Abstract
Background
Hizentra® (IGSC 20 %) is a 20 % liquid IgG product approved for subcutaneous administration in adults and children 2 years of age and older who have primary immunodeficiency disease (PIDD). There is limited information about the use of IGSC 20 % in very young children including those less than 5 years of age.
Methods
A retrospective chart review involved 88 PIDD infants and children less than 5 years of age who received Hizentra®.
Results
The mean age at the start of Hizentra® was 34 months (range 2 to 59 months). IGSC 20 % was administered weekly to 86 infants (two additional infants received twice weekly and three times weekly infusions, respectively) and included an average of 63 infusions (range 6–182) for an observation period up to 45.5 months. Infusion by manual delivery occurred in 15 patients. The mean dose was 674 mg/kg/4 weeks. The mean IgG level was 942 mg/dL while on IGSC 20 %, compared to a mean trough IgG level of 794 mg/dL (p < 0.0001) during intravenous or subcutaneous IgG administration prior to IGSC 20 %. Average infusion time was 47 (range 5–120) minutes, and the median number of infusion sites was 2 (range 1–4). Local reactions were mostly mild and observed in 36/88 (41 %) children. No serious adverse events were reported. A significant increase in weight percentile (7 % ± 19.2, p = 0.0012) among subjects was observed during IGSC 20 % administration. The rate of serious bacterial infections was 0.067 per patient-year while receiving IGSC 20 %, similar to previously reported efficacy studies.
Conclusions
Hizentra® is effective in preventing infections, and is well tolerated in children less than age 5 years.
Keywords: Immunoglobulin, Subcutaneous, Immunodeficiency, Children, Safety, Efficacy
Introduction
Primary immunodeficiency diseases (PIDD) confer increased risk of recurrent respiratory, skin, and gastrointestinal infections [1–5]. Intravenous IgG replacement therapy (IVIG), indicated for patients with antibody deficiency, is commonly given every 3–4 weeks [6–10]. Subcutaneously-administered IgG (SCIG) has increasingly been used as alternative replacement therapy for PIDD patients [11–15]. Because of low infusion volume and ease of administration, SCIG is well suited for children.
Hizentra® (IGSC 20 %), a 20 % liquid SCIG formulation, is approved for adults and children greater than 2 years of age with PIDD [16–21]. The higher concentration allows a lower infusion volume and shorter infusion time. Experience in children less than 5 years of age is limited [22, 23].
We evaluated the efficacy and safety of IGSC 20 % in children less than age 5 years. Because growth restriction in early childhood has been associated with a high infectious disease burden [24–26], weight and height percentiles during IGSC 20 % infusions were assessed.
Methods
Patients
A retrospective review of Hizentra® replacement therapy was performed in 88 infants and children under age 5 years from 9 North American centers. Patients were included if they received >1 dose of IGSC 20 % prior to age 5 years, regardless of previous replacement. Patients with protein losing conditions or concomitant treatment with IVIG while on IGSC 20 % were excluded. Study data were managed using Research Electronic Data Capture (REDCap) program hosted at Carolinas Medical Center [27]. The study was approved by the Institutional Review Board at all participating institutions.
Efficacy and Safety Assessments
Study assessments included rates of serious bacterial infections (SBIs) (bacteremia/sepsis, meningitis, pneumonias, osteomyelitis, septic arthritis, visceral abscesses), non-serious infections, adverse events (AEs), local reactions, volume and duration of infusions, number of infusion sites, and serum IgG levels [28]. Rate of infusions was assessed for pump or manual delivery, and a cut-off value of >20 ml/h was used for rapid delivery based on previously published studies [13, 23, 29].
Growth Parameter Assessments
Weight and body length at the start and end of treatment were recorded. Only patients with values at both time points were included. Results were provided for all patients, 4 subgroups described by gender and age at start of Hizentra® (females ≤24 months, females >24–59 months, males ≤24 months, and males >24–59 months), and those who received hematopoietic stem cell transplantation (HSCT, n = 15) or gene therapy (n = 1). The difference in mean weight and length percentile was evaluated using a t-test. The analysis of covariance was used to test for sex differences.
Results
Study Population
Demographics and underlying PIDD diagnoses are shown (Table 1). Fifty-three of the 88 infants were male and the most common race was white. The predominant PIDD conditions included T-cell or combined immunodeficiency (n = 26), primary antibody deficiency (n = 19), and isolated hypogammaglobulinemia with repeated infections (n = 25). The mean age at the start of Hizentra® was 34 (range 2–59) months.
Table 1.
Demographics, Characteristics, and Diagnosis for 88 PIDD Infants and Children at the Start of Hizentra®
| Patient characteristic | 34 Patients, Aged 0 to <2 Years, No. (%) | 54 Patients, Aged 2 to 5 Years, No. (%) | 88 Patients Total, No. (%) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 23 (26) | 30 (34) | 53 (60) |
| Female | 11 (13) | 24 (27) | 35 (40) |
| Race, n (%) | |||
| White | 29 (33) | 41 (46) | 70 (80) |
| Black or African American | 4 (5) | 4 (5) | 8 (9) |
| Asian | 1 (1) | 3 (3) | 4 (4) |
| Other | 0 (0) | 6 (7) | 6 (7) |
| Age and Growth Parameters | |||
| Age, months, mean (range) | 11 (2–23) | 44 (25–59) | 34 (2–59) |
| Height, cm, mean (range) | 70.0 (50.6–91) | 94.5 (76.3–116.5) | 84.3 (50.6–116.5) |
| Weight, kg, mean (range) | 8.7 (4.1–15.5) | 15.0 (9.3–28.4) | 12.5 (4.1–28.4) |
| Diagnosis, n (%) | |||
| T-cell and combined immunodeficiencies | |||
| Severe combined immunodeficiency | 6 (6.8) | 8 (9) | 14 (16) |
| Hyperimmunoglobulin M syndrome | 0 (0) | 1 (1) | 1 (1) |
| 22q11 deletion | 0 (0) | 5 (5.7) | 5 (5.7) |
| Ataxia-telangiectasia | 0 (0) | 2 (2) | 2 (2) |
| Wiscott-Aldrich syndrome | 1 (1) | 0 (0) | 1 (1) |
| Combined immunodeficiency, unspecified | 3 (3.4) | 0 (0) | 3 (3.4) |
| Total | 10 (11.4) | 16 (18.2) | 26 (29.6) |
| Predominantly antibody deficiencies | |||
| Common variable immunodeficiency | 0 (0) | 5* (6) | 5* (6) |
| X-linked agammaglobulinemia | 7 (8) | 4 (4.5) | 11 (12.5) |
| Immunoglobulin G subclass deficiency | 0 (0) | 2 (2) | 2 (2) |
| Autosomal recessive agammaglobulinemia | 0 (0) | 1 (1) | 1 (1) |
| Total | 7 (8) | 12 (14) | 19 (22) |
| Disorders of innate immunity | |||
| NF-κB essential modulator (NEMO) | 2 (2) | 1 (1) | 3 (3) |
| Total | 2 (2) | 1 (1) | 3 (3) |
| Other | |||
| Hypogammaglobulinemia with specific antibody deficiency | 1 (1) | 7 (8) | 8 (9) |
| Isolated Hypogammaglobulinemia with repeated infections | 11 (12.5) | 14 (16) | 25 (28.4) |
| Transient hypogammaglobulinemia of infancy | 2 (2) | 2 (2) | 4 (4.5) |
| Hyperimmunoglobulin E syndrome (STAT3) | 0 (0) | 2 (2) | 2 (2) |
| Netherton syndrome | 1 (1) | 0 (0) | 1 (1) |
| Total | 15 (17) | 25 (28) | 40 (45) |
Infusion Parameters
Most infants received weekly IGSC 20 % therapy (n = 86) for 1.5–45.5 (average 14.25) months, one was infused twice weekly (by pump) for 10.25 months, and one was infused three times weekly (by manual delivery) for 8 months (Table 2). Seventy-two of 88 patients received prior Ig therapy including 48 patients who received IVIG, and 24 patients who received SCIG (Vivaglobin®, 16 %, CSL Behring). Sixteen patients (18 %) were naïve to Ig therapy prior to starting Hizentra®.
Table 2.
Summary of 5572 Hizentra® Infusions for 88 Children
| 34 Patients, Aged 0 to <2 Years | 54 Patients, Aged 2 to <5 Years | 88 Patients Total | |
|---|---|---|---|
| Median dosage, mg/kg/4 weeks (range) | 753 (400–2000) | 602 (260–2000) | 674 (260–2000) |
| Mean number of infusions (range) | 69 (10–182) | 60 (6–137) | 63 (6–182) |
| Mean infusion time, minutes (range) | 49 (5–120) | 46 (5–120) | 47 (5–120) |
| Median number of infusion sites (range) | 2.0 (1–3) | 2.0 (1–4) | 2.0 (1–4) |
| Mean volume per patient, mL (range) | 8.3 (4–13) | 11.2 (5–38) | 10.1 (4–38) |
| Mean volume per site, mL (range) | 4.5 (1.5–13.5) | 5.7 (2.5–20.6) | 5.2 (1.5–20.6) |
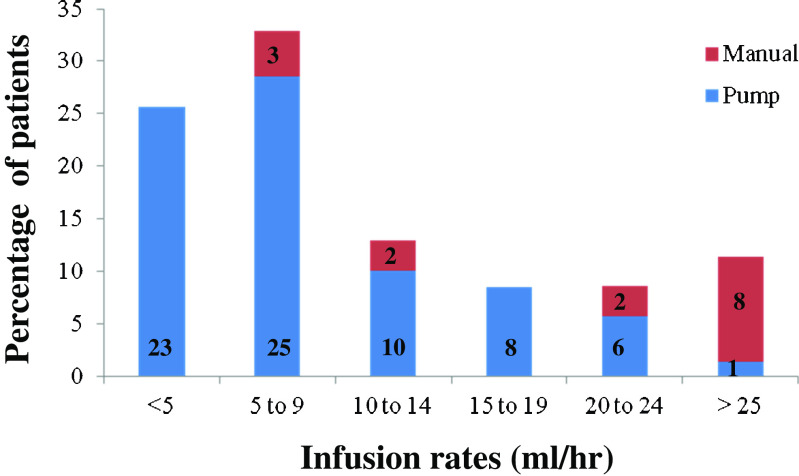
Median cumulative monthly IGSC 20 % dosage was 674 mg/kg, compared to a monthly dosage of 552 mg/kg of Ig prior to IGSC 20 % treatment. Infusion rates (Fig. 1) of <20 ml/h occurred in 66/73 (90 %) (median 5.9 ml/h, range 1.3–18.9) subjects infused by pump compared to 5/15 (33 %) (median 10 ml/h, range 7.5–12) subjects infused by manual delivery, respectively. Rates >20 ml/h occurred in 7 of 73 (10 %) (median 20.6 ml/h, range 20–39.8) subjects infused by pump compared to 10/15 (67 %) (median 29.7 ml/h, range 22.3–89.8) infused by manual delivery.
Fig. 1.
Infusion rates for 88 pediatric patients receiving Hizentra® by pump or manual delivery. Data are presented as the percentage of patients and number of patients within each category
Of 46 patients receiving IVIG before being changed to IGSC 20 %, 20 underwent dose adjustment by a factor of 1.53 (as recommended by the FDA) while 12 patients received the same IVIG and IGSC 20 % dose and 9 patients received a weekly dose lower than calculated for IVIG; 5 patients received dosing above the recommended 1.53 adjustment factor. Mean IgG levels were higher for the 88 patients receiving IGSC 20 % (942 mg/dL) compared to the 72 patients receiving IVIG or SCIG therapy prior to being switched to IGSC 20 % (794 mg/dL) (p < 0.0001). Thirty-three patients had IgG levels for review during IVIG and subsequent IGSC 20 % administration: the mean IgG trough level on IVIG was 720 mg/dL while mean IgG level on IGSC 20 % was 943 mg/dL (p < 0.0001, paired t-test).
For 72 patients who transitioned to Hizentra® from another IgG therapy, reasons for change included difficult IV access (n = 26), family/patient preference (n = 31), discontinuation of prior product (Vivaglobin®) (n = 22), side effects (n = 5), transportation problems (n = 3), more reliable dosing (n = 1), and discontinuation of a peripherally-inserted central catheter (n = 1).
Sites of administration included thigh (58 %), abdomen (24 %), thigh and abdomen (17 %), and arm and thigh (1 %). Hizentra® was administered by pump (n = 73) or manual delivery (n = 15). Manual delivery was most often accomplished using a 23-, 25-, or 27-guage butterfly needle set and the mean volume per site was 4.7 mL (range 2.0–7.5 mL). Needle length was reported in 55 patients and the most frequently used size was 6 mm (80 %), followed by 4 mm (15 %) and 9 mm (5 %). The median number of administration sites was 2 (range 1–4 sites). After a short instruction phase, Hizentra® was administered at home by a parent or caregiver administered in all but two patients who received administration by a home care nurse or other provider.
Efficacy
The incidence of SBIs and non-serious infections per patient-year (Table 3), respectively, were 0.067 and 0.29 during Hizentra® administration, 0.22 and 0.37 prior to Hizentra® administration (including 72 patients previously treated with Ig), and 0.25 and 0.40 prior to any Ig therapy.
Table 3.
Comparison of Number and Rate of Infections Per Patient-Year Occurring During Treatment Prior to and during Hizentra® (N = 88)
| Prior to Hizentra®a | Hizentra® | |||
|---|---|---|---|---|
| Infection Type | Total Number of Infections | Rate of Infections Per Patient-Year | Total Number of Infections | Rate of Infections Per Patient-Year |
| Serious Bacterial Infections | ||||
| Bacteremia/sepsis | 20 | 0.09 | 3 | 0.03 |
| Bacterial meningitis | 2 | 0.01 | 0 | 0 |
| Pneumonia, bacterial | 42 | 0.20 | 3 | 0.03 |
| Osteomyelitis/septic arthritis | 0 | 0 | 1 | 0.01 |
| Visceral abscess (liver,lung,brain) | 3 | 0.01 | 0 | 0 |
| Total | 47 | 0.22 | 7 | 0.067 |
| Non-Serious Infections | ||||
| Urinary tract infection | 8 | 0.04 | 0 | 0 |
| Pneumonia, viral | 10 | 0.05 | 3 | 0.03 |
| Ear infection | 6 | 0.03 | 3 | 0.03 |
| Gastroenteritis | 11 | 0.05 | 7 | 0.06 |
| Abscess/cellulitis | 8 | 0.04 | 4 | 0.04 |
| Bronchiolitis/tracheitis | 16 | 0.07 | 11 | 0.10 |
| Sinusitis | 1 | 0.005 | 3 | 0.03 |
| Viremia (CMV, EBV) | 6 | 0.03 | 0 | 0 |
| Lymphadenitis | 1 | 0.005 | 0 | 0 |
| Fever | 4 | 0.02 | 0 | 0 |
| Other | 6b | 0.03 | 0 | 0 |
| Total | 77 | 0.37 | 31 | 0.29 |
aIncludes 72 patients who received IVIG (n = 48) or SCIG other than Hizentra® (n = 24)
bOther infections included acute respiratory distress syndrome (n = 1), necrotizing enterocolitis (n = 1), necrotizing fasciitis of the chest wall (n = 1), Staphylococcus aureus peritonitis (n = 1), and infections site-not-specified (Klebsiella, n = 1; Enterobacter, n = 1)
Tolerability
Replacement therapy with IGSC 20 % was well tolerated; 41 patients experienced no local AEs (Table 4). Local reactions were mostly mild and observed in 36 children. Upper respiratory tract infections were reported in 15 (17 %) patients. Other AEs included pruritus (n = 3), irritability (n = 3), and one patient each with bronchitis, pyrexia, sinusitis, and otitis. There were no serious AEs.
Table 4.
Summary of Adverse Events
| Adverse Event | 34 Patients, Aged 0 to <2 Years, No. (%) | 54 Patients, Aged 2 to <5 Years, No. (%) | 88 Patients Total, No. (%) |
|---|---|---|---|
| None | 20 (59) | 21 (39) | 41 (47) |
| Any adverse eventa | 14 (41) | 33 (61) | 47 (53) |
| Local reactionb | 9 (26) | 27 (50) | 36 (41) |
| Upper respiratory tract infection | 1 (3) | 14 (26) | 15 (17) |
| Pruritus | 1 (3) | 2 (4.7) | 3 (3) |
| Bronchitis | 0 (0) | 1 (2) | 1 (1) |
| Pyrexia | 0 (0) | 1 (2) | 1 (1) |
| Sinusitis | 0 (0) | 1 (2) | 1 (1) |
| Otitis media | 0 (0) | 1 (2) | 1 (1) |
| Irritability | 3 (9) | 0 (0) | 3 (3) |
aMultiple adverse events occurred in some patients
bBased on 16 MedDRA preferred terms
IGSC 20 % was discontinued in 14 (15.9 %) patients prior to age 5 years. Reasons for discontinuation included resolved immune deficiency (n = 6), physician discontinuation (n = 4), parental refusal (n = 2), intolerance related to pruritus (n = 1), and prolonged hospitalization following renal transplantation (n = 1). One patient expired of unrelated causes.
Growth Parameter Assessments during Hizentra®
Weight and length percentiles were assessed at the beginning and end of Hizentra® observation period. Among 85 patients for whom weight parameters were available, there was a statistically significant increase in overall weight percentile with a mean increase of 7 % (standard deviation [SD] = 19.2 %) (p = 0.0012) (Fig. 2A-D) over the observation period (mean 427 days). Those with an initial weight below 30th percentile saw the most significant gains. The largest increase in weight percentile was in males ≤24 months with a mean of 18 % (SD = 24) (p = 0.0009). Among 74 patients for whom length parameters were available, there was an overall increase in length percentile of 2 % (SD = 18), but this did not reach statistical significance (p = 0.36) (Fig. 3A-D). The greatest increase in length percentile occurred in males ≤24 months with a mean of 9 % (SD = 21) (p = 0.052). For 16 patients who underwent HSCT or gene therapy, smaller increases in growth were seen compared to those who did not (Table 5). The difference between males and females was statistically significant for change in length (p = 0.018) but not weight (p = 0.099) regardless of age at baseline.
Fig. 2.
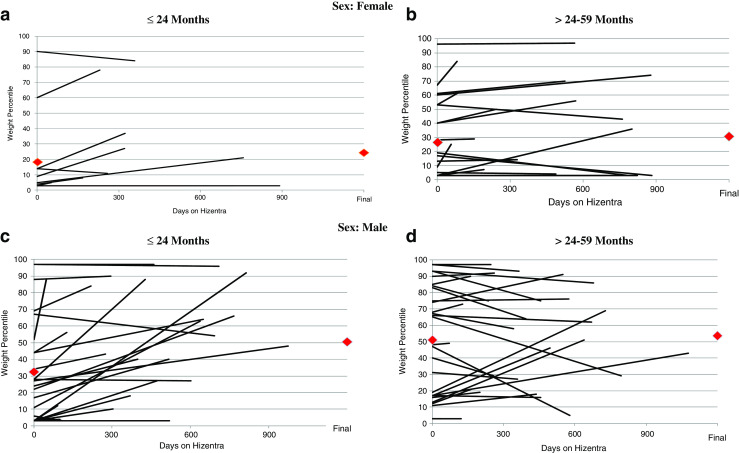
(A-D): Weight percentile measurements are shown at the start and completion of Hizentra® therapy for 86 patients. A and B include females (<=24 and >24–59 months, respectively). C and D similarly show males (<=24 and >24–59 months, respectively). Each line represents 1 patient, and red diamonds ( ) represent mean weight percentile at first and last measurement on Hizentra®
) represent mean weight percentile at first and last measurement on Hizentra®
Fig. 3.
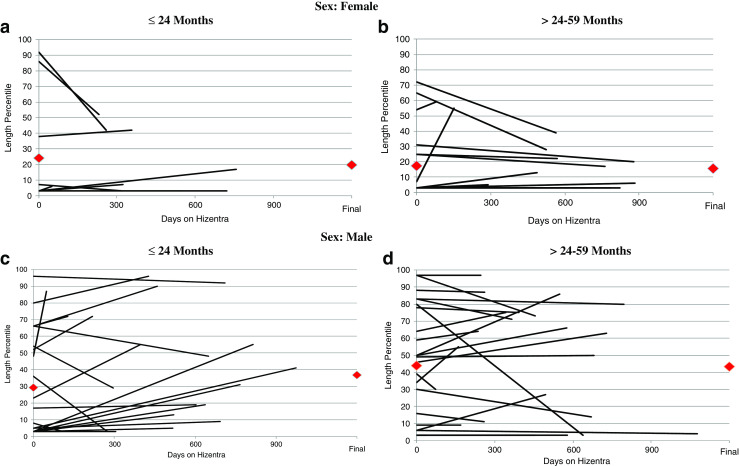
(A-D): Length percentile measurements are shown at the start and completion of Hizentra® therapy for 74 patients. A and B include females (<=24 and >24–59 months, respectively). C and D similarly show males (<=24 and >24–59 months, respectively). Each line represents 1 patient, and red diamonds ( ) represent mean length percentile at first and last measurement on Hizentra®
) represent mean length percentile at first and last measurement on Hizentra®
Table 5.
Comparison of Changes in Weight and Height Percentiles Among Transplanted and Non-transplanted Patients During Treatment with Hizentra®
| Change from Baseline | |||||
|---|---|---|---|---|---|
| Weight Percentile | Height Percentile | ||||
| Group | Transplant* Status | N | Mean (SD) | N | Mean (SD) |
| Male | |||||
| ≤ 24 months | Transplant | 4 | 8.0 (6.6) | 4 | 2.3 (27.0) |
| No Transplant | 20 | 20.3 (25.2) | 18 | 10.7 (20.0) | |
| Overall | 24 | 18.2 (23.5) | 22 | 9.2 (20.9) | |
| > 24 months | Transplant | 6 | −0.8 (26.3) | 6 | 2.5 (9.3) |
| No Transplant | 22 | 1.5 (17.7) | 18 | 2.3 (14.2) | |
| Overall | 28 | 1.0 (19.3) | 24 | 2.3 (12.9) | |
| Female | |||||
| ≤ 24 months | Transplant | 3 | 19.7 (2.9) | 3 | −11.3 (20.0) |
| No Transplant | 8 | −2.4 (6.3) | 7 | −8.1 (19.4) | |
| Overall | 11 | 3.6 (11.6) | 10 | −9.1 (18.5) | |
| > 24 months | Transplant | 3 | −5.7 (9.0) | 3 | 3.3 (5.8) |
| No Transplant | 19 | 5.6 (10.7) | 15 | −2.7 (18.7) | |
| Overall | 22 | 4.0 (11.0) | 18 | −1.3 (17.3) | |
*Included patients who underwent hematopoietic stem cell transplantation (n = 15) or gene therapy (n = 1)
Discussion
This is the first large retrospective study to examine efficacy and safety of SCIG in very young children. In 88 children less than 5 years of age, including 34 children less than 2 years of age, Hizentra® was safe and efficacious with an annualized rate of SBIs of 0.067 per patient-year. This rate is similar to those observed in older children and adults [16, 17, 19–21]. Mean IgG levels were higher during IGSC 20 % administration (942 mg/dL) compared to IgG levels in the same cohort during receipt of IVIG (794 mg/dL) (p < 0.0001). Higher trough IgG levels are known to be associated with a significant decrease in serious infections for PIDD patients [9]. The higher mean dose of IGSC 20 % (674 mg/kg/4 weeks) compared to IVIG may have been due to the converting factor of 1.53 or higher when switch from IVIG to IGSC 20 %. Higher IgG serum levels (17–22 %) on IGSC 20 % compared to IVIG have been previously reported [16, 17], and with equal dosing 1:1, SCIG will result in 13 % average high serum IgG levels compared to IVIG [18]. However, most immunologists believe it is not necessary to give higher doses of Hizentra® including those patients less than age 5 years.
Hizentra® was well tolerated in PIDD patients. Local reactions were mostly mild. Systemic AEs were infrequent and there were no serious AEs. Only one patient discontinued treatment due to pruritus. Low rates (29 %) of local reactions observed in children less than age 2 years may have been related to lower infusion volumes [16, 17, 21]. Manual administration of IGSC 20 % in 15 (17 %) patients without a pump device was tolerated without difficulty. Mean infusion time (47 min) was less than that reported for IGSC 20 % in adolescents (60 min) and adults (85 min) [17] and among children and adults (76 min) [16]. This was likely related to smaller volumes administered to smaller patients, and to some patients who received rapid infusion by manual delivery.
Weight percentile among 85 subjects increased significantly (7 %, p = 0.0012) during the observation period (mean 427 days), and those below the 30th percentile for weight saw the largest gains. Similar observations have been reported in young children during catch-up growth after a period of high infectious disease burden or inadequate dietary intake [24, 25, 30–32]. A smaller increase in length percentile was observed (2 %) in 74 subjects, although this was not statistically significant. Previous observations suggest stunted growth in children requires recovery in weight before resuming linear growth [24, 30–32]. Perhaps a longer observation period in the current study would have seen larger gains in length percentiles. Patients receiving HSCT or gene therapy had smaller gains in growth which may have been related to complications associated with treatment, although this was not assessed. Data from this study support the observation that poor growth seen among PIDD patients, most likely related to prior frequent and severe infections, can be reversed with optimal immunoglobulin replacement.
In summary, Hizentra® was effective in preventing infections in PIDD infants and children less than age 5 years. Infusions were associated with an overall increase in weight and length percentiles. Hizentra® is well tolerated with no serious adverse events and is suitable for immunoglobulin replacement in very young children. Hizentra® may also be well-suited for small pediatric patients in whom venous access is difficult.
Acknowledgments
This study was supported through an Investigator Initiated Research grant awarded to Niraj Patel, funded by CSL Behring LLC, King of Prussia, PA, USA.
References
- 1.Buckley RH. Primary immunodeficiency diseases due to defects in lymphocytes. N Engl J Med. 2000;343:1313–1324. doi: 10.1056/NEJM200011023431806. [DOI] [PubMed] [Google Scholar]
- 2.Chapel H, Geha R, Rosen F. Primary immunodeficiency diseases: an update. Clin Exp Immunol. 2003;132:9–15. doi: 10.1046/j.1365-2249.2003.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochs HD, Smith CIE, Puck JM. Primary immunodeficiency diseases: a molecular and genetic approach. 3rd. New York: Oxford University Press Inc, USA; 2014. [Google Scholar]
- 4.Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, Cunningham-Rundles C, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2014;5:162. doi: 10.3389/fimmu.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 6.Quartier P, Debre M, De Blic J, de Sauvarzac R, Sayegh N, Jabado N, et al. Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. J Pediatr. 1999;134:589–596. doi: 10.1016/S0022-3476(99)70246-5. [DOI] [PubMed] [Google Scholar]
- 7.Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2002;109:1001–1004. doi: 10.1067/mai.2002.124999. [DOI] [PubMed] [Google Scholar]
- 8.Church JA, Borte M, Taki H, Nelson RP, Sleasman JW, Knutsen AP, et al. Efficacy and safety of privigen in children and adolescents with primary immunodeficiency. Pediatr Asthma Allergy Immunol. 2009;22:53–62. doi: 10.1089/pai.2009.0005. [DOI] [Google Scholar]
- 9.Eijkhout HW, van der Meer JW, Kallenberg CG, Weening RS, van Dissel JT, Sanders LA, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med. 2001;135:165–174. doi: 10.7326/0003-4819-135-3-200108070-00008. [DOI] [PubMed] [Google Scholar]
- 10.Stein MR, Nelson RP, Church JA, Wasserman RL, Borte M, Vermyle C, et al. Safety and efficacy of Privigen®, a novel 10 % liquid immunoglobulin preparation for intravenous use, in patients with primary immunodeficiencies. J Clin Immunol. 2009;29:137–44. [DOI] [PubMed]
- 11.Abrahamsen TG, Sandersen H, Bustness A. Home therapy with subcutaneous immunoglobulin infusions in children with congenital immunodeficiencies. Pediatrics. 1996;98:1127–1131. [PubMed] [Google Scholar]
- 12.Berger M. Principles of and advances in immunoglobulin replacement therapy for primary immunodeficiency. Immunol Allergy Clin N Am. 2008;28:413–437. doi: 10.1016/j.iac.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardulf A, Nicolay U, Asensio O, Bernatowska E, Bock A, Carvalho BC, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies – a prospective, multi-national study. J Clin Immunol. 2006;26:177–185. doi: 10.1007/s10875-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 14.Ochs HD. Gupta S, kiessling, Nicolay U, Berger M. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–273. doi: 10.1007/s10875-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 15.Berger M. Subcutaneous administration of IgG. Immunol Allergy Clin N Am. 2008;28:413–437. doi: 10.1016/j.iac.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Jolles S., Bernatowska E., de Gracia J., Borte M., Cristea V., Peter H.H., Belohradsky B.H., Wahn V., Neufang-Hüber J., Zenker O., Grimbacher B. Efficacy and safety of Hizentra® in patients with primary immunodeficiency after a dose-equivalent switch from intravenous or subcutaneous replacement therapy. Clinical Immunology. 2011;141(1):90–102. doi: 10.1016/j.clim.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Borte M, Pac M, Serban M, Gonzalez-Quevedo T, Grimbacher B, Jolles S, et al. Efficacy and safety of 20 % SCIg, a new 20 % immunoglobulin preparation for subcutaneous administration, in pediatric patients with primary immunodeficiency. J Clin Immunol. 2011;31:752–761. doi: 10.1007/s10875-011-9557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger M, Jolles S, Orange JS, Sleasman JW. Bioavailability of IgG administered by the subcutaneous route. J Clin Immunol. 2013;33:984–990. doi: 10.1007/s10875-013-9876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagan JB, Fasano MB, Spector S, Wasserman RL, Melamed I, Rojavin MA, et al. Efficacy and safety of a new 20 % immunoglobulin preparation for subcutaneous administration, IgPro20, in patients with primary immunodeficiency. J Clin Immunol. 2010;30:734–745. doi: 10.1007/s10875-010-9423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasserman RL, Melamed I, Nelson RP, Knutsen AP, Fasano MB, Stein MR, et al. Pharmacokinetics of subcutaneous IgPro20 in patients with primary immunodeficiency. Clin Pharmacokinet. 2011;50:405–414. doi: 10.2165/11587030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Kanegane H, Imai K, Yamada M, Takada H, Ariga T, Bexon M, et al. Efficacy and safety of IgPro20, a subcutaneous immunoglobulin, in Japanese patients with primary immunodeficiency diseases. J Clin Immunol. 2014;34:204–211. doi: 10.1007/s10875-013-9985-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher Joel L., Patel Niraj C. Subcutaneous Immunoglobulin Replacement Therapy with Hizentra® is Safe and Effective in Two Infants. Journal of Clinical Immunology. 2012;32(3):474–476. doi: 10.1007/s10875-011-9645-0. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro R. Subcutaneous immunoglobulin: rapid push vs. infusion pump in pediatrics. Pediatr Allergy Immunol. 2013;24:49–53. doi: 10.1111/pai.12026. [DOI] [PubMed] [Google Scholar]
- 24.Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol. 1998;148:497–506. doi: 10.1093/oxfordjournals.aje.a009675. [DOI] [PubMed] [Google Scholar]
- 25.Kabir I. Malek Ma, mazumder RN, Rahman MM, mahalanabis D. Rapid catch-up growth of children fed a high-protein diet during convalescence from shigellosis. Am J Clin Nutr. 1993;57:441–445. doi: 10.1093/ajcn/57.3.441. [DOI] [PubMed] [Google Scholar]
- 26.Goodgame RW, Kimball K, Ou CN, White AC, Genta RM, Lifschitz CH, et al. Intestinal function and injury in acquired immunodeficiency syndrome-related cryptosporidiosis. Gastroenterology. 1995;108(4):1075–1082. doi: 10.1016/0016-5085(95)90205-8. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FDA Guidance for Industry, Safety, efficacy, and pharmacokinetic studies to support marketing of immune globulin intravenous (human) as replacement therapy for primary humoral immunodeficiency. 2008. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/ucm078526.pdf
- 29.Thomas MJ, Brennan VM, Chapel HH. Rapid subcutaneous immunoglobulin infusions in children. Lancet. 1993;342:1432–1433. doi: 10.1016/0140-6736(93)92798-X. [DOI] [PubMed] [Google Scholar]
- 30.Richard SA, Black RE, Checkley W. Revisiting the relationship of weight and height in early childhood. Adv Nutr. 2012;3:250–254. doi: 10.3945/an.111.001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golden MH. Is complete catch-up possible for stunted malnourished Children? Eur J Clin Nutr. 1994;48:S58–S70. [PubMed] [Google Scholar]
- 32.Ashworth A. Growth rates in children recovering from protein-calorie malnutrition. Br J Nutr. 1969;23:835–845. doi: 10.1079/BJN19690094. [DOI] [PubMed] [Google Scholar]