Abstract
Untreated hyperthyroidism and high-dose thyroid hormone are associated with osteoporosis, and increased bone mineral density (BMD) has been demonstrated in postmenopausal females with hypoparathyroidism. Studies on the effect of suppressive levothyroxine (LT4) therapy on BMD and bone metabolism after total thyroidectomy in patients with differentiated thyroid carcinoma have presented conflicting results, and few studies in relation to the status of hypoparathyroidism have been studied. One hundred postmenopausal women and 24 premenopausal women on LT4 suppression therapy were included in this study. BMD of lumbar spine and femur and bone turnover markers were measured at the baseline and during the follow-up period up to 18 months using dual energy X-ray absorptiometry. Biochemical marker of bone resorption was measured by urine deoxypyridinoline and bone formation by serum osteocalcin. The age ranged from 36 to 64 years old. Thyroid stimulating hormone (TSH) was suppressed during the study. The results showed that BMD of femur and lumbar spine were not significantly changed in both pre- and postmenopausal women except femur neck in postmenopausal women without hypoparathyroidism. Patients with hypoparathyroidism had higher BMD gain than those without hypoparathyroidism in total hip (1.25 vs. -1.18%, P=0.015). Biochemical markers of bone turnover, serum osteocalcin, and urine deoxypyridinoline did not show significant change. In conclusion, patients with well differentiated thyroid carcinoma are not at a great risk of bone loss after LT4 suppressive therapy. The state of hypoparathyroidism is associated with increased BMD, particularly in postmenopausal women.
Keywords: Bone density, Hypoparathyroidism, Postmenopause, Thyroid neoplasms, Thyroxine
INTRODUCTION
The incidence of differentiated thyroid cancer (DTC) is rapidly increasing, although mortality is stable.[1] Suppression of thyroid stimulating hormone (TSH) is required in an effort to decrease the risk of tumor recurrence.[2] Because hyperthyroidism accelerates bone turnover and shortens the normal bone remodeling cycle, it was expected that suppressive levothyroxine (LT4) therapy might decrease the bone mineral density (BMD).[3]
Although many studies in patients with suppressive LT4 therapy for DTC have been reported, there was no uniform answer to this suspicion. While the majority of reports concluded that suppressive LT4 therapy has no deteriorating effect on BMD,[4,5,6,7,8] some studies reported opposite conclusions in which LT4 suppressive therapy showed a slightly negative effect on bone metabolism.[9,10,11,12,13] Several of these studies evaluated the influence of menopausal status and LT4 dose as well.[9,10,11]
Most studies excluded patients with hypoparathyroidism as considering a confounding variable. No study on the effects of thyroid hormone on BMD and biochemical marker of bone turnover according to the presence of hypoparathyroidism and estrogen status has been reported. Even though it occurs in 1 to 10% of patients permanently after thyroidectomy, transient hypoparathyroidism is more common.[14]
Therefore, the aim of our study is to evaluate the effect of suppressive therapy with LT4 on BMD in relation to post-operative hypoparathyroidism in Korean female patients who underwent total thyroidectomy due to DTC.
METHODS
1. Patients
The research utilized patients' information between January, 2003 and April, 2015, based on digitalized medical records of Inha University hospital. Of all patients who had undergone total or near total thyroidectomy during the period, only those who checked BMD at post-operation and repeated BMD exam from 12 to 18 month from the baseline exam were included. Only female patients were selected, and their states of menopause at the time of the first BMD were confirmed. Postmenopausal women were defined as women with amenorrhea for the 12 months following the final menstrual period and who did not receive hormone replacement therapy (HRT). Patients who underwent thyroid lobectomy or had a history of treatment of osteoporosis using bisphosphonate, selective estrogen receptor modulators, calcitonin, and intermittent parathyroid hormone (PTH) were excluded. Patients who had been taking medication of calcium supplementation and/or active form of vitamin D were included in the list of study. Fifty postmenopausal, 6 premenopausal women who had undergone total thyroidectomy for diagnosed thyroid cancer and were diagnosed postoperative hypoparathyroidism were selected. Fifty postmenopausal and 18 postmenopausal women who had intact parathyroid function, and matched based on age and body weight, with the women with hypoparathyroidism were included in the study. A total of 112 patients (90%) had been previously treated with iodine-131 ablation therapy according to the indication. All included patients had been taking LT4 in order to maintain the serum TSH concentration between 0.1 and 0.5 mU/L.
The occurrence of hypoparathyroidism after surgery was defined as re-occurrence of hypocalcemia after cessation of active form of vitamin D or dose de-escalation and also refractory hypocalcemia to medication. Serum PTH was confirmed in all patients.
2. Methods
BMD of the lumbar spine (lumbar vertebrae, L1-L4), total hip, and femoral neck was measured at baseline (post-operative) and during the follow-up period (from 12 to 18 months) using dual energy X-ray absorptiometry (DXA; Hologic QDR-4500, Hologic, Inc., Bedford, MA, USA). The coefficient of variation for BMD measurement at our center is 1.41% in the lumbar spine and 1.89% in the femur. All serum tests including TSH, free T4, intact PTH (iPTH) and bone markers were measured at the time when the first BMD was performed and the latter one was performed. Biochemical marker of bone resorption was measured by urine deoxypyridinoline (mmol/cr) and bone formation by serum osteocalcin (radioimmunoassay [RIA], ng/mL). iPTH was determined by immunoradiometric assay (IRMA; Nicholos Institute Diagonostics, San Juan Capistrano, CA, USA). Dose of LT4 was titrated by the level of TSH according to the guideline of American Association of Thyroid.[2]
3. Statistical analysis
All results were expressed as mean±standard deviation (SD) unless indicated. Statistical analyses were performed using SPSS version 12.1 (SPSS Inc., Chicago, IL, USA). The characteristics of the participants were compared according to group using independent samples Student's t-tests or Mann-Whitney U test for continuous measures and χ2 tests for categorical measures. The BMD at baseline and follow-up were compared using paired t test. Logistic regression was used for categorical variables. Observations were considered significant if two-sided P-values were < 0.05.
RESULTS
1. General characteristics of the study population
Of 124 subjects in the study population, 24 women were premenopausal status and 100 women were postmenopausal at the time of the first BMD. Thus subjects were subdivided into 4 different subgroups by menopausal state and presence of hypoparathyroidism. The baseline characteristics of each subgroup are shown in Table 1. Age ranged from 36 to 64 at the time of the earlier BMD. As expected, baseline characteristics of patients with hypoparathyroidism were not different except the serum concentration of PTH compared to those without hypoparathyroidism in both premenopausal and postmenopausal groups respectively.
Table 1. The baseline characteristics of study population.
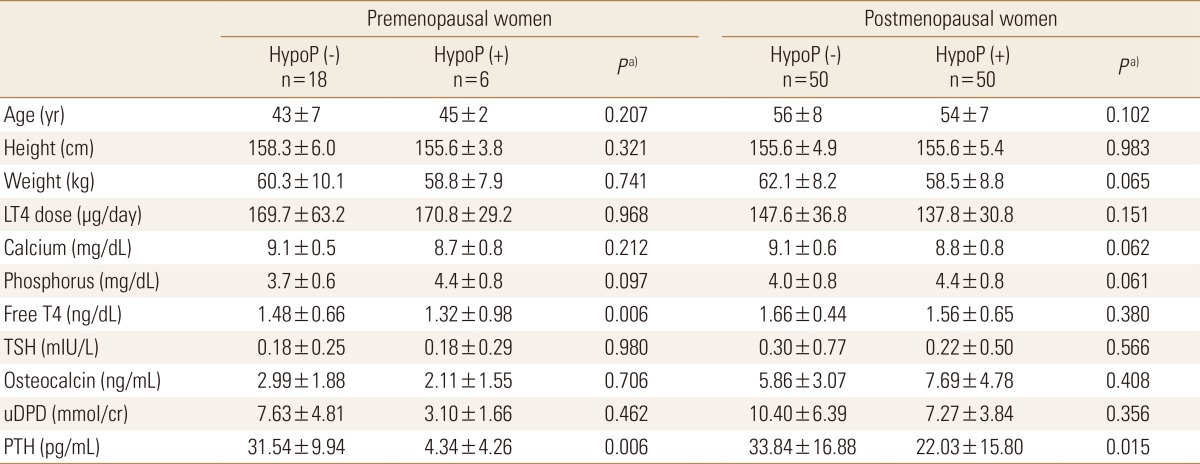
a)P-values were obtained using the χ2 test for categorical variables and the Mann-Whitney U test for continuous variables and represent the significances of differences between patients with and without hypoparathyroidism.
HypoP, hypoparathyroidism; LT4, levothyroxine; PTH, parathyroid hormone; TSH, thyroid stimulating hormone; uDPD, urine deoxypyridinoline.
2. Postoperative changes in BMD
The value of BMD at the early postoperative period (baseline) was compared with later one. In all patients, follow-up BMD of lumbar spine and femur neck decreased from baseline exam significantly (P<0.001 and P=0.018, respectively) (Table 2). However, when same analysis was done in 4 groups according to menopausal status and hypoparathyroidism, there were no significant differences between levels at baseline and follow-up period on all three sites, except femur neck in postmenopausal women without hypoparathyroidism (P<0.001).
Table 2. Bone mineral density changes in patients with thyroid cancer.
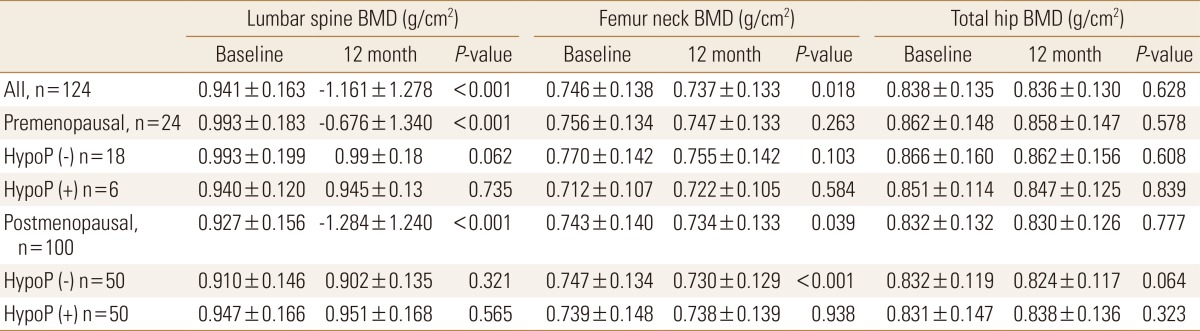
P-values were obtained using paired t-test.
BMD, bone mineral density; HypoP, hypoparathyroidism.
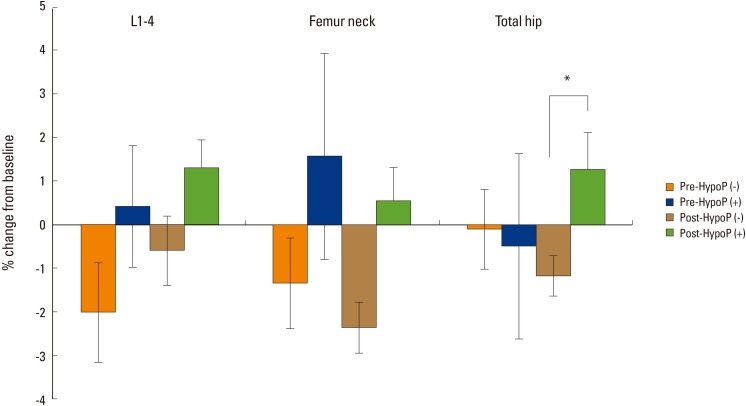
The mean change of BMD after thyroidectomy was calculated as percent change between levels at baseline and follow-up (Fig. 1). In women with hypoparathyroidism, Percent change of BMD of hip in patients with hypoparathyroidism increased compared with those in women without hypoparathyroidism in the postmenopausal group (1.25 vs. -1.18%, P=0.015). Percent change of lumbar spine BMD of patient without hypoparathyroidism was not different significantly compared with those with hypoparathyroidism in both pre- and postmenopausal women.
Fig. 1. Bone mineral density changes (%) according to menopausal status and presence of hypoparathyroidism *P<0.05. Pre-HypoP (-), premenopausal patients without hypoparathyroidism; Pre-HypoP (+), premenopausal patients with hypoparathyroidism; Post-HypoP (-), postmenopausal patients without hypoparathyroidism; Post-HypoP (-), postmenopausal patients with hypoparathyroidism.
3. Changes of serum level of PTH and calcium
Since hypoparathyroidism is usually accompanied by hypocalcemia and low level of PTH, hypocalcemia would have been expected in patients with hypoparathyroidism. As expected, there were significant differences in the follow up level of serum calcium and phosphorus (P=0.001, P=0.001, respectively), not only the level of PTH in relation to hypoparathyroidism. However level of serum TSH showed no significant difference between patients with and without hypoparathyroidism in pre-, postmenopausal women (P=0.407, P=0.825, respectively). Baseline or follow up TSH, PTH or age could not predict BMD change in all groups on multiple regression study.
4. Changes in biochemical markers of bone turnover
The levels of serum osteocalcin and urine deoxypyridinoline were not significantly changed. Follow up serum osteocalcin level was higher in patients without hypothyroidism compared to those with hypoparathyroidism in postmenopausal women (Table 3). For all patients enrolled in this study, osteocalcin and urine deoxypyridinoline were not associated with BMD change in lumbar and femur.
Table 3. Variables of bone metabolism and bone turnover markers at follow up.
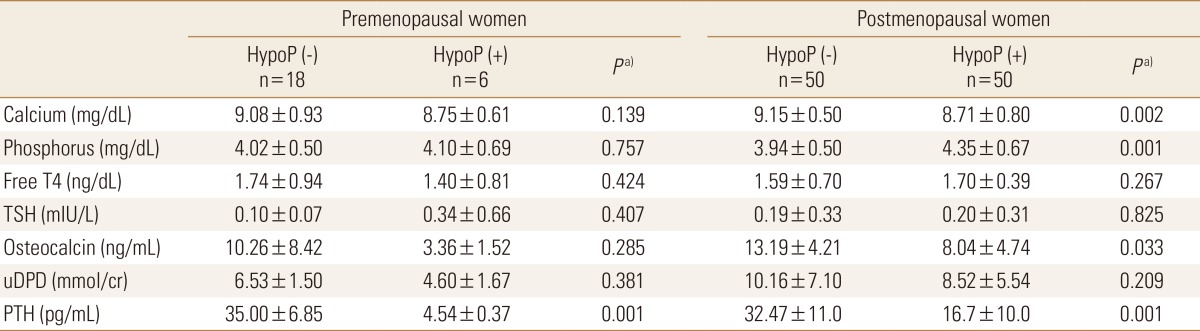
a)P-values were obtained using the Mann-Whitney U test for continuous variables and represent the significances of differences between patients with and without hypoparathyroidism.
HypoP, hypoparathyroidism; TSH, thyroid stimulating hormone; uDPD, urine deoxypyridinoline; PTH, parathyroid hormone.
DISCUSSION
Our study showed no deleterious effect of suppressive LT4 treatment in women with DTC, regardless of their estrogen status, and a protective effect was found in women with post-operative hypoparathyroidism. Even recommendations for management of thyroid carcinoma have no definite suggestion with regard to monitoring TSH level to prevent accelerated bone turnover.[15] Thyroid hormone excess increases osteoclastic and osteoblastic activities, both in vitro studies.[16] Although the majority of investigations showed that a carefully monitored LT4 suppressive therapy is not associated with bone loss in premenopausal women with differentiated thyroid cancer, change of BMD in postmenopausal women was still conflicting.
LT4 suppressive therapy was associated with bone loss in postmenopausal women,[6,8] which could be prevented by either calcium supplementation or intranasal calcitonin.[10] LT4 suppressive therapy accelerates bone loss, particularly in postmenopausal women and exclusively during the early post-thyroidectomy period.[11] On the other hand, Reverter et al.[7] reported that long-term suppressive LT4 treatment did not affect skeleton in women regardless of estrogen status.
However, most previous studies did not include patients with hypoparathyroidism, because hypoparathyroidism is regarded as a confounding factor. PTH plays an important role in regulation of bone metabolism. It regulates calcium homeostasis by affecting intestinal calcium absorption, renal calcium excretion, and the rate of bone resorption.[17]
In our study, percent change of BMD in patients with hypoparathyroidism increased significantly in total hip compared to patients with normal parathyroid function. Chan et al.[18] reported that the state of hypoparathyroidism either idiopathic or post-thyroidectomy is associated with increased BMD, most notably at the spine. However that study included 6 patients with post-thyroidectomy hypoparathyroidism and 8 with idiopathic hypoparathyroidism. In addition, their age ranged from 23 to 57 years old and menopause was not considered. In another study, BMD was increased in postmenopausal females at the lumbar spine and the proximal femur with hypoparathyroidism after thyroidectomy, but it was not statistically significant and a cross-sectional study.[19] Increase of BMD may either be attributable to hypoparathyroidism or to treatment with active form of vitamin D and calcium supplementation.[20,21] An increase in bone mineralization secondary to suppressed bone turnover can increase BMD. Accelerated bone loss can be attenuated in patients after menopause, indicating a reduced remodeling rate with calcium and vitamin D.[22] Although high dose calcium and vitamin D supplement means severe hypoparathyroidism status, dose of this correlated with BMD. Vitamin D receptors have been found in osteoblasts and in normal subjects, vitamin D stimulates both the number and activity of osteoblasts, also increased BMD.[23,24]
Previous studies have focused on menopausal state and endogenous and drug induced hyperthyroidism. Meta-analyses have shown a significant reduction in BMD only in postmenopausal women on long-term LT4 suppressive therapy that was more marked on cortical bone than on trabecular bone.[25] The study group included cancer and hypothyroidism, and studied BMD alone. In addition, changes of BMD could be different according to the replacement or suppressive treatment.
Bone marker study did not show significant change during the follow up period, and did not show correlation with percent change of BMD. Serum osteocalcin increased during treatment with calcium and vitamin D in patients with hypoparathyroidism.[26]
Currently there are no studies concerning the effect of LT4 on BMD and bone turnover marker according to menopausal and parathyroid status. Therefore our study is the first study considering the effect of thyroid hormone on BMD and biological bone turnover marker in relation to parathyroid state and estrogen status.
The limitations of our study include small sample size and the fact that all participants were enrolled at a single center. Because our study was retrograde, a thyroid hormonal status during the follow-up period was not strictly controlled. The second limitation is that the follow up period was not long enough to evaluate the long-term effect of LT4 suppression on bone metabolism. Further studies are necessary to clarify the long term effect of TSH suppression in multiple centers. Also, baseline serum vitamin D level was not measured, which could have differently affected BMD changes.
In summary, we have shown little deleterious effect of LT4 suppressive treatment, suggesting that patients with LT4 suppressive therapy are not at great risk of bone loss. In addition, in patients with hypoparathyroidism, it showed an improved BMD which might be explained in part by supplementation of calcium and active vitamin D metabolites. Further series of intervening studies to prove this opinion is required.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Brito JP, Davies L. Is there really an increased incidence of thyroid cancer? Curr Opin Endocrinol Diabetes Obes. 2014;21:405–408. doi: 10.1097/MED.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 3.Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19:35–63. [PubMed] [Google Scholar]
- 4.Lee MY, Park JH, Bae KS, et al. Bone mineral density and bone turnover markers in patients on long-term suppressive levothyroxine therapy for differentiated thyroid cancer. Ann Surg Treat Res. 2014;86:55–60. doi: 10.4174/astr.2014.86.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heijckmann AC, Huijberts MS, Geusens P, et al. Hip bone mineral density, bone turnover and risk of fracture in patients on long-term suppressive L-thyroxine therapy for differentiated thyroid carcinoma. Eur J Endocrinol. 2005;153:23–29. doi: 10.1530/eje.1.01933. [DOI] [PubMed] [Google Scholar]
- 6.Larijani B, Gharibdoost F, Pajouhi M, et al. Effects of levothyroxine suppressive therapy on bone mineral density in premenopausal women. J Clin Pharm Ther. 2004;29:1–5. doi: 10.1046/j.0269-4727.2003.00508.x. [DOI] [PubMed] [Google Scholar]
- 7.Reverter JL, Holgado S, Alonso N, et al. Lack of deleterious effect on bone mineral density of long-term thyroxine suppressive therapy for differentiated thyroid carcinoma. Endocr Relat Cancer. 2005;12:973–981. doi: 10.1677/erc.1.01072. [DOI] [PubMed] [Google Scholar]
- 8.Marcocci C, Golia F, Bruno-Bossio G, et al. Carefully monitored levothyroxine suppressive therapy is not associated with bone loss in premenopausal women. J Clin Endocrinol Metab. 1994;78:818–823. doi: 10.1210/jcem.78.4.8157704. [DOI] [PubMed] [Google Scholar]
- 9.Jódar E, Begoña López M, Garcia L, et al. Bone changes in pre- and postmenopausal women with thyroid cancer on levothyroxine therapy: evolution of axial and appendicular bone mass. Osteoporos Int. 1998;8:311–316. doi: 10.1007/s001980050069. [DOI] [PubMed] [Google Scholar]
- 10.Kung AW, Yeung SS. Prevention of bone loss induced by thyroxine suppressive therapy in postmenopausal women: the effect of calcium and calcitonin. J Clin Endocrinol Metab. 1996;81:1232–1236. doi: 10.1210/jcem.81.3.8772604. [DOI] [PubMed] [Google Scholar]
- 11.Kim MK, Yun KJ, Kim MH, et al. The effects of thyrotropin-suppressing therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Bone. 2015;71:101–105. doi: 10.1016/j.bone.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Mohammadi B, Haghpanah V, Tavangar SM, et al. Modeling the effect of levothyroxine therapy on bone mass density in postmenopausal women: a different approach leads to new inference. Theor Biol Med Model. 2007;4:23. doi: 10.1186/1742-4682-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazokopakis EE, Starakis IK, Papadomanolaki MG, et al. Changes of bone mineral density in pre-menopausal women with differentiated thyroid cancer receiving L-thyroxine suppressive therapy. Curr Med Res Opin. 2006;22:1369–1373. doi: 10.1185/030079906X115612. [DOI] [PubMed] [Google Scholar]
- 14.Youngwirth L, Benavidez J, Sippel R, et al. Parathyroid hormone deficiency after total thyroidectomy: incidence and time. J Surg Res. 2010;163:69–71. doi: 10.1016/j.jss.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 15.Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737–744. doi: 10.1089/thy.1998.8.737. [DOI] [PubMed] [Google Scholar]
- 16.Lee MS, Kim SY, Lee MC, et al. Negative correlation between the change in bone mineral density and serum osteocalcin in patients with hyperthyroidism. J Clin Endocrinol Metab. 1990;70:766–770. doi: 10.1210/jcem-70-3-766. [DOI] [PubMed] [Google Scholar]
- 17.De Sanctis V, Soliman A, Fiscina B. Hypoparathyroidism: from diagnosis to treatment. Curr Opin Endocrinol Diabetes Obes. 2012;19:435–442. doi: 10.1097/MED.0b013e3283591502. [DOI] [PubMed] [Google Scholar]
- 18.Chan FK, Tiu SC, Choi KL, et al. Increased bone mineral density in patients with chronic hypoparathyroidism. J Clin Endocrinol Metab. 2003;88:3155–3159. doi: 10.1210/jc.2002-021388. [DOI] [PubMed] [Google Scholar]
- 19.Duan Y, De Luca V, Seeman E. Parathyroid hormone deficiency and excess: similar effects on trabecular bone but differing effects on cortical bone. J Clin Endocrinol Metab. 1999;84:718–722. doi: 10.1210/jcem.84.2.5498. [DOI] [PubMed] [Google Scholar]
- 20.Dawson-Hughes B, Dallal GE, Krall EA, et al. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med. 1990;323:878–883. doi: 10.1056/NEJM199009273231305. [DOI] [PubMed] [Google Scholar]
- 21.Reid IR, Ames RW, Evans MC, et al. Effect of calcium supplementation on bone loss in postmenopausal women. N Engl J Med. 1993;328:460–464. doi: 10.1056/NEJM199302183280702. [DOI] [PubMed] [Google Scholar]
- 22.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 23.Haussler MR, Haussler CA, Jurutka PW, et al. The vitamin D hormone and its nuclear receptor: molecular actions and disease states. J Endocrinol. 1997;154(Suppl):S57–S73. [PubMed] [Google Scholar]
- 24.Cranney A, Weiler HA, O'Donnell S, et al. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr. 2008;88:513s–519s. doi: 10.1093/ajcn/88.2.513S. [DOI] [PubMed] [Google Scholar]
- 25.Uzzan B, Campos J, Cucherat M, et al. Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab. 1996;81:4278–4289. doi: 10.1210/jcem.81.12.8954028. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins F, Escobar-Jiménez F, Jódar E, et al. Bone mineral density in hypoparathyroid women on LT4 suppressive therapy. Effect of calcium and 1,25(OH)2 vitamin D3 treatment. J Musculoskelet Neuronal Interact. 2003;3:71–76. [PubMed] [Google Scholar]