Abstract
For patients at high risk for surgery, carotid artery stenting (CAS) is a viable alternative to help reduce risk of stroke for patients with high-grade carotid artery stenosis; however, a higher incidence of perioperative stroke has been observed in patients undergoing stenting compared to those undergoing open surgery. Intravascular ultrasound (IVUS) is commonly used during coronary artery procedures to help evaluate lesions and to guide stent placement. Multiple groups have sought to determine whether IVUS could also be used during CAS. While IVUS has been shown to be both feasible and safe during CAS, there is limited evidence that demonstrates direct improvement in procedural outcomes. Further studies focusing on clinical outcomes should be conducted in order to justify routine use of this technology during CAS.
Keywords: stroke, carotid stenosis, carotid stent, internal carotid artery, intravascular ultrasound, microemboli, ultrasound
Carotid revascularization procedures are effective in decreasing risk of stroke for patients with high-grade carotid artery stenosis. While carotid endarterectomy (CEA) remains the gold standard for treatment, carotid artery stenting (CAS) has emerged as an alternative for patients at high risk for surgery. Results of the CREST trial revealed similar composite end points of stroke, myocardial infarction, and death between CEA and CAS, but CAS had a higher risk of perioperative stoke.1 Stroke is one of the leading causes of death in the United States, and a significant economic impact on our health care systems.2 Improving the outcomes of these procedures and decreasing the risk for stroke in all carotid interventions is an important goal.
One of the major challenges and contributors to risk for stroke during CAS is the manipulation of wires and catheters across the lesion and within the artery. Despite the use of embolic protection devices (EPDs), we and others at our institution (overlapping author groups) have observed a high incidence of microembolization for patients with CAS, as identified on diffusion-weighted magnetic resonance imaging (DW-MRI) performed following stenting.3 4 5 Embolic events may occur during aortic arch manipulation before EPD deployment, or during catheterization of the carotid artery itself.6 Carotid plaques that are vulnerable to rupture have higher embolic risk.7 Identifying vulnerable plaques before interventions can help clinicians with the decisions on the types of interventions, stents, and EPD; however, often these types of lesions cannot be accurately identified by traditional imaging techniques such as computed tomographic angiography, MRI, or duplex ultrasound (DUS).
Intravascular ultrasound (IVUS) has been suggested as an adjunct to CAS to help identify high-risk plaques. IVUS is an imaging modality in which a small ultrasound probe is affixed to the end of a special catheter. This device permits internal imaging of the artery to provide cross-sectional measurements and morphological data. In addition, the virtual histology (VH) mode reveals the histology of the tissue. VH–IVUS identifies the following four tissue subtypes: fibrous, fibrofatty, necrotic, and dense calcium. The CAPITAL (Carotid Artery Plaque Intravascular Ultrasound Evaluation) study outlines different plaque types based on the location and arrangement of these tissue subtypes within a lesion.8 The major descriptive plaque types include pathological intimal thickening, fibroatheroma, calcified fibroatheroma, thin-cap fibroatheroma (TCFA), and calcified thin-cap fibroatheroma (CaTCFA). Using this histological data, VH–IVUS may provide the interventionist with more information about the plaque. This additional information could potentially result in improvement in procedure technique and a reduction of adverse outcomes such as stroke.
IVUS is commonly used during coronary artery procedures to help identify lesion characteristics and guide proper stent placement9; however, use of IVUS remains limited in the carotid artery despite evidence that it is both feasible and safe.10 11 12 13 14 15 Reviews of the use of IVUS in the carotid artery were conducted by Schiro and Wholey16 in 2008, Inglese et al17 in 2009, and Politi et al in 201118; however, several additional prospective studies have since been conducted to examine utility of IVUS during CAS including our own study in 2014. Therefore, herein, we will review the current literature on VH–IVUS in the carotid artery and discuss our own experience. Our focus is primarily on the use of IVUS intraoperatively.
The Technique
The IVUS catheter utilizes ultrasound technology where a small ultrasound probe is mounted on the end of the catheter. There are several types of IVUS catheters available commercially and we largely use a Volcano s5VH Ultrasound Imaging System (Volcano Corporation, Rancho Cordova, CA) along with Eagle Eye Gold (Volcano Corporation) coronary imaging catheters for carotid artery evaluation. The IVUS catheter is a monorail catheter, advancing along a 0.014″ wire. Once the catheter reaches a relatively normal segment of carotid artery distal to the lesion, the VH mode is activated and the catheter is carefully withdrawn at a rate of approximately 1 mm/s. The morphology of the lesion is recorded and analyzed offline. Fig. 1 shows a diseased carotid artery. Grayscale image (Fig. 1A) delineates luminal contour and VH image (Fig. 1B) shows fibrous (green), fibrofatty (light green), necrotic (red), and calcified (white) tissue composition.
Fig. 1.
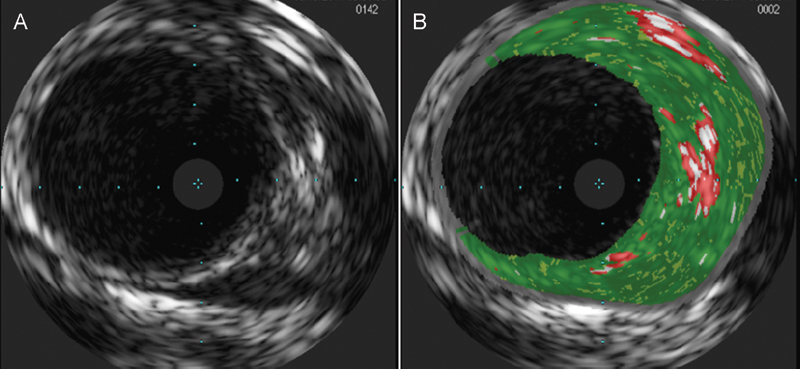
(A) Grayscale and (B) virtual histology images reveal a diseased carotid artery.
The Benefits
Multiple groups have found VH–IVUS to be useful during CAS. IVUS provides a three-dimensional visualization of luminal contour, whereas angiography is limited to the two-dimensional plane. In our own experience, we found the additional information provided by IVUS to be helpful in stent placement for some patients.11 For one patient in our study cohort, a longer stent was chosen after IVUS revealed a more extensive plaque than what was visualized on angiography. Other groups have also modified their treatment after reviewing IVUS imaging.10 14 Bandyk and Armstrong found that IVUS revealed the need to use larger balloons during poststenting angioplasty, as the lumen diameter of the stent was found to be smaller than visualized by angiography. In our study, we performed IVUS before stent placement, so we do not know whether this may have also been the case in our cohort; however, we observed similar results to the study by Bandyk when using IVUS in the lower extremity.19 For patients undergoing superficial femoral artery endovascular treatment, we found that IVUS revealed significant residual stenosis following angioplasty and/or stenting, despite satisfactory angiographic results. Admittedly, it remains unclear whether there is a significant benefit to using larger balloons or performing additional angioplasty to obtain greater lumen diameters. These findings highlight the need for additional long-term follow-up studies and for further endovascular device refinement.
Detection of plaque vulnerability is another important advantage that VH–IVUS can provide. As described in the CAPITAL study, plaque types that include a “thin-cap” arrangement of tissue, including TCFA and CaTCFA, are of highest risk for rupture. Occurrence of cerebral microemboli may indicate presence of such an unstable or vulnerable carotid plaque. Several studies, including our own, looked at incidence of microemboli as an outcome to correlate with VH–IVUS morphologic findings.11 20 21 22 Timaran et al found a relationship between IVUS-identified plaque calcification and incidence of microemboli; however, other groups, including our own, did not replicate this finding. Yamada et al found a correlation between fibrofatty plaque composition and microembolization. Another study by Matsumoto et al found an association between fibrofatty plaque volume and the amount of debris aspirated during stenting. While we did not find any significant correlations between IVUS-defined plaque type and incidence of postoperative microemboli, we did find a trend with fibrous IVUS plaque composition and microemboli (p = 0.099). Together with Yamada and Matsumoto's findings, this suggests that softer plaques are more vulnerable and should be treated with caution.
These studies largely analyzed plaque composition in regard to volume of calcified, fibrous, necrotic, and fibrofatty tissue types, rather than basing analyses on plaque classifications described in the CAPITAL study. In our study, we did look for correlations between thin-capped plaques and incidence of microemboli, but did not find any significant relationships. This may have been because of our small sample size. A more recent study by González et al had a larger cohort and found a relationship between IVUS identified thin-capped plaques and timing of intervention.23 Vulnerable thin-capped plaques were observed more frequently in symptomatic patients undergoing intervention closer to their ischemic episode compared with those who had more delayed treatment or were asymptomatic. This finding suggests that there may be some benefit in allowing a ruptured plaque time to heal before subjecting it to stenting.
VH–IVUS may also be helpful for its offline use to retrospectively characterize carotid lesions. While improving procedural outcomes is the primary goal, a better understanding of carotid plaque morphology could be an indirect benefit. Collecting and analyzing a large series of IVUS images may improve our understanding of carotid artery morphology. Tsurumi et al studied the composition of plaque at the location of the minimal lumen in comparison to the entire plaque and found that the two did not significantly differ.24 In our study, we also did not observe significant differences between the minimal lumen area and the plaque as a whole. Nevertheless, both of our cohorts were relatively small and larger studies would be needed to extend and validate this finding.
The Challenges
In our experience, a big challenge to successfully implementing VH–IVUS during CAS procedures is the lack of automated border detection. Existing IVUS software requires the user to manually correct the lumen and vessel borders on the ultrasound image, which can be time consuming and prone to error. While border editing can be accomplished in a relatively short time frame by a skilled operator, a fully-automated and accurate border detection program would be more practical. A 2012 study by Siewiorek et al also discusses this limitation.25 We feel that a lack of better developed analysis software discourages more widespread adoption of this technology during CAS.
While the CAPITAL study validates IVUS-derived plaque type with histological findings, there are no standard quantitative measures that can be used to categorize plaque type. Determination of plaque type is largely operator driven, and similar to border editing is thus prone to variation and error despite interpretation standards set by the American College of Cardiology.26 In our own experience, we found it challenging to determine which category certain plaques should fall under. Every plaque is unique, and while some may fit clearly into one category over another, others are less straightforward. In the study by González et al, they used two independent readers to determine plaque classification, which helps to reduce variability in interpretation. Regardless, a quantitative approach could help resolve discrepancies between readers and better stratify plaque risk.
A practical consideration for interventionists contemplating the use of IVUS during CAS is time. Including IVUS analysis does add additional time to the length of the procedure; however, we feel that this added time is generally insignificant. The IVUS catheter leverages the 0.014″ wire that is commonly used during CAS, so there is no need for wire exchange. Most lesions are < 15 mm, so at a pullback rate of 0.5 mm/s, it should take less than a minute to image the plaque and its surrounding tissue. Often, motorized pullback is performed at even faster rates of 1 mm/s. Bandyk and Armstrong demonstrated that while procedure time did increase, less contrast agent was used during IVUS-assisted procedures; they felt that this was because of the fewer angiogram runs to confirm stent size and placement. Nevertheless, the risk of additional catheter passages because of the IVUS is a relevant concern, and minimizing the length of time performing manipulations within the carotid artery is important.
IVUS is also limited in its use as a diagnostic tool. Although IVUS can provide more information about a plaque than DUS, its use is limited to an inpatient setting. As IVUS is an interventional procedure, it would not be possible to conduct as an outpatient diagnostic service as DUS is done. IVUS must be performed by a trained interventionist, whereas DUS can be performed by technologists.
Another limitation of IVUS to acknowledge is cost. Endovascular equipment is expensive, and IVUS catheters are no exception. The computerized imaging system itself is an additional cost. However, if it can be demonstrated that utilizing IVUS during CAS can produce more favorable clinical outcomes, then these additional equipment costs would be warranted. Specifically, if IVUS can help decrease the length of hospital stay or readmission, then its use may be financially rationalized. To our knowledge, there are no studies looking at length of hospital stay following IVUS-assisted CAS compared with CAS without IVUS; such a study may help to clarify these cost–benefit issues.
Conclusion
In summary, VH–IVUS has the potential for improving clinical outcomes for patients undergoing CAS. Use of IVUS during CAS has been shown to feasible and safe, however, sufficient benefit of its use remains to be proven. In our experience, the biggest benefit to IVUS has been its ability to guide optimal stent placement within the carotid artery. Nevertheless, limitations to its widespread use remain, including its cost and limited analysis capabilities. Further studies looking at long-term clinical outcomes are warranted, as well as the development of better analytical software.
References
- 1.Brott T G, Hobson R W II, Howard G. et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363(1):11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin E J, Go A S. et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Bonati L H, Jongen L M, Haller S. et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS) Lancet Neurol. 2010;9(4):353–362. doi: 10.1016/S1474-4422(10)70057-0. [DOI] [PubMed] [Google Scholar]
- 4.Tedesco M M, Lee J T, Dalman R L. et al. Postprocedural microembolic events following carotid surgery and carotid angioplasty and stenting. J Vasc Surg. 2007;46(2):244–250. doi: 10.1016/j.jvs.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Hitchner E, Gillis K. et al. Prospective neurocognitive evaluation of patients undergoing carotid interventions. J Vasc Surg. 2012;56(6):1571–1578. doi: 10.1016/j.jvs.2012.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pieniazek P, Musialek P, Kablak-Ziembicka A. et al. Carotid artery stenting with patient- and lesion-tailored selection of the neuroprotection system and stent type: early and 5-year results from a prospective academic registry of 535 consecutive procedures (TARGET-CAS) J Endovasc Ther. 2008;15(3):249–262. doi: 10.1583/07-2264.1. [DOI] [PubMed] [Google Scholar]
- 7.Verhoeven B, Hellings W E, Moll F L. et al. Carotid atherosclerotic plaques in patients with transient ischemic attacks and stroke have unstable characteristics compared with plaques in asymptomatic and amaurosis fugax patients. J Vasc Surg. 2005;42(6):1075–1081. doi: 10.1016/j.jvs.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Diethrich E B, Pauliina Margolis M, Reid D B. et al. Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study. J Endovasc Ther. 2007;14(5):676–686. doi: 10.1177/152660280701400512. [DOI] [PubMed] [Google Scholar]
- 9.Lee J T, White R A. Basics of intravascular ultrasound: an essential tool for the endovascular surgeon. Semin Vasc Surg. 2004;17(2):110–118. doi: 10.1053/j.semvascsurg.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Bandyk D F, Armstrong P A. Use of intravascular ultrasound as a “Quality Control” technique during carotid stent-angioplasty: are there risks to its use? J Cardiovasc Surg (Torino) 2009;50(6):727–733. [PubMed] [Google Scholar]
- 11.Hitchner E, Zayed M A, Lee G, Morrison D, Lane B, Zhou W. Intravascular ultrasound as a clinical adjunct for carotid plaque characterization. J Vasc Surg. 2014;59(3):774–780. doi: 10.1016/j.jvs.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irshad K, Millar S, Velu R, Reid A W, Diethrich E B, Reid D B. Virtual histology intravascular ultrasound in carotid interventions. J Endovasc Ther. 2007;14(2):198–207. doi: 10.1177/152660280701400212. [DOI] [PubMed] [Google Scholar]
- 13.Wehman J C, Holmes D R Jr, Ecker R D. et al. Intravascular ultrasound identification of intraluminal embolic plaque material during carotid angioplasty with stenting. Catheter Cardiovasc Interv. 2006;68(6):853–857. doi: 10.1002/ccd.20871. [DOI] [PubMed] [Google Scholar]
- 14.Clark D J, Lessio S, O'Donoghue M, Schainfeld R, Rosenfield K. Safety and utility of intravascular ultrasound-guided carotid artery stenting. Catheter Cardiovasc Interv. 2004;63(3):355–362. doi: 10.1002/ccd.20188. [DOI] [PubMed] [Google Scholar]
- 15.Sangiorgi G, Bedogni F, Sganzerla P. et al. The Virtual histology In CaroTids Observational RegistrY (VICTORY) study: a European prospective registry to assess the feasibility and safety of intravascular ultrasound and virtual histology during carotid interventions. Int J Cardiol. 2013;168(3):2089–2093. doi: 10.1016/j.ijcard.2013.01.159. [DOI] [PubMed] [Google Scholar]
- 16.Schiro B J, Wholey M H. The expanding indications for virtual histology intravascular ultrasound for plaque analysis prior to carotid stenting. J Cardiovasc Surg (Torino) 2008;49(6):729–736. [PubMed] [Google Scholar]
- 17.Inglese L, Fantoni C, Sardana V. Can IVUS-virtual histology improve outcomes of percutaneous carotid treatment? J Cardiovasc Surg (Torino) 2009;50(6):735–744. [PubMed] [Google Scholar]
- 18.Politi L, Aprile A, Rollini F. et al. Carotid plaque characterisation by IVUS-VH during carotid stenting: the “eyes wide shut” between plaque morphology and symptoms. Minerva Cardioangiol. 2011;59(6):591–600. [PubMed] [Google Scholar]
- 19.Hitchner E, Zayed M, Varu V, Lee G, Aalami O, Zhou W. A prospective evaluation of using IVUS during percutaneous superficial femoral artery interventions. Ann Vasc Surg. 2015;29(1):28–33. doi: 10.1016/j.avsg.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Timaran C H, Rosero E B, Martinez A E, Ilarraza A, Modrall J G, Clagett G P. Atherosclerotic plaque composition assessed by virtual histology intravascular ultrasound and cerebral embolization after carotid stenting. J Vasc Surg. 2010;52(5):1188–1194. doi: 10.1016/j.jvs.2010.05.101. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto S, Nakahara I, Higashi T. et al. Fibro-fatty volume of culprit lesions in Virtual Histology intravascular ultrasound is associated with the amount of debris during carotid artery stenting. Cerebrovasc Dis. 2010;29(5):468–475. doi: 10.1159/000297962. [DOI] [PubMed] [Google Scholar]
- 22.Yamada K, Yoshimura S, Kawasaki M. et al. Prediction of silent ischemic lesions after carotid artery stenting using virtual histology intravascular ultrasound. Cerebrovasc Dis. 2011;32(2):106–113. doi: 10.1159/000328231. [DOI] [PubMed] [Google Scholar]
- 23.González A, López-Rueda A, Gutiérrez I. et al. Carotid plaque characterization by virtual histology intravascular ultrasound related to the timing of carotid intervention. J Endovasc Ther. 2012;19(6):764–773. doi: 10.1583/JEVT-12-3914MR2.1. [DOI] [PubMed] [Google Scholar]
- 24.Tsurumi A, Tsurumi Y, Hososhima O, Matsubara N, Izumi T, Miyachi S. Virtual histology analysis of carotid atherosclerotic plaque: plaque composition at the minimum lumen site and of the entire carotid plaque. J Neuroimaging. 2013;23(1):12–17. doi: 10.1111/j.1552-6569.2012.00748.x. [DOI] [PubMed] [Google Scholar]
- 25.Siewiorek G M, Loghmanpour N A, Winston B M, Wholey M H, Finol E A. Reproducibility of IVUS border detection for carotid atherosclerotic plaque assessment. Med Eng Phys. 2012;34(6):702–708. doi: 10.1016/j.medengphy.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mintz G S, Nissen S E, Anderson W D. et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37(5):1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]


