Abstract
Studies on sirtuins (SIRT), a family of proteins with deacetylase activity, have provided convergent evidence of the key role of these enzymes in aging-linked physiological functions. The link between SIRT1 and longevity has emerged in model organism but few data are available in humans, in particular relying on longitudinal studies. Here, we assessed whether a genetic variant within SIRT1 gene promoter (rs12778366) was associated to human longevity. We analyzed 586 genomic DNA (gDNA) collected in the study “Treviso Longeva” (TRELONG), including elderly over 70 years of age from the municipality of Treviso, a town in the Northeast of Italy, with a 11-year follow-up. We genotyped SIRT1 rs12778366 by real-time polymerase chain reaction (RT-PCR) allelic discrimination assay. A cross-sectional analysis performed by comparing people over and under 85 years of age did not evidence association between rs12778366 and longevity. When we performed a longitudinal analysis considering mortality as dependent variable, we did not observe an association of rs12778366 with longevity in the whole population (corrected P-value = 0.33). However, when we stratified the TRELONG subjects according to circulating level of interleukin-6 (IL-6), a predictor of disability and mortality, we found that rs12778366 (TC+CC) carriers were at increased risk of mortality in comparison to the TT reference group (corrected P-value = 0.03, HR 1.47). Our data do not support a major role of rs12778366 in human longevity, but the stratified analysis on IL-6 suggests that this variant may be involved in the detrimental effect of high circulating IL-6 in the elderly.
Keywords: SIRT1, rs12778366, longevity, prospective study, genetics
Introduction
Human sirtuins (SIRTs) are a 7-member protein family sharing NAD+-dependent deacetylase activity. Sirtuins’ targets are transcription factors or structural proteins that are relevant for basic physiologic mechanisms, longevity and age-linked diseases [1,2]. The most studied SIRT is SIRT1, a nuclear protein involved in longevity in model organism, and relevant for defensive mechanisms against oxidative stress, inflammation and cancer also in humans [3-8]. Genetic variants of SIRT1 have been studied in the field of human longevity with no univocal results. Flachsbart et al. compared German long-lived individuals (mean age: 98.3 years) with younger subjects (mean age: 67.2 years) by using a tag-SNP study design with negative results [9]. A similar conclusion was drawn by two other European studies (the Leiden 85-Plus study [10] and the Rotterdam study [11]) and by another study in Askenazi Jews [12]. However, positive association of SIRT1 with longevity-related traits or longevity were reported. Shimoyama et al. found an association between SIRT1 genetic variants, body fat and blood pressure [13], while in a Dutch population carriers of the C-minor allele of rs12778366 (T/C) SIRT1 promoter variant had a significantly reduced mortality risk compared to the T-carriers, an effect that was gender-independent and present even in smokers and overweight/obese subjects [14]. So, it is possible that SIRT1 modulates long-term survival or at least basic physiologic features relevant for longevity in humans.
Starting from the latter positive association, we decided to assess the role of rs12778366 in an ongoing prospective study enrolling an elderly population (TRELONG study) [15].
Materials and methods
Population
The TRELONG study has been described in details elsewhere [15]. The study design envisioned the selection of the 13,861 Treviso inhabitants over 70 years of age from the residents listed in the Registry Office of Treviso, the systematic sampling planning to include at least 100 participants according to gender and 10-year-age group up to 100 years, and all available people > 100. A total of 668 participants were selected, 311 men and 357 women (mean age 84.0 ± 8.0 years, range 70.0-105.5 years). A 11-year follow-up was then performed. A blood sample was collected and each participant was administered a structured interview assessing clinical, lifestyle and demographic information. The study protocol, blood collection procedure and the questionnaire to be administered at home were submitted to and approved by the ethical committee of the National Institute on Research and Care of the Elderly (INRCA, Italy). The protocol included an written informed consent for clinical and genetic studies.
Biological samples preparation and SIRT1 rs12778366 genotyping
Fasting peripheral blood samples (30 mL) were collected by venipuncture; one aliquot was used to separate mononuclear cells (PBMC) by a standard Ficoll centrifugation procedure. PBMC pellets were washed with ice-cold PBS, divided into aliquots and stored at -80°C for further analysis. From the enrolled population of 668 subjects, 590 gave their consent to blood collection and PBMC preparation. Genomic DNA was extracted from PBMC pellet using a vacuum-based semi-automated nucleic acid extractor (AB6100, Applied Biosystems, Foster City, CA, USA), checked for concentration by a UV-spectrophotometer (Eppendorf, Hamburg, Germany) and stored at 4°C.
To assess rs12778366, a gDNA aliquot (about 20 ng) was used in an allelic discrimination assay using a real-time PCR apparatus and TaqMan technology according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). The successful genotyping rate was around 99.3% (586 genotypes/590 available samples). Subjects’ genotypes were independently confirmed in a random sample representing 10% of the population, with 100% replication rate.
Statistical analysis
Genotypic and allelic frequency distributions and departure from Hardy-Weinberg equilibrium were assessed by χ2-test. Calculations were done using GraphPad Prism program ver. 5.04.
Survival curves were estimated by the Kaplan-Meier method. Hazard ratios (HR) were calculated using Cox proportional hazard model. The proportional hazard has been tested using Schoenfeld’s residuals test, and it has never been rejected, thus confirming the suitability of the model. Multivariate regression analysis was performed considering mortality as dependent variable. These statistic analyses were computed using the package “survival” of the “R” software. After correction for possible confounders (diabetes, cardiovascular diseases, cerebral vasculopaties, cancer, cholesterol level, education, age and gender), results were considered significant at P < 0.05, using two-tailed tests of significance.
Results
Cross-sectional analysis
In the whole population, rs12778366 genotypic distribution respected the Hardy-Weinberg equilibrium (HWE) (P=0.89, for χ2-test assessing departure from HWE). We began evaluating the correlation of SIRT1 rs12778366 with longevity by splitting the TRELONG population around 85 years of age, starting from the assumption that people over 85 years might be informative and considered as long-living subjects, as supported also by previous evidence from the TRELONG study itself [16,17]. Results of this analysis are shown in Table 1. Genotypic and allelic frequenciesdid not differ between the two groups, even when we combined the TC and CC carriers to increase sample size. We tried also to stratify according to gender, founding no association (data not shown).
Table 1.
Cross-sectional analysis of the genotypic and allelic frequencies of SIRT1 single nucleotide polymorphisms rs12778366 in the TRELOONG population
| SNP (no.) | Genotype no. (%) | Allele no. (%) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ≤ 85 y | > 85 y | χ2 statistics | ≤ 85 y | > 85 y | χ2 statistics | |||
| rs12778366 (n=586) | TT | 261 (80.1) | 201 (77.3) | χ2=0.72 | T | 583 (89.4) | 458 (88.1) | χ2=0.56 |
| TC | 61 (18.7) | 56 (21.5) | d.f.=2 | C | 69 (10.6) | 62 (11.9) | d.f.=1 | |
| CC | 4 (1.2) | 3 (1.2) | P=0.69 | P=0.45 | ||||
| rs12778366 dominant model (n=586) | TT | 261 (80.1) | 201 (77.3) | χ2=0.65 | ||||
| TC+CC | 65 (19.9) | 59 (22.7) | d.f.=1 | |||||
| P=0.41 | ||||||||
Abbreviations: no.; number; 85 y: 85 years of age; d.f, degree of freedom; P: P-value.
Prospective analysis
To take advantage from the prospective design of the TRELONG study, we analyzed rs12778366 considering mortality as dependent variable. The last available vital status of the TRELONG study is reported in Table 2. Dead participants showed a significant risk profile in comparison to living people, including male gender, cognitive decline (as indicated by reduced mean mini-mental state examination -MMSE-score), and increased mean circulating level of the pro-inflammatory cytokine interleukin-6 (IL-6), that can be considered a marker of disability and mortality [18]. When we plot survival curves according to the SNP genotype, due to the low frequency of C-allele, we grouped TC and CC carriers in a dominant effect model hypothesis (Figure 1A), also basing on reported analysis [14]. As mortality might be influenced by several other variables, we controlled for TRELONG prevalent disorders (diabetes, cardiovascular diseases, cerebral vasculopaties and cancer), risk factors (cholesterol level, education), age and gender. We found no association between rs12778366 and survival. In fact, by considering the TT-homozygous carriers group as reference, the hazard ratio (HR) and confidence interval (CI) of the TC+CC group was 1.12 (0.88-1.43), with associated P-value =0.3. To investigate whether we had a different outcome in males or females, we performed a gender-stratified analysis, correcting for all the variables influencing survival listed above and considering TT-carriers as reference. Survival plots of the TRELONG population according to gender are shown in Figure 1B, 1C. We had no significant effect, as in males the HR (CI) was 1.13 (0.81-1.59), with associated P-value of 0.55; in females it was 1.1 (0.78-1.56), with P-value of 0.45.
Table 2.
Characteristics of participants to the TRELONG study at baseline (2003) by vital status on April 2nd, 2014
| Status on April 2nd. 2014 | Alive | Dead | P-value |
|---|---|---|---|
|
|
|||
| Mean ± SD or % | Mean ± SD or % | ||
| Number (%) | 214 (32.04%) | 454 (67.96%) | |
| Males. n (%) | 84 (39.25%) | 227 (50.00%) | 0.012 |
| Age. median (range) (years) | 76.90 (73.60-81.45) | 88.20 (80.93-92.88) | < 0.0001 |
| BMI (kg/m2) | 25.65 ± 4.02 | 24.31 ± 4.11 | 0.0001 |
| BMI < 18.5 (underweight) | 1.4% | 7.5% | 0.003 |
| 18.5 ≤ BMI < 25 (normal weight) | 42.52% | 43.61% | 0.856 |
| 25 ≤ BMI < 30 (overweight) | 41.12% | 29.74% | 0.005 |
| BMI > 30 (obese) | 12.62% | 8.15% | 0.091 |
| Smoking status smoking (1) | 41.12 | 41.41% | 1.0 |
| Never smoking (0) | |||
| SPPB | 7.995 ± 3.57 | 4.115 ± 3.57 | < 0.0001 |
| CCI | 4.439 ± 1.43 | 6.392 ± 1.96 | < 0.001 |
| MMSE ≤ 24. n (%) | 31 (14.49%) | 183 (46.26%) | < 0.0001 |
| APOE ε4/4 and 4/3. n (%) | 27 (15.00%) | 66 (16.92%) | 0.649 |
| IL-6 median (range) (pg/mL) | 0.59 (0.33-1.30) | 1.12 (0.54-2.61) | < 0.001 |
BMI: body mass index; SPPB: short physical performance battery score; CCI: Charlson co-morbidity index; APOE: apolipoprotein E; IL-6: interleukin-6.
Figure 1.
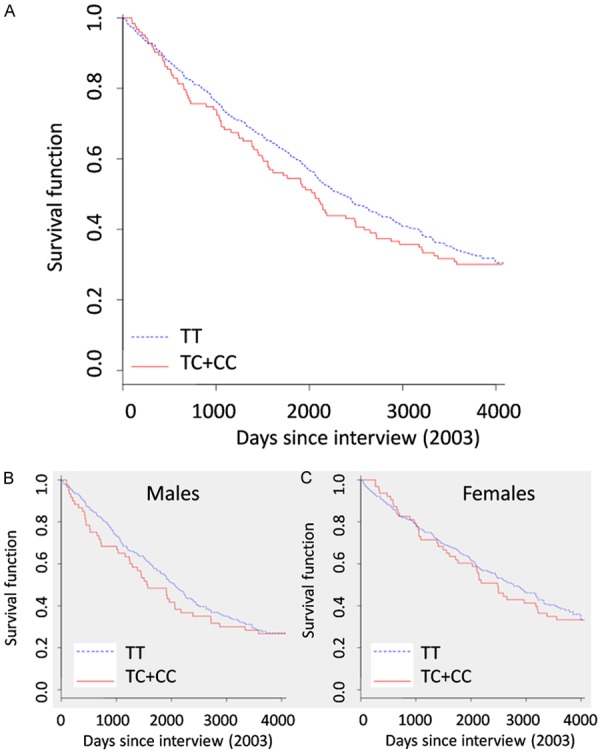
Survival curves of the TRELONG population according to rs12778366 genotype. A. Survival plot of the whole TRELONG population, considering the TT group as reference vs. the TC+CC carriers. The reported P-value was corrected for possible confounding factors affecting longevity (age, gender, education level, cholesterol level, cardiovascular disease, vascular cerebropaties, diabetes and cancer). B, C. Survival plots of the TRELONG population stratified according to rs12778366 genotype and gender. The associated P-value was corrected as above.
These results did not rule out that the effect of rs12778366 may be relevant for a TRELONG population component group. To verify this hypothesis, we stratified the study according to parameters that were significantly different between alive and dead subjects (Table 2), as body mass index (BMI), blood pressure or fasting glucose level, (considering as overweight/obese people with BMI > 25), a cognitive performance measure (MMSE, cut-off for cognitive impairment < 24) [19], or measures of disability and frailty, as the short physical performance battery (SPPB) score (cut-off value of 6) and the Charlson co-morbidity index (CCI) (cut-off score of 6) [15]. The results are summarized in Table 3. We were not able to highlight a significant variation in the HRs of the considered features. Moreover, the presence of the rs12778366 polymorphism did not correlate with different mean values of continuous variables (systolic blood pressure level of TT carriers vs. TC+CC carriers: 144.6 ± 19.8 and 143.0 ± 20.0 mmHg, respectively, P=0.33; fasting glucose level for TT carriers vs. TC+CC carriers: 106.3 ± 33.3 and 102.3 ± 32.1 mg/dL, respectively, P=0.25). Finally, we stratified the population according to the circulating level of interleukin-6 (IL-6). We found an increased mortality in TC+CC carries in comparison to TT reference group in the high IL-6 sub-population (considering as cut-off value IL-6 plasma level ≥ 0.93 pg/mL) (Table 3).
Table 3.
Stratified longitudinal analysis of mortality in the TRELONG population. Hazard ratio (HR) for mortality calculated according to rs12778366 genotype and smoking status, BMI, SPPB, CCI, MMSE and circulating IL-6. TT-carriers were considered as reference. The reported P-values were corrected for age, gender, education level, cholesterol level, cardiovascular disease, vascular cerebropaties, diabetes and cancer
| Stratification | HR (95% CI) | P-value |
|---|---|---|
| BMI < 18.5 | 1.016 (0.403-2.563) | 0.973 |
| 18.5 ≤ BMI < 25 | 1.165 (0.820-1.656) | 0.393 |
| 25 ≤ BMI < 30 and BMI ≥ 30 | 0.916 (0.605-1.389) | 0.680 |
| SPPB score < 6 | 1.272 (0.968-1.671) | 0.085 |
| SPPB score ≥ 6 | 0.793 (0.478-1.316) | 0.370 |
| CCI < 6 | 1.149 (0.839-1.573) | 0.386 |
| CCI ≥ 6 | 1.104 (0.763-1.597) | 0.600 |
| MMSE ≤ 24 | 1.232 (0.868-1.748) | 0.243 |
| MMSE > 24 | 1.065 (0.766-1.480) | 0.707 |
| IL-6 < 0.93 (pg/mL) | 0.948 (0.650-1.382) | 0.780 |
| IL-6 ≥ 0.93 (pg/mL) | 1.476 (1.038-2.098) | 0.030 |
BMI: body mass index; SPPB: short physical performance battery score; CCI: Charlson comorbidity index; MMSE: mini-mental state examination; IL-6: interleukin-6.
Discussion
The contribution to human longevity of genetic variants of SIRT1 is a matter of debate and the overall negative results suggest that in humans the relevance of this component on a so complex phenotype may be limited or hard to highlight. We addressed a specific aspect of SIRT1 variability (rs12778366) in relation to longevity, starting from available evidence [14], and despite our robust prospective design we were unable to find an association in the entire TRELONG population or after stratification according to glucose fasting level, analysis performed in an attempt to parallel what reported by Figarska et al [14]. We acknowledge that the TRELONG population is different from that enrolled in the above cited study, as the TRELONG design was centered on elderly subjects while Figarska et al. followed for 18 years a general population-based cohort. This difference may be relevant, as it suggests that the positive effect of this variant on human longevity and health status is age-dependent and may magnify its action in a defined age bracket, a situation that is not uncommon [20]. In fact, a similar scenario was reported for instance for dementia, where some relevant risk factors (i.e. BMI or apolipoprotein E genotype) may vary their association or magnitude effect when assessed in a younger population or in the elderly [21,22].
The above interpretation of our finding of no association between SIRT1 rs12778366 and longevity may also be the right key to interpret the increased mortality of TRELONG subjects having the rs12778366 C-allele and elevated levels of IL-6. This cytokine is a marker of disability and mortality whose detrimental effect has already been described in the study [18], and is supportive of a general pro-inflammatory background with aging and disease. SIRT1 genetic variability and enzymatic function have been reported to deal with systemic inflammation [23,24] and more directly with IL-6 as SIRT1 regulates the acetylation state of NF-kB, a transcription factor promoting IL-6 expression [25,26]. Consequently, in the TRELONG patients having the rs12778366 C-variant and increased values of circulating IL-6, rs12778366 may be an additional risk factor contributing to mortality by acting on SIRT1 protein level that in turn is related to the NF-kB/IL-6 expression pattern. A direct measure of SIRT1 mRNA level in TRELONG patients may help in assessing this hypothesis and supporting a functional correlation between SIRT1 rs12778366 C-variant and SIRT1 transcription. Unfortunately, we did not have suitable material to perform this analysis.
In summary, we were unable to confirm in an Italian elderly population the reported effect of rs12778366 on human longevity, but we found some evidence that SIRT1 promoter genetic variability may have a different impact on survival and health status in aged people in comparison to younger ones.
Acknowledgements
This work was supported by “Fondazione Italo Monzino”, Milan, Italy and by “Interdisciplinary Geriatric Research Foundation (FORGEI)” and “Fondazione Veneto Banca”.
Disclosure of conflict of interest
None.
References
- 1.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–38. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polito L, Kehoe PG, Forloni G, Albani D. The molecular genetics of sirtuins: association with human longevity and age-related diseases. Int J Mol Epidemiol Genet. 2010;1:214–225. [PMC free article] [PubMed] [Google Scholar]
- 3.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 4.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 5.Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 6.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M, Kozaki K, Akishita M, Ouchi Y, Kaneki M. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- 9.Flachsbart F, Croucher PJ, Nikolaus S, Hampe J, Cordes C, Schreiber S, Nebel A. Sirtuin 1 (SIRT1) sequence variation is not associated with exceptional human longevity. Exp Gerontol. 2006;41:98–102. doi: 10.1016/j.exger.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Kuningas M, Putters M, Westendorp RG, Slagboom PE, van Heemst D. SIRT1 gene, age-related diseases, and mortality: the Leiden 85-plus study. J Gerontol A Biol Sci Med Sci. 2007;62:960–965. doi: 10.1093/gerona/62.9.960. [DOI] [PubMed] [Google Scholar]
- 11.Zillikens MC, van Meurs JB, Sijbrands EJ, Rivadeneira F, Dehghan A, van Leeuwen JP, Hofman A, van Duijn CM, Witteman JC, Uitterlinden AG, Pols HA. SIRT1 genetic variation and mortality in type 2 diabetes: interaction with smoking and dietary niacin. Free Radic Biol Med. 2009;46:836–841. doi: 10.1016/j.freeradbiomed.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Han J, Atzmon G, Barzilai N, Suh Y. Genetic variation in Sirtuin 1 (SIRT1) is associated with lipid profiles but not with longevity in Ashkenazi Jews. Transl Res. 2015;165:480–481. doi: 10.1016/j.trsl.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimoyama Y, Suzuki K, Hamajima N, Niwa T. Sirtuin 1 gene polymorphisms are associated with body fat and blood pressure in Japanese. Transl Res. 2011;157:339–347. doi: 10.1016/j.trsl.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Figarska SM, Vonk JM, Boezen HM. SIRT1 polymorphism, long-term survival and glucose tolerance in the general population. PLoS One. 2013;8:e58636. doi: 10.1371/journal.pone.0058636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallucci M, Ongaro F, Bresolin F, Bernardi U, Salvato C, Minello A, Amici GP, Barasciutti E, Mazzuco S, Gajo GB, De Angeli S, Forloni GL, Albani D, Zanardo A, Regini C. The Treviso Longeva (Trelong) study: a biomedical, demographic, economic and social investigation on people 70 years and over in a typical town of North-East of Italy. Arch Gerontol Geriatr. 2007;44(Suppl 1):173–192. doi: 10.1016/j.archger.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Albani D, Batelli S, Polito L, Prato F, Pesaresi M, Gajo GB, De Angeli S, Zanardo A, Galimberti D, Scarpini E, Gallucci M, Forloni G. Interleukin-6 plasma level increases with age in an Italian elderly population (“The Treviso Longeva”-Trelong-study) with a sex-specific contribution of rs1800795 polymorphism. Age. 2009;31:155–162. doi: 10.1007/s11357-009-9092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albani D, Mazzuco S, Polito L, Batelli S, Biella G, Ongaro F, Gustafson DR, Antuono P, Gajo G, Durante E, Caberlotto L, Zanardo A, Siculi M, Gallucci M, Forloni G. Insulin-like growth factor 1 receptor polymorphism rs2229765 and circulating interleukin-6 level affect male longevity in a population-based prospective study (Treviso Longeva-TRELONG) Aging Male. 2011;14:257–264. doi: 10.3109/13685538.2011.607521. [DOI] [PubMed] [Google Scholar]
- 18.Gallucci M, Amici GP, Ongaro F, Gajo GB, De Angeli S, Forloni GL, Albani D, Prato F, Polito L, Zanardo A, Regini C. Associations of the plasma interleukin 6 (IL-6) levels with disability and mortality in the elderly in the Treviso Longeva (Trelong) study. Arch Gerontol Geriatr. 2007;44(Suppl 1):193–198. doi: 10.1016/j.archger.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Moskalev AA, Aliper AM, Smit-McBride Z, Buzdin A, Zhavoronkov A. Genetics and epigenetics of aging and longevity. Cell Cycle. 2014;13:1063–1077. doi: 10.4161/cc.28433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolppanen AM, Ngandu T, Kåreholt I, Laatikainen T, Rusanen M, Soininen H, Kivipelto M. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimers Dis. 2014;38:201–209. doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- 22.Rusted JM, Evans SL, King SL, Dowell N, Tabet N, Tofts PS. APOE e4 polymorphism in young adults is associated with improved attention and indexed by distinct neural signatures. Neuroimage. 2013;65:364–373. doi: 10.1016/j.neuroimage.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Consiglio CR, Juliana da Silveira S, Monticielo OA, Xavier RM, Brenol JC, Chies JA. SIRT1 promoter polymorphisms as clinical modifiers on systemic lupus erythematosus. Mol Biol Rep. 2014;41:4233–4239. doi: 10.1007/s11033-014-3294-3. [DOI] [PubMed] [Google Scholar]
- 24.Vachharajani VT, Liu T, Brown CM, Wang X, Buechler NL, Wells JD, Yoza BK, McCall CE. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J Leukoc Biol. 2014;96:785–796. doi: 10.1189/jlb.3MA0114-034RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo L, Li S, Zhao Y, Qian P, Ji F, Qian L, Wu X, Qian G. Silencing angiopoietin-like protein 4 (ANGPTL4) protects against lipopolysaccharide-induced acute lung injury via regulating SIRT1 /NF-kB pathway. J Cell Physiol. 2015;230:2390–402. doi: 10.1002/jcp.24969. [DOI] [PubMed] [Google Scholar]
- 26.Niederer F, Ospelt C, Brentano F, Hottiger MO, Gay RE, Gay S, Detmar M, Kyburz D. SIRT1 overexpression in the rheumatoid arthritis synovium contributes to proinflammatory cytokine production and apoptosis resistance. Ann Rheum Dis. 2011;70:1866–1873. doi: 10.1136/ard.2010.148957. [DOI] [PubMed] [Google Scholar]


