If the antigen is recognized as pathogenic, the T-cells release cytokines to allow themselves to differentiate into cytotoxic phenotypes and then secrete chemokines to recruit more leukocytes from the bloodstream. B-cells also produce complementary antibodies to help target the pathogen for destruction if its antigens are recognized from past infections.7
Many of these functions are dysregulated in cancer. Tumor cells and their cohorts secrete agents that induce immunological tolerance (eg, lactic acid, indoleamine 2,3-dioxygenase [IDO], and various cytokines), attract immunosuppressive immune cells such as M2 macrophages, alter their cell attributes to avoid notice (eg, by suppressing antigen presentation or becoming elusive mesenchymal-like cells), and skew immune cell function by triggering immunosuppressive pathways. Simultaneously, they constitutively proliferate by upregulating signaling pathways that trigger growth (eg, the estrogen-induced growth pathway in breast cancer). As a result, there are many factors at play that have to be considered in breast cancer therapy in order to better improve patient survival, tumor reduction, and overall well-being.
Respiration Alterations in the Tumor Microenvironment
The work of Husain et al shows that tumor-secreted non-cytokine chemicals recruit immune suppressor cells to the tumor’s microenvironment.8 Ordinarily in mammals, under aerobic conditions, glucose is taken into the cells by glucose transporters (GLUTs), metabolized through the Embden– Meyerhof–Parnas (EMP) glycolysis pathway to produce two molecules of pyruvate and two molecules of adenosine triphosphate (ATP) per molecule of glucose. The pyruvate is then modified to acetyl-CoA and fed into the mitochondria for utilization by the aerobic respiration pathway (also known as the tricarboxylic acid cycle or Kreb’s cycle) where oxygen is the terminal electron acceptor. However, in cancer cells under aerobic conditions, GLUT activity is upregulated and the pyruvate undergoes fermentation to produce lactate, an acidic waste product. This fermentative alternate metabolism in the presence of oxygen is facilitated by the enzyme lactate dehydrogenase-A (LDH-A) and is called the Warburg effect.9,10 Incidentally, this process also gives cancer the ability to lower the microenvironment’s pH.8
By imitating hypoxic conditions, tumor cells significantly upregulate expression of hypoxia-inducible factor 1-alpha (HIF-1-alpha) and nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-kB). These are transcription factors expressed in hypoxic conditions to trigger expression of LDH-A to ferment pyruvate to lactate. Tumor cells do this by producing reactive oxygen species (ROS) in the tumor microenvironment in order to induce a hypoxia response.11 Upregulation of HIF-1-alpha also has the effect of increasing expression of GLUT proteins and monocarboxylate transporters (MCTs), in order to increase glucose flux through the respiratory cycles to compensate for loss of some of the ATP production from fermentation (as opposed to aerobic respiration) and to efflux lactate, a monocarboxylate, to prevent lactate buildup in the cytosol.9,12 It has been hypothesized that lactate is not just a waste product of cellular metabolism but serves another purpose. Husain et al8,12 performed a detailed investigation into the role of lactate in the tumor microenvironment. They found that lactate aids in the recruitment of myeloid-deprived suppressor cells (MDSCs), which in turn induce immunosuppressive regulatory T-cells (Tregs), leading to overall suppression of the immune response to tumors.
The oxidative stress induced by HIF-1-alpha also induces cancer cells to undergo autophagy. Autophagy is further activated by the upregulation of glutaminolysis, which causes cancer cells to consume excess glutamine and secrete ammonia (another trigger of the hypoxia response) into the microenvironment.10 Autophagy allows cancer cells to consume secreted lactate and other secreted chemicals (eg, glutamate produced by glutaminolysis) and put them through gluconeogenesis to derive more glucose in a process normally operative under starvation conditions.10 The induced autophagy also facilitates consumption of degraded organelles and neighboring cell components by tumor cells to further fuel their progression.10
The Role of Lactate
It has been established that MDSCs, Tregs, and M2 macrophages are recruited to tumors to aid them in immune system escape. Previous experiments have shown that secreted factors like vascular endothelial growth factor (VEGF), IL-10, and TGF-beta aid in the recruitment of immunosuppressive cells, but the role of lactate in this process has only recently been described. It has also been established in the past that the Warburg effect in tumors results in retarded maturation of APCs, a key middleman in alerting naïve T-cells to a pathogen.8 Now, the two studies were linked directly. To test whether lactate production by tumor cells and extrusion into the tumor microenvironment results in tumor growth, Husain et al8 used a lentiviral shRNA system to knockdown LDH-A in tumor cells, while the control cancer cells had empty shRNA vectors. Mice were injected with pancreatic cancer cells modified with either LDH-A shRNA or empty shRNA. It was found that the LDH-A knockdown tumors were smaller, had significantly more natural killer cell toxicity in the tumor microenvironment, and significantly reduced MDSCs in the microenvironment (indicated by the presence of antigens CD11b and Gr1 in mice and CD33 in human beings, according to Gabrilovich and Nagaraj).8,13 These results were bolstered by in vitro experiments, where isolated human and mouse natural killer cells were treated with lactate at varying ratios of effector to target. In most cases, lactate-treated cells had significantly lowered cytotoxicity through knockdown of the expression of cytolytic molecules (eg, granzyme, a protease that induces cell apoptosis). Mouse-derived MDSCs also significantly reduced CD4+ T-cell proliferation and expression of CD8+ T-cell activity. It was also found that treating mouse bone marrow cells with lactate in addition to two other known factors of MDSC differentiation (IL-6 and GM-CSF) significantly increased the presence of MDSCs compared to the two other factors alone as well as significantly augmenting suppression of natural killer cell cytotoxicity.
In an attempt to lower lactate production by altering the diet, Husain et al gave a group of mice a ketogenic diet (low carbohydrate) to reduce glucose flux. Although it had no statistically significant impact on tumor volume, it significantly increased the presence of CD4+ and CD8+ T-cells and significantly decreased the presence of MDSCs and Tregs as determined by flow cytometry.12 The LDH-A knockdown had the most effect overall, but shRNA therapy is tricky in patients as it can have off-target cytotoxic effects, especially on the nervous system. One method that shows promise is the placing of the sequences of shRNA, which cause toxicity into miRNA scaffolding and expressing this complex using an adeno-associated virus vector in vivo.14 Use of the CRISPR/Cas system to effectively delete hypoxia-inducing genes in cancer cells also presents a highly promising prospect for future cancer therapy research.
Marchiq et al performed knockdown of MCTs and/or basigin (BSG) to find the right combination that would cause cancer cells to undergo a deadly combination of ATP production collapse and oxidative stress. Knockdown of MCT4 sensitized the cells to MCT1 inhibitors (eg, AZD3965) and greatly reduced lactate efflux. BSG knockdown greatly reduced both MCT1 and MCT4 activity significantly as they are both dependent on BSG for proper protein folding and delivery to the cell membrane.9 However, in both cases, the cancer cells compensated for the excess of lactate and pyruvate by ramping up oxidative phosphorylation in the mitochondria to process pyruvate. In order to block this alternative metabolic pathway, Marchiq et al treated the BSG-null cells with MCT inhibitors and the benign antidiabetic drugs such as metformin and phenformin, known for inhibiting mitochondrial complex I. This caused the cell supply of ATP to fall by 80% (by 60% without the MCT inhibitors). As a result, the cancer cells underwent necrotic death as they were enervated, thus providing a novel anticancer therapy option.9
Lactate has also been shown to trigger cells in the microenvironment to stimulate chronic inflammation and angiogenesis, to damage the surrounding tissue and promote tumor progression. Lactate induces T-cells and macrophages in the microenvironment to secrete the cytokine IL-17a, triggering inflammation that increases blood flow to the microenvironment and augments vascular permeability.15 Lactate also induces endothelial cells in the microenvironment to secrete VEGF to cause growth of blood vessels to the microenvironment (normally this happens in hypoxic conditions) to provide more oxygen for the cancer cells’ respiration and to give them a route through which to metastasize to another part of the body.15 This creates an even greater incentive to knockdown lactate production in therapeutic regimens.
The Role of IDO
The catabolic enzyme indoleamine-2,3-dioxygenase (IDO) in the tumor microenvironment plays a role in immune escape. Normally, IDO catalyzes the conversion of tryptophan to kynurenine to inhibit T-cell activation to help prevent an immune response to the mammalian fetus during pregnancy.16 Unlike other forms of immune suppression, IDO is not used to prevent autoimmunity but to create tolerance toward a necessary nonself, such as an embryo or a tissue graft. This has been demonstrated in experiments with pregnant mice where inhibition of IDO expression led to the fetus eliciting an immune response from the parent.16 IDO-induced immunological tolerance is achieved by depleting the microenvironment of tryptophan, a key nutrient required by T-cells for proliferation upon being presented as nonself antigens by APCs, and by producing kynurenine, a catabolite that helps suppress T-cell activation and recruits Tregs.16 Owing to these effects, it is no surprise that IDO is often found to be overexpressed in many types of cancer, particularly breast cancer. It is found to be significantly upregulated in both cancer cells and in tumor-associated dendritic cells; the former directly inactivates T-cells, while the latter induces CD4+ cells to become Tregs.16 Cancer cells upregulate IDO by inhibiting expression of the tumor suppressor gene Bin1, which encodes a protein that blocks transcription factors (such as STAT and NF-kB) from switching on IDO gene transcription.16 The IDO inhibitor 1-methyl-tryptophan (1MT) was effective in inhibiting breast cancer growth in mouse models and showed signs of regression if combined with the chemotherapy drug, paclitaxel.16
The Role of Estrogen
Estrogen receptor-alpha-positive (ER-alpha+) breast cancer represents roughly 70% of breast cancer cases, and the corresponding hormone estrogen plays a unique role in the survival and progression of breast cancer cells not seen in most other forms of cancer.17 Estrogen is a key growth factor in healthy mammary cells, and this attribute is abused by cancer. In ERalpha+ cells, estrogen receptors (ERs) are upregulated by Notch signaling in the tumor microenvironment compared to healthy cells, eliciting a powerful pro-growth (increased cell division) and anti-immune response.18 The strongest agent in the immune escape of ER-alpha+ cells is the estrogen-dependent molecule proteinase inhibitor-9 (PI-9).17 This molecule inhibits the protease, granzyme B, in cytotoxic T lymphocytes (CTLs) and natural killer cells from inducing apoptosis in the cancer cells.17 Therefore, a normally highly effective antitumor response is rendered impotent. Combined with the pro-proliferation response induced by estrogen, ER-alpha+ tumor cells develop their characteristic malignancy and resilience. There is a class of selective ER modulator (SERM) drugs already widely used, but the majority of patients who undergo this treatment show increased SERM resistance over time as the tumor relies on other pathways that induce growth and immune escape.17 Furthermore, there are ER-negative breast cancers such as triple-negative breast cancer (TNBC) (which lacks three of the most novel receptor targets) that further increase the need for personally tailored therapeutic strategies.
The Role of Fibroblasts in the Tumor Microenvironment
Another component of the tumor microenvironment is the population of fibroblasts known as carcinoma-associated fibroblasts (CAFs). Normally, fibroblasts are associated with construction of the extracellular matrix through secretion of molecular structures like proteoglycan, collagen, vimentin, and actin (depending on the type of fibroblast). Tumor microenvironment-secreted TGF-beta and platelet-derived growth factor (PDGF) induce local fibroblasts and their bone marrow-derived precursors to become CAFs.13 CAFs represent a motley assortment of fibroblasts, including some that secrete muscle actin fibers, some that secrete collagen, and some that secrete proteoglycan, as proved by fibroblast marker assays in tumors.19 By secreting these extracellular matrix molecules, CAFs remodel the tumor microenvironment to aid in organizing the outgrowth of the tumors, store growth factors, and epigenetically disrupt surrounding healthy tissue.20 CAFs can also play a housekeeping role in the tumor microenvironment by removing toxic metabolites produced by the cancer cells.21 They also produce a variety of pleiotropic tumor progression factors.
When induced by cell membrane-bound Notch receptor-ligand signaling, breast cancer CAFs secrete VEGF and fibroblast growth factor (FGF) in the presence of HIF-1-alpha signaling to induce circulatory system endothelial cells to the tumor space for neoangiogenesis.18,21 The capillaries made by neoangiogenesis draw blood to the tumor with an abundance of oxygen and nutrients needed for their excessive growth patterns. CAFs increase their numbers in the microenvironment by secreting the chemokine CXCL12 into the blood stream to attract more fibroblasts to the tumor space.21 Furthermore, the Notch signaling cascade also upregulates the expression of mitogenic cyclin D1, antiapoptotic survivin, growth-inducing ER, and human epidermal growth factor receptor 2 (HER2).18 CAFs also assist the tumor in the recruitment of tumor-associated macrophages (TAMs), MDSCs, and Tregs to the microenvironment by secreting the chemokine CXCL14.21 Even more detrimentally, they can augment cancer cell motility and metastasis through the production of the protease MMP2 to allow cancer cells to become more mesenchymal-like, secretion of the growth factors TGF-beta and insulin growth factor (IGF), production of the inflammation-inducing extracellular matrix glycoprotein osteopontin, and secretion of the chemokines CXCL12 and CCL5.21 More specific to breast cancer, CAFs secrete FGF-2 and FGF receptor 2 in order to constitutively activate breast cancer cell progesterone receptors (PRs) and induce malignant tumor growth independent of the host’s hormone secretion levels.22 They also use the Wnt/beta-catenin signaling pathway to induce adipocytes to become fibroblasts and develop an extracellular matrix conducive to the movement of metastatic cells.18 Finally, and perhaps most deviously, Notch signaling by breast cancer CAFs has the downstream effect of inducing the antioncogenic p53 protein in noncancer stromal cells.18 This decreases mitosis of healthy stromal cells, increases their senescence, and makes it easier for the tumor to infiltrate the surrounding tissue. This dangerous diversity of pro-progression and malignancy factors contributed by CAFs make them an attractive novel target in cancer therapeutics.
There are a variety of ways to combat CAFs in vivo. Clinical inhibition of the tyrosine kinase activities of PDGF receptors (key to the recruitment of CAFs) by drugs such as sorafenib, sunitinib, and imatinib are under development and may prevent the recruitment of CAFs to the tumor microenvironment.21 Also, there are ER, PR, and HER2 inhibitors available such as lapatinib ditosylate, trastuzumab, toremifene, and megestrol acetate to reduce CAF growth and chemoresistance.18 Further back in the drug development pipeline are pharmacological inhibitors of TGF-beta and CXCL14 activity, which would block two key secretions of CAFs in tumor progression.21 In studies reported by Giulianelli et al, mice treated with drug PD173074, which inhibits FGF receptor (FGFR) tyrosine kinase activity, prevented the constitutive promotion of breast cancer malignancy.22 Another very promising therapeutic strategy being developed is the targeting of Notch signaling in breast cancer cells and their CAFs in order to downregulate the pro-growth, pro-survival, and pro-metastasis pathways described earlier. The oral drug R04929097 is in phase I testing, and it blocks Notch signaling by inhibiting the functionality of gamma-protease (the integral membrane enzyme that assembles Notch across the cell membrane).18 It shows promise, but makes the patient ill because of the reduction of other gamma-protease-dependent proteins. In order to reduce these off-target effects, another target for inhibition being proposed is of the breast cancer-specific Notch-induced transcription factor CSL.18 Indeed, such drugs that inhibit the functionality of CAFs would have a broad effect of crippling the tumor microenvironment and rendering the tumor more isolated and vulnerable. Targeting upstream pathways like Notch can have positive effects on prognosis even for patients with TNBC (characterized by the absence of the novel targets ER, HER2, and PR) by also removing survivin and cyclin D1 from the equation.18
The Role of the Epithelial–Mesenchymal Transition
Another issue that needs to be dealt with is the use of epithelial–mesenchymal transition (EMT) by cancer cells to promote their migration and metastasis,11 a deadly feature of breast cancer. EMT is normally induced during wound healing where epithelial cells near the region of damage gain mesenchymal traits and migrate into the wound to help replace the damaged cells.23 This is induced by upregulation of TGF-beta, FGFs, and PDGFs to induce expression of the EMT-inducing factor Snail,24 downregulation of E-cadherin expression, expression of Snail by upregulation of HIF-1-alpha, and solid stress and alterations of the extracellular matrix.11,23 In order to block EMT of cancer cells, Marcucci et al suggest inhibition of the hedgehog signaling pathway, an upstream regulator of Snail and EMT, and inhibition of the AMPK pathway to prevent autophagy.11
EMT is also facilitated by mesenchymal stromal cells (MSCs) in the tumor microenvironment. The TGF-beta and VEGF induction pathways associated with MSCs have a role in reducing E-cadherin expression and augmenting mesenchyme markers in their associated cells.4 This combined with the pro-angiogenic properties of MSCs can aid in breast cancer cell dispersal and metastasis, rendering epithelial mammary cancer cells motile. These mesenchymal-like cancer cells can enter the vasculature extended by angiogenesis and travel to other compartments in the body to form secondary tumors. Making matters worse, cancer cells that undergo EMT show augmented chemoresistance and have altered antigenic properties that the adaptive immune system does not recognize as cancer cells.23
Antiangiogenesis in Tumor Therapy
One common function of the tumor microenvironment is the induction of angiogenesis by the triggering of hypoxia responses (eg, through expression of HIF-1-alpha and the production of ROS), which regulate the secretion of growth factors such as VEGFs, FGFs, and HGFs by MDSCs, TAMs, and CAFs. Angiogenesis is a key process in embryogenesis and wound healing by developing vasculature to develop areas of the body in the former and restoring blood vessels to areas of tissue damage in the latter.25 It is critical in wound healing so as to feed the regrown tissue and prevent tissue necrosis, which is potentially deadly as it leads to severe bacterial infection. However, angiogenesis is usurped by cancer in order to promote growth and metastasis. Although chaotic and not architecturally well set up, angiogenesis in the tumor vicinity is nonetheless effective in providing fast growing tumor cells with an essential supply line of much needed nutrients. To distinguish this process from normal blood vessel construction, cancer-induced angiogenesis is also known as neoangiogenesis. The CAFs and TAMs secrete large amounts of VEGF, while tumor-associated endothelial cells produce large amounts of VEGF receptors (VEGFRs) to constitutively activate angiogenesis, producing primitive and leaky blood vessels to provide the tumor with a glut of oxygen and nutrients as well as to provide an avenue through which they can disperse and metastasize.25 Therefore, antiangiogenesis is a prominent area of research in cancer therapy. The first antiangiogenic drug, bevacizumab, was approved in 2003 and was developed from an anti-VEGF neutralizing monoclonal antibody regimen that was shown to bind to and inhibit the activity of metastatic CRC-secreted VEGF in mouse models.25 It was later expanded for use in patients with metastatic non-small cell lung cancer and breast cancer. This opened the door for more antiangiogenic drugs that target the process from different angles. For instance, sorafenib and sunitinib inhibit the tyrosine kinase activation of VEGFRs, preventing the induction of angiogenesis in tumor-associated endothelial cells.25 Another set of drugs called vascular disrupting agents bypass the role of VEGF by destroying the blood vessels extended to the tumor space. ASA404, for example, induces apoptosis of tumor-associated endothelial cells and destroys the tumor’s vasculature, effectively starving the cancer cells.25
Antiangiogenesis drugs, like all cancer drugs, ultimately show signs of failure as some cancers develop resistance. Two common methods of resistance to antiangiogenic drugs are the secretion of factors to augment VEGF binding to try to outcompete antibodies for VEGFRs and the use of alternative angiogenic pathways (eg, Notch–Delta interactions).25 The tumor microenvironment plays an indispensable role in conferring antiangiogenesis resistance by such means as upregulating EGFR to induce endothelial cell proliferation, MDSC-secreted granulocyte colony-stimulating factor induction of the alternative angiogenesis factor Bv8, and CAF-secreted PDGF and CXCL12.20 Pharmacological inhibitors for these alternate pathways exist and can be combined with VEGF inhibition for a more effective method of antiangiogenic therapy. RNA therapy to downregulate tumor angiogenesis-related genes and tumor-directed micelle delivery of anti-angiogenic agents is also in early development.25,26
Survival and Immune Tolerance
Estrogen-dependent expression of PI-9 and Notch-dependent expression of survivin have already been described as paracrine-induced methods of breast cancer immune escape and anti-apoptosis, but there are also intrinsic factors to consider. One of these is the downregulation of BAX-alpha in breast cancer cells, a key factor in the apoptotic cascade triggered by the binding of Fas to the Fas ligand.17 Breast cancer also further prevents cell death by raising expression of the immune suppression ligands, programmed death ligand 1 (PD-L1) and HLA-E (or G), which bind to their receptors on antitumor lymphocytes to negate an effective immune response.17 Also augmented is the expression of the soluble molecule MICA, which degrades the granzyme B of natural killer cells independent of estrogen.17 Rescuing these expression modulations through miRNA therapy or ectopic expression has been found to restore the proper antitumor immune response and make breast cancer cells more susceptible to apoptosis-triggering chemotherapy. However, many of these breast cancer cell traits are also recapitulated in the MDSCs and Tregs recruited to the tumor space and have to be accounted for as well in any therapeutic regimen.
MDSCs in the Tumor Microenvironment
MDSCs are a diverse collection of less-differentiated macrophages, granulocytes, and dendritic cells that have the ability to suppress the immune response.8 Differentiated from hematopoietic stem cells, they are blocked from final differentiation by tumor-secreted factors such as IL-6, GM-CSF, and M-CSF. These trigger the JAK2/STAT3 signal transduction pathway in these progenitor cells to inhibit further differentiation.13 As a result, there is a tenfold increase in MDSC concentration in the blood of cancer patients versus healthy patients. Once recruited to the tumor microenvironment, most take on a granulocytic phenotype, which have an upregulated STAT3 pathway that allows them to produce large amounts of ROS and ARG1 to cause posttranslational modification of T-cell receptors to inactivate the T-cells.13 The other subsets, monocytic MDSCs, have an upregulated STAT1 pathway to express Arg1 and iNOS, two enzymes that deplete arginine (the latter making nitric oxide from it) in order to nonspecifically stunt T-cell functionality and proliferation, as well as to promote damaging chronic inflammation.8,27 Serafini et al27 found that Arg1 activity seemed to have the effect of halting CD8+ T-cell proliferation and recruiting Tregs to the tumor microenvironment. MDSCs also express the receptor IL4R-alpha and TGF-beta, which induce anergy in tumor-specific T-cells.27
In metastatic human breast cancer grown in Severe Combined Immunodeficient mice lacking functional B and T lymphocytes, it was found that the induced expression of IL-6 by the cancer cells recruited MDSCs to the primary tumor microenvironment and to the spleen, liver, and metastatic lung compartments.28 These IL-6-induced MDSCs in turn secrete IL-6 and soluble IL-6Ralpha into the microenvironment, creating a feedback loop of augmented IL-6 expression as seen in vivo and in vitro.28 ADAM proteases secreted by the MDSCs in the microenvironment also induced cells to shed the membrane IL-6Ralpha in order to make more soluble IL-6Ralpha.28 This IL-6 feedback loop was observed in metastasizing cancer cells but not in nonmetastasizing cancer cells. These findings elucidated IL-6 signaling as an upstream indicator of breast cancer aggressiveness and metastasis and a novel target in breast cancer therapy. The MDSCs also phosphorylate components of the STAT3 pathway in the microenvironment to inactivate T-cells in addition to the other protumorigenic activities of recruited MDSCs.13,28 MDSCs have also been found to nitrate MHC-I molecules on breast cancer cells to prevent them from presenting antigens to cytotoxic T-cells, making the cells resistant to the immune system.17
The Role of Regulatory T-cells
Recruited to the tumor microenvironment by cytokines secreted by MDSCs and their derivatives, Tregs further augment the immunosuppressive activities of the microenvironment. Tregs are seemingly further selected by significant production of ROS secreted by MDSCs, chemicals that cause damaging inflammation to the region, but against which Tregs and MDSCs are resilient. Some studies indicate that the ROS also helps induce the immunosuppressive activities of Tregs in the microenvironment.29 In mouse cancer models, CD4+ helper T-cells were found to be induced to differentiate into Tregs by upregulation of CTLA-4, a helper T-cell receptor that suppresses other T-cells.13 Marked with the antigen FoxP3, these Tregs are tasked with blocking tumor-specific T-cell activation. This function is normally reserved for preventing autoimmunity, but in this case, it complements the role of MDSCs in blocking the antitumor immune response.
In breast cancer, the glycan-binding protein galectin-1 (Gal1) has been positively correlated to Treg counts and immunosuppression in the tumor microenvironment.30 Gal1 secreted in the cancer microenvironment (by tumor and stromal cells) interacts with the surface glycoproteins of immune cells and skews them toward immune suppression by lectin– glycan binding.30 This activity was found in both the primary tumor site and lung metastatic compartments. Gal1 was also found to downregulate antitumor effector T-cells and to be upregulated by TGF-beta, a common cytokine in the tumor microenvironment.30
Anti-MDSC and Anti-Treg Therapies
In a study reported by Serafini et al, sildenafil mesylate (commercially known as Viagra®) was able to reduce expression of IL-4Ralpha, iNOS, Arg1, and induction of Tregs by MDSCs as part of its off-target effect of inhibiting immunosuppressive pathways.27 Blocking the IL-6 signaling pathway with anti-IL-6R antibodies or with the protein gp130-Fc has also been demonstrated to reduce the activity of the STAT3 pathway and neutralize the potency of the MDSCs in the tumor microenvironment.28 Deficiency of vitamin A and/or its metabolite retinoic acid was common in patients and mouse models with high MDSC, impeding their differentiation. Administration of all-trans retinoic acid was shown to rescue this deficiency and resulted in the MDSCs either differentiating into dendritic cells or undergoing apoptosis.13,29 A more drastic measure is use of the chemotherapy drug gemcitabine, which has been shown in mouse models to decimate the MDSC count and rescue the antitumor immune response.13 The use of anti-Gr1 antibodies in murine models has also been shown to deplete MDSC counts in the lungs and prevent the formation of metastatic outgrowth there.28 There is general agreement that therapeutically targeting MDSCs can lead to more positive patient prognoses.
In murine and in vitro models, inhibition of Gal1 in the primary breast cancer tumor decreases Treg counts, which can in turn reverse immunosuppression activity and help prevent the cancer from metastasizing.30 However, drugs that block Gal1 also target other, non-cancer-related lectin–glycan interactions. Engineering a neutralizing antibody that specifically targets Gal1 that is delivered to the primary tumor could be highly effective.30 Effective inhibition of Gal1 would restore the balance in favor of antitumor immune cell phenotypes in order to isolate and shrink the primary tumor.
Immunotherapy in Breast Cancer Cells
Vaccines that immunologically target breast cancer cells are a common therapeutic utilized today. One class of these is monoclonal antibodies, an immune system agent that can be engineered to specifically target certain receptor activation by blocking ligand binding or preventing receptor dimerization.31 Since antibodies can be designed with extremely high specificity and binding strength, they can have very few off-target effects on patients and thus are a rapidly growing field in immunotherapy. They also have the added benefit of making it easier for CTLs and natural killer cells to target cancer cells. An effective early discovery was trastuzumab, an approved antibody drug that binds to domain IV of HER2 and prevents its dimerization with HER3, which therefore inhibits mitogenesis.31 Trastuzumab has been determined to have an incredible combinatorial effect with other drugs. It has been found to have an increased effect when combined with pertuzumab, an antibody that binds to domain II of HER2 and inhibits dimerization, by further decreasing HER2 activation without significantly increasing toxicity.31 Trastuzumab can even be made to target trastuzumab-resistant breast cancer cells by conjugating it with the drug emtansine, which enters the cancer cell and breaks down its microtubules.31 Another method of overcoming trastuzumab resistance is the inhibition of pro-growth IGF-1R activity through antibodies like dalotuzumab (currently in phase I trials) in combination with trastuzumab, which has shown promise.31 Strong inhibition of HER2 activity, however, appears to increase HER3 activity, HER2’s heterodimer that directly triggers the mitogenic signaling cascade.31 This requires the development of anti-HER3 antibody drugs. None are currently on the market, but phase I trials are occurring for antibodies like MM-111 and U3-1287 that block HER3 activity.31 There are no conclusive results yet, but the combination of anti-HER2 and anti-HER3 antibodies could provide an extremely effective therapeutic regimen that can go with chemotherapy. However, all the above only has a significant effect with HER2+ breast cancer cases (~20% of all cases).31 Therefore, other considerations need to be made for other subtypes of breast cancer, such as TNBC.
A novel target in TNBC cancer patients is the growth-inducing EGFR receptor, which is upregulated in about half of the TNBC patients.31 Anti-EGFR monoclonal antibodies, cetuximab and panitumumab, are in phase II trials with chemotherapy drugs, but limited response was seen in the former drug and tests are inconclusive in the latter drug at this time.31 Further results combined with a better understanding of EGFR’s structure and likely weaknesses are crucial in developing new antibody-related therapy that significantly improves the prognosis of TNBC patients. One possible way of going about this is the development of neutralizing antibodies that bind to the EGFR and negate its tyrosine kinase activity. Also early in the pipeline for both TNBC and HER2+ breast cancer are anti-PD-1 and anti-PD-L1 antibodies.31 Constitutive activation of the PD-1 pathway in nearby T-cells is one key way by which breast cancer avoids the immune response, and it has been found in vitro that blockage of this pathway restores the antitumor activities of T-cells. Antibodies, such as MPDL3280A, were developed for this purpose, but there are no conclusive results yet.31 Just like with EGFR, anti-PD-1/anti-PD-L1 neutralizing antibodies could also be looked at as a possible therapeutic. Neutralizing or monoclonal antibodies that target and negate CTLA-4 receptors on tumor microenvironment T-cells should also be looked at as an immunotherapeutic way of restoring the antitumor immune response and could be combined with anti-PD-1 drugs to have a more pronounced effect.
Other forms of vaccine drugs are being looked at in the long fight against breast cancer. The design of tumor-associated antigen (TAA) peptides that elicit an immune response is a likely candidate.32 They are easy to manufacture, easy to deliver to the tumor space, and have low toxicity. However, they require coadministration with an immunological adjuvant, can only target certain immune cells (eg, CD4+ or CD8+), are limited to only HLA-A2 MHC-I complex antigens, and have a short half-life.32 Work on broadening their effect to trigger both CD4+ and CD8+ T-cells is something currently being worked on in order to make this line of therapy more potentially viable. Also being worked on are methods of programing the TAA peptides into dendritic cells through techniques such as transfection so as to trigger a broad antitumor response and commit the TAAs to immunological memory.32 Treating breast cancer cells with DNA vectors also has a lot of potential. Delivered in viral vectors, lipoproteins, or engineered nanoparticles, the breast cancer DNA can be recombined to upregulate expression of antigens that trigger a broad antitumor immune response.32 One promising early example is the use of poxviral vectors to deliver DNA to breast cancer cells to express modified immunogenic HER2 receptors, triggering an antitumor immune response.32 Finally, and most simply, is the possibility of using cancer cells themselves to vaccinate patients. Here, breast cancer cells would be taken from the patient or from a cell line, programed to express immunogenic molecules, and injected into the patient to elicit a broad and complex antitumor immune response.32 The drawback here is that it will be hard to monitor whether the inoculation was successful and is receiving the desired response. Nonetheless, the numerous forms of immunotherapy being explored seem to promise new drugs that increase patient survival with little added toxicity.
Concluding Remarks
Hypoxia-like conditions stimulated by cancer cells lead to overproduction of lactate, which suppresses the adaptive immune system from being activated in response to the cancer. Metabolic products secreted by tumor cells aid in the recruitment of MDSCs and Tregs to help maintain immunosuppression in the tumor space through methods such as arginine depletion and cytokine pathways (Fig. 1). Induction of hypoxia responses also causes the cancer cells to commit autophagy to further increase their nutrient consumption. The Warburg effect also contributes to chronic inflammation to increase blood flow to the vicinity and attract MSCs to be sequestered in the microenvironment. Some of the MDSCs also differentiate into immunosuppressive TAMs that promote M2-like wound-healing proliferation and M1-like chronic inflammation. The hypoxia-like conditions in the tumor microenvironment also induces endothelial cells and MSCs to secrete angiogenic factors to bring capillaries to help nourish and facilitate possible metastatic dissemination of the tumor. Also, CAFs are at play, which secrete numerous pleiotropic factors that promote tumor growth and metastasis. Metastasis is further assisted by the MSC-mediated EMT process and the creation of cancer stem cells that can restore the cancer after it has been decimated by chemotherapy and radiotherapy. Some cancer types also have ligands on their cell membranes that trigger an immune inactivation response by interacting with CD8+ cell, CTLA-4 and PD-1 receptors. TAMs also express these immunosuppressive ligands.
Figure 1.
Summary of the various immune suppression activities by cancer cells and the cancer microenvironment. Actions that directly trigger immune suppression are boxed.
The survival and proliferation strategies adopted by tumor cells need to be undone by picking apart interactions between tumor cells and other cell types in the tumor microenvironment. Blocking of CD4+ differentiation into Tregs, terminal differentiation of monocytes to M1 macrophages, inhibiting MCT and mitochondrial complex I activity, rescuing p53 deficiency to prevent dysregulation of MSCs and cancer cells, blocking lymphocyte inactivation receptors, inhibition of the recruitment or functionality of CAFs, blocking key cancer signaling pathways, and knockdown of chronic inflammation and arginine depletion are all very viable proposals to treat cancer in the future. The traditional broad-spectrum chemotherapy methods only serve to buy a little more time for the patient. In order to give cancer patients significantly longer, healthier, and more comfortable lives, treatment strategies have to be tailored toward each cancer type to minimize off-target effects, prevent metastasis, and maximize cancer cell destruction as long-term goals rather than merely sending the patient into temporary remission. Newer therapeutic opportunities such as those presented by immunotherapy may complement chemotherapy without adding to the patient’s toxicity burden. If it advances far enough, most forms of breast cancer will be undermined and more patients will live longer, healthier lives.
Acknowledgments
This review was written in part fulfillment of a Master’s degree.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1043 words, excluding any confidential comments to the academic editor.
FUNDING: DB was supported by the Epstein Fund. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: EDR and DB. Agreed with manuscript conclusions: EDR and DB. Made critical revisions and approved the final version: EDR and DB. Both authors reviewed and approved the final manuscript.
REFERENCES
- 1.Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21(1):3–10. doi: 10.1016/j.cytogfr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torroella-Kouri M, Rodríguez D, Caso R. Alterations in macrophages and monocytes from tumor-bearing mice: evidence of local and systemic immune impairment. Immunol Res. 2013;57(1–3):86–98. doi: 10.1007/s12026-013-8438-3. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Janeway CA. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Hou J, Han Z, et al. One cell, multiple roles: contribution of mesenchymal stem cells to tumor development in tumor microenvironment. Cell Biosci. 2013;3(5):1–10. doi: 10.1186/2045-3701-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edin S, Wikberg ML, Rutegård J, Oldenborg PA, Palmqvist R. Phenotypic skewing of macrophages in vitro by secreted factors from colorectal cancer cells. PLoS One. 2013;8(9):1–10. doi: 10.1371/journal.pone.0074982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. [Google Scholar]
- 7.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191(3):1486–1495. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 9.Marchiq I, Le Floch R, Roux D, Simon MP, Pouyssegur J. Genetic disruption of lactate/H+ symporters (MCTs) and their subunit CD147/BASIGIN sensitizes glycolytic tumor cells to phenformin. Cancer Res. 2014;75(1):171–180. doi: 10.1158/0008-5472.CAN-14-2260. [DOI] [PubMed] [Google Scholar]
- 10.Sotgia F, Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Lisanti MP. Understanding the Warburg effect and the prognostic value of stromal caveolin-1 as a marker of a lethal tumor microenvironment. Breast Cancer Res. 2011;13:213. doi: 10.1186/bcr2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcucci F, Bellone M, Caserta CA, Corti A. Pushing tumor cells towards a malignant phenotype: stimuli from the microenvironment, intercellular communications and alternative roads. Int J Cancer. 2013;135(6):1265–1276. doi: 10.1002/ijc.28572. [DOI] [PubMed] [Google Scholar]
- 12.Husain Z, Seth P, Sukhatme VP. Tumor-derived lactate and myeloid-derived suppressor cells: linking metabolism to cancer immunology. Oncoimmunology. 2013;2(11):e26383. doi: 10.4161/onci.26383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Nagaraj S. Myeloid-derived-suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBride JL, Boudreau RL, Harper SQ, et al. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi. Proc Natl Acad Sci U S A. 2008;105(15):5868–5873. doi: 10.1073/pnas.0801775105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschhaeuser F, Sattler UGA, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 16.Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27:3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Shapiro DJ. The immune system and inflammation in breast cancer. Mol Cell Endocrinol. 2014;382:673–682. doi: 10.1016/j.mce.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nwabo Kamdje, AH Seke, Etet PF, Vecchio L, et al. New targeted therapies for breast cancer: a focus on tumor microenvironmental signals and chemoresistant breast cancer. World J Clin Cases. 2014;2(12):769–786. doi: 10.12998/wjcc.v2.i12.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5(12):1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 20.Shekhar MPV, Pauley R, Heppner G. Host microenvironment in breast cancer development: extracellular matrix–stromal cell contribution to neoplastic phenotype of epithelial cells in the breast. Breast Cancer Res. 2003;5(3):130–135. doi: 10.1186/bcr580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth— bystanders turning into key players. Curr Opin Genet Dev. 2009;19(1):67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Giulianelli S, Cerliani JP, Lamb CA, et al. Carcinoma-associated fibroblasts activate progesterone receptors and induce hormone independent mammary tumor growth: a role for the FGF-2/FGFR-2 axis. Int J Cancer. 2008;123(11):2518–2531. doi: 10.1002/ijc.23802. [DOI] [PubMed] [Google Scholar]
- 23.Biddle A, Mackenzie IC. Cancer stem cells and EMT in carcinoma. Cancer Metastasis Rev. 2012;31(1):285–293. doi: 10.1007/s10555-012-9345-0. [DOI] [PubMed] [Google Scholar]
- 24.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shojaei F. Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett. 2012;320(2):130–137. doi: 10.1016/j.canlet.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Gong C, Deng S, Wu Q, et al. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials. 2013;34(4):1413–1432. doi: 10.1016/j.biomaterials.2012.10.068. [DOI] [PubMed] [Google Scholar]
- 27.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid derived suppressor cells promote cross-tolerance in B cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh K, Lee OY, Shon SY, et al. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013;15:R79. doi: 10.1186/bcr3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poschke I, Mougiakakos D, Kiessling R. Camouflage and sabotage: tumor escape from the immune system. Cancer Immunol Immunother. 2011;60:1161–1171. doi: 10.1007/s00262-011-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalotto-Moreno T, Croci DO, Cerliani JP, et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013;73(3):1107–1117. doi: 10.1158/0008-5472.CAN-12-2418. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Garcia J, Munoz-Couselo E, Cortes J, Scaltriti M. Therapeutic antibodies in breast cancer. Semin Oncol. 2014;41(5):576–588. doi: 10.1053/j.seminoncol.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Milani A, Sangiolo D, Aglietta M, Valabrega G. Recent advances in the development of breast cancer vaccines. Breast Cancer (Dove Med Press) 2014;6:159–168. doi: 10.2147/BCTT.S38428. [DOI] [PMC free article] [PubMed] [Google Scholar]