Abstract
AIM: To investigate the relationship between p53 codon 72 polymorphism and human papillomavirus (HPV) type 16 infection in Kazakh’s esophageal cancer (EC) in Xinjiang, China.
METHODS: Encoding regions of p53 codon 72 and HPV-16 E6 were amplified by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and polymerase chain reaction (PCR) methods using pairs of primary esophageal squamous cell carcinoma (SCC) tissue and corresponding normal mucosa, which were collected from 104 patients of Kazakh in Xinjiang, China.
RESULTS: Only arginine allele was detected in 70.1% (39/55) of HPV-16-E6- positive cases but only in 40.8% (20/49) of HPV-16-E6-negative cases (P < 0.05; OR, 3.53; 95%CI, 1.57-7.98). In contrast, such a significant correlation between p53 polymorphism and HPV infection was not evident in corresponding normal mucosae. The allele frequency of Arg allele in cancer cases (0.68) was higher than that in normal mucosa samples (0.54) (P < 0.05; OR, 1.80; 95%CI, 1.21-2.69).
CONCLUSION: p53 codon 72 Arg homozygous genotype is one of the high-risk genetic factors for HPV-associated SCC of Kazakh. Individuals carrying Arg allele compared to those with Pro allele have an increased risk for esophageal SCC.
INTRODUCTION
Esophageal cancer is common in several areas of central Asia, including Xinjiang Uygur Autonomous Region,China. The incidence of Kazakh’s EC is the highest among population in Xinjiang and its age-adjusted mortality rate up to 91/100000 has been reported in Kazakh’s population[1]. The population size of Kazakh was estimated to be 13 million around the world and 10 million Kazakh distributed in Kazakhstan and 2 million in Xinjiang, China. The population in the present study was a Kazakh isolated community located at the Northeast of Xinjiang. The genetic homogeneity and geography stability of the population, along with shared exposure to common environmental variables, provide an excellent opportunity for the study of genetic influence on EC. These cancers are mostly SCC , and show a high frequency of mutation in the p53 tumor suppressor[2]. Epidemiological studies have suggested that a number of risk factors are involved in the carcinogenesis of Kazakh’s SCC, including deficiencies in vitamins and minerals, consumption of pickled foods and environmental exposure to specific nitrosamines, etc[3,4]. Viral infections, in particular HPV infection, have been reported in esophageal cancers from China, and HPV DNA has been detected in 0%-60.0% of cancer tissues by polymerase chain reaction analysis[5,6]. HPV is implicated in the pathogenesis of squamous cell carcinoma of the cervix and esophagus. HPV-16 encodes E6 protein, which binds to cellular tumor-suppresser protein p53 and directs degradation through the ubiquitin pathway[7]. This event is mediated by another cellular protein termed E6-AP, a component of the ubiquitin pathway[8,9]. The arginine allele at codon 72 of p53 was found to be more susceptible to degradation by HPV E6 protein than the proline allele in vivo, thus resulting in a high frequency of esophageal SCC in individuals homozygous for arginine at the codon[8]. On the basis of these experiments, it has been widely assumed that p53 is functionally inactivated by the viral E6 protein in HPV-associated cancer cells and that infection with high-risk HPV types leads to the same phenotype as a loss of p53 function because of p53 gene mutations or direct degradation[9].The association of p53 codon 72 polymorphism with HPV-16-associated esophagus SCC risk has been studied by several groups but with inconsistent results. Kawaguchi et al[10] reported that the form of p53 protein carrying an Arg residue at this position in HPV-16/18 positive samples was found to be significantly more susceptible to degradation by E6 protein than that in HPV-16/18 negative samples. There are controversial results from several clinical studies of esophagus SCC[11,12]. A part of Kazakh’s esophageal SCC correlated with the presence of HPV-16/18[13]. To our knowledge, p53 polymorphism in Kazakh’s esophageal SCC has apparently not been documented. In this study, we investigated the genotypic frequency of p53 codon 72 polymorphism and HPV-16 E6 in Kazakh’s esophageal SCC patients in Xinjiang, China. The data we obtained seemed to be the first regarding the association of this polymorphism with HPV-associated risk for cancer of the esophagus.
MATERIALS AND METHODS
Tissue specimens
Pairs of primary Kazakh’s esophageal SCC tissue and corresponding normal mucosa were obtained from 63 patients who underwent surgery in the Department of Surgery, 1st Teaching Hospital of Xinjiang Medical University, from 1999 to 2003, and from 41 patients who underwent surgery in Department of Surgery, the People’s Hospital of Xinjiang Uygur Autonomous Region, China, between 1998 and 2000. No patient had been given treatment prior to the study. In all cases the histopathological type of tumors was squamous cell carcinoma. Cancer tissues and well-separated normal esophageal mucosae obtained from surgically resected esophageal SCC patients were fixed in 40 g/L formaldehyde and embedded in paraffin. Genomic DNA was prepared by proteinase K digestion and phenol/ chloroform extraction, followed by ethanol precipitation, as described by Greer et al[14].
HPV detection and identification
First, as a control, purified genomic DNA was successfully amplified by PCR using primers specific for the β-globin gene, indicating a suitable quality and quantity of DNA. PCR analysis was then performed using HPV-16 E6 oligodeoxynucleotide primers as follows: HPV-16E6 forward, 5’-GCAAGCAACAGTT ACTGCGA-3’ and reverse, 5’-CAACAAGACATACATCGACC -3’. Amplified PCR products were then determined by electrophoresis on 15 g/L agarose gels stained with ethidium bromide. Finally, the gels were analyzed by DC-2000 image system (Bio-Rad, USA).
Analysis of codon 72 polymorphism
p53 exon 4 (codons 33-125) containing codon 72 was amplified by PCR using oligodeoxynucleotide primers 5’-TGAGGACC TGGTCC TCTGAC-3’ (forward) and 5’-AGAGGAATCCCAAA GTTCCA-3’ (reverse), under the following conditions: denaturation at 94 °C for 30 s, primary annealing at 54 °C for 30 s, and extension at 72 °C for 30 s for 35 cycles. PCR products (412 bp) were digested overnight at 37 °C with Acc II, which was cut within the sequence corresponding to the Arg codon (CGC) at position 72 to generate two fragments of 252 bp and 160 bp[15]. The DNA fragments were then resolved by electrophoresis on 30 g/L agarose gels stained with ethidium bromide. Presence of uncut (412 bp) DNA was indicative of the Pro allele and heterozygous for Arg/Pro genotypes showed three fragments of 412, 252 and 160 bp.
Statistical analysis
Chi-square test was used to examine the correlation between the p53 codon 72 polymorphism of the esophageal SCC patients and the presence of HPV-16E6 by SPSS software(12.0).
RESULTS
Frequency of HPV-16E6 among Kazakh’s esophageal SCC patients
Pairs of 104 DNA sample from primary Kazakh’s esophageal SCC tissues and corresponding normal mucosae were analyzed for the presence of oncogenic HPV-16-E6 using PCR methods (Figure 1). The frequency of HPV-16-E6 gene in cancer cases (52.9%) was higher than that in corresponding normal mucosae (39.4%) (Table 1, Table 2). These results were similar to previous reports[16].
Figure 1.
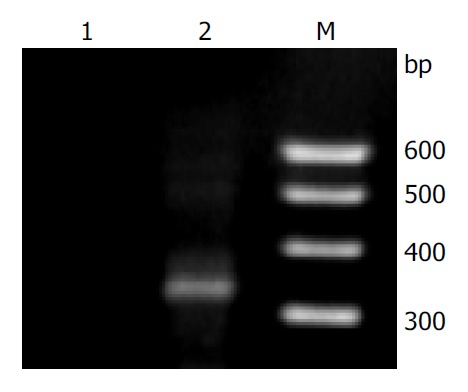
Agarose gel electrophoresis of HPV-16 E6 PCR-am-plified fragments. Lane M: 100 bp DNA ladder marker; Lane 1: negative sample; and Lane 2: positive sample.
Table 1.
Arg and Pro alleles of p53 in SCCs of Kazakh’s esopha-gus (n, %)
| Pro | Pro/Arg | Arg | |
| Esophageal SCC (n = 104) | 21 (20.2) | 25 (24.0) | 58 (55.8) |
| HPV16E6 positive (n = 55) | 8 (14.5) | 8 (14.5) | 39 (71.0)a |
| HPV16E6 negative (n = 49) | 13 (26.5) | 16 (32.7) | 20 (40.8) |
P < 0.05 vs negative.
Table 2.
Arg and Pro alleles of p53 in normal mucosae of Kazakh’s esophagus (n, %)
| Pro | Pro/Arg | Arg | |
| Normal mucosa (n = 104) | 28 (26.9) | 40 (38.5) | 36 (34.6) |
| HPV16E6 positive (n = 41) | 10 (24.4) | 18 (43.9) | 13 (31.7) |
| HPV16E6 negative (n = 63) | 10 (15.9) | 30 (47.6) | 23 (36.5) |
Arg allele at the codon 72 in HPV-associated esophageal SCC
PCR-RFLP was carried out on 104 DNA samples from primary Kazakh’s esophageal SCC tissues and corresponding normal mucosae to analyze the association between the p53 codon 72 polymorphism and HPV-associated esophageal SCC by DC-2000 image system (Figure 2). It showed a typical pattern of codon 72 evaluated by restriction analysis. Presence of the Pro allele resulted in resistance of the PCR amplified DNA fragment to digestion by Acc II. The comparision between the distribution of p53 alleles at codon 72 in HPV-positive esophageal SCC with the HPV-negative group is shown in Table 1. The frequency of presence of Arg allele alone from the Kazakh’s esophageal cancer specimens was similar (55.8%, 58 of 104) to other population cases of esophageal cancer[10]. Moreover, there was a marked difference in the frequency of Pro/Arg alleles between HPV-positive and HPV-negative groups. p53 Arg allele alone was detected in 71.0% (39/55) in the HPV-positive group, whereas in 40.8% (20/49) in the HPV-negative group (P < 0.05; OR, 3.53; 95%CI, 1.57-7.98) (Table 1). The allele frequency of Arg alleles in cancer cases (0.68) was higher than that in normal mucosa samples (0.54) (P < 0.05;OR, 1.80; 95%CI, 1.21-2.69).
Figure 2.
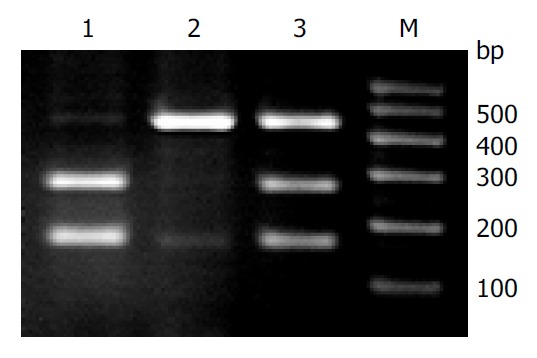
Restriction analysis of p53 codon 72 polymorphism. The PCR product from the proline alleles had a single band with a fragment of 412 bp in length. The arginine was cleaved by Acc II, yielding two small fragments (252 and 160 bp). Lane M: 100 bp DNA ladder marker; Lane 1: homozygous for arginine; Lane 2: homozygous for proline; and Lane 3: di-gested sample, heterozygous for the polymorphism.
Arg allele at the codon 72 in surrounding normal mucosae in HPV-associated esophageal cancer
Differences in p53 polymorphism in the corresponding normal mucosae were not significant between HPV-positive and -negative tissues (P > 0.05; OR, 0.81; 95%CI, 0.35-1.86) (Table 2).
DISCUSSION
Infection with human papilloma virus is an important etiological factor in the development of SCC and it has been proposed that individuals homozygous for Arg/Arg at codon 72 of p53 are several times more susceptible to HPV-mediated cancer[10,17]. In agreement with the result, studies in India and Mexico also found a strong increase in SCC risk associated with p53 polymorphism and the presence of HPV infection[18,19]. In China similar research has been carried out on esophageal SCC, ovarian carcinoma, and breast carcinoma[20]. However, several studies conducted in different countries failed to reproduce this observation[21-25].This polymorphism has been shown to vary with ethnic and geographical distribution.However, its influence has not been elucidated in the Kazakh population.
In the present study, the frequency of HPV-16-E6 gene in Kazakh’s esophageal SCC cases was higher than that in corresponding normal mucosae, suggesting that there is a trend towards an association between the carcinogenesis of Kazakh’s esophageal SCC and the presence of HPV-16 infection. These results were similar to previous report[16], which suggested that infection with HPV-16 might be involved in carcinogenesis of Kazakh’s esophageal SCC. In addition, the distribution of p53 codon 72 Arg homozygous genotype in Kazaks’s esophageal SCC was significantly higher than that in corresponding normal mucosae, indicating that an individual homozygous for p53 Arg would be more likely to develop esophageal SCC than a Pro/Arg heterozygote or a Pro homozygote. Furthermore, it is noteworthy that the distribution of p53 codon 72 Arg homozygous genotype in HPV positive samples of Kazakh’s esophageal SCC was at a 3.53-folds higher risk for the development of esophageal SCC compared with HPV negative samples. In contrast, such a significant correlation between p53 polymorphism and HPV infection was not evident in corresponding normal mucosae. From the above analyses, when stratified with HPV infection, the frequency of p53 codon 72 Arg homozygous genotype was at a 1.48-folds increased risks for developing Kazakh’s esophageal SCC compared with p53 Arg homozygosity (Arg/Arg) solely. Therefore, this implied p53 codon 72 Arg polymorphism in combination with HPV infection could increase the risk of development of SCC in Kazakhs.
p53 tumor-suppresser protein accmulates rapidly through post-transcriptional mechanisms and is also activated as a transcriptional factor, thus leading to growth arrest or apoptosis when DNA damage has occurred[26]. The ubiquitin-dependent proteolytic pathway plays a major role in selective protein deregulation. E6 oncoprotein of oncogenic HPV-16/18 might use this cellular proteolytic system to target p53 protein[7] and bind to a cellular protein of E6-AP, and the E6-AP complex might interact with p53, resulting in the rapid ubiquitin-dependent degradation of p53[27]. The level and half-life of p53 in E6 immortalized cell lines or in HPV-positive cervical carcinoma cells have been reported to be generally decreased[28,29]. Certain HPV types such as HPV-16/18 found in SCC of esophagus suggested a model by which E6 degraded cell growth control by elimination of the p53 tumor suppresser protein and led to HPV-associated esophageal SCC[10,30].
In conclusion, the current study reveals the potential role of the polymorphism of p53 at codon 72 in HPV-associated carcinogenesis of esophageal SCC in Kazakh population. Individuals carrying Arg alleles compared to those with Pro alleles have an increased risk for esophageal SCC.
ACKNOWLEDGMENTS
We thank Professor Zhen-Zhu Sun (Department of Pathology, the People’s Hospital of Xinjiang Uygur Autonomous Region, China ) for providing the samples of Kazakh’s esophageal SCC.
Footnotes
Supported by Xinjiang Key Laboratory Foundation, No. XJDX0202-2003-05
Edited by Kumar M and Wang XL Proofread by Xu FM
References
- 1.Zhang YM. The distribution of esophageal cancer in Xinjiang. Xinjiang Yixueyuan Xuebao. 1988;11:139–144. [Google Scholar]
- 2.Liang YY, Estève A, Martel-Planche G, Takahashi S, Lu SH, Montesano R, Hollstein M. p53 mutations in esophageal tumors from high-incidence areas of China. Int J Cancer. 1995;61:611–614. doi: 10.1002/ijc.2910610505. [DOI] [PubMed] [Google Scholar]
- 3.Muñoz N, Wahrendorf J, Bang LJ, Crespi M, Thurnham DI, Day NE, Ji ZH, Grassi A, Yan LW, Lin LG. No effect of riboflavine, retinol, and zinc on prevalence of precancerous lesions of oesophagus. Randomised double-blind intervention study in high-risk population of China. Lancet. 1985;2:111–114. doi: 10.1016/s0140-6736(85)90223-5. [DOI] [PubMed] [Google Scholar]
- 4.Lin K, Shen W, Shen Z, Cai S, Wu Y. Estimation of the potential for nitrosation and its inhibition in subjects from high- and low-risk areas for esophageal cancer in southern China. Int J Cancer. 2003;107:891–895. doi: 10.1002/ijc.11506. [DOI] [PubMed] [Google Scholar]
- 5.He D, Zhang DK, Lam KY, Ma L, Ngan HY, Liu SS, Tsao SW. Prevalence of HPV infection in esophageal squamous cell carcinoma in Chinese patients and its relationship to the p53 gene mutation. Int J Cancer. 1997;72:959–964. doi: 10.1002/(sici)1097-0215(19970917)72:6<959::aid-ijc7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Chen B, Yin H, Dhurandhar N. Detection of human papillomavirus DNA in esophageal squamous cell carcinomas by the polymerase chain reaction using general consensus primers. Hum Pathol. 1994;25:920–923. doi: 10.1016/0046-8177(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 7.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 8.Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huibregtse JM, Scheffner M, Howley PM. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol Cell Biol. 1993;13:4918–4927. doi: 10.1128/mcb.13.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaguchi H, Ohno S, Araki K, Miyazaki M, Saeki H, Watanabe M, Tanaka S, Sugimachi K. p53 polymorphism in human papillomavirus-associated esophageal cancer. Cancer Res. 2000;60:2753–2755. [PubMed] [Google Scholar]
- 11.Peixoto Guimaraes D, Hsin Lu S, Snijders P, Wilmotte R, Herrero R, Lenoir G, Montesano R, Meijer CJ, Walboomers J, Hainaut P. Absence of association between HPV DNA, TP53 codon 72 polymorphism, and risk of oesophageal cancer in a high-risk area of China. Cancer Lett. 2001;162:231–235. doi: 10.1016/s0304-3835(00)00643-1. [DOI] [PubMed] [Google Scholar]
- 12.Lu Z, Chen K, Guo M. [Detection of HPV in human esophageal cancer in high-incidence area and its correlation with p53 expression] Zhonghua Zhongliu Zazhi. 2001;23:220–223. [PubMed] [Google Scholar]
- 13.Zou SY, Si JY, Liu XM, Tang XP. PCR in detdction of human papillomavirus DNA in esophageal carcinoma in Xingjiang. Aizheng. 1998;17:32–34. [Google Scholar]
- 14.Greer CE, Whee Le CM, Manos MM. PCR Primer A Labora-tory Manual. Cold Spring Harbor Laboratory Press. 1995. pp. 64–69. [Google Scholar]
- 15.de la Calle-Martin O, Fabregat V, Romero M, Soler J, Vives J, Yague J. AccII Polymorphism of the p53 gene. Nucleic Acids Res. 1990;18:4963. [PMC free article] [PubMed] [Google Scholar]
- 16.Chang F, Syrjänen S, Shen Q, Cintorino M, Santopietro R, Tosi P, Syrjänen K. Human papillomavirus involvement in esophageal carcinogenesis in the high-incidence area of China. A study of 700 cases by screening and type-specific in situ hybridization. Scand J Gastroenterol. 2000;35:123–130. doi: 10.1080/003655200750024272. [DOI] [PubMed] [Google Scholar]
- 17.Ojeda JM, Ampuero S, Rojas P, Prado R, Allende JE, Barton SA, Chakraborty R, Rothhammer F. p53 codon 72 polymorphism and risk of cervical cancer. Biol Res. 2003;36:279–283. doi: 10.4067/s0716-97602003000200017. [DOI] [PubMed] [Google Scholar]
- 18.Saranath D, Khan Z, Tandle AT, Dedhia P, Sharma B, Contractor R, Shrivastava S, Dinshaw K. HPV16/18 prevalence in cervical lesions/cancers and p53 genotypes in cervical cancer patients from India. Gynecol Oncol. 2002;86:157–162. doi: 10.1006/gyno.2002.6735. [DOI] [PubMed] [Google Scholar]
- 19.Sifuentes Alvarez A, Reyes Romero M. [Risk factors for cervico-uterine cancer associated to HPV: p53 codon 72 polymorphism in women attending hospital care] Ginecol Obstet Mex. 2003;71:12–15. [PubMed] [Google Scholar]
- 20.Li T, Lu ZM, Guo M, Wu QJ, Chen KN, Xing HP, Mei Q, Ke Y. p53 codon 72 polymorphism (C/G) and the risk of human papillomavirus-associated carcinomas in China. Cancer. 2002;95:2571–2576. doi: 10.1002/cncr.11008. [DOI] [PubMed] [Google Scholar]
- 21.Humbey O, Cairey-Remonnay S, Guérrini JS, Algros MP, Mougin C, Bittard H, Aubin F. Detection of the human papillomavirus and analysis of the TP53 polymorphism of exon 4 at codon 72 in penile squamous cell carcinomas. Eur J Cancer. 2003;39:684–690. doi: 10.1016/s0959-8049(02)00835-3. [DOI] [PubMed] [Google Scholar]
- 22.Inserra P, Abrahamsen M, Papenfuss M, Giuliano AR. Ethnic variation of the P53 codon 72 polymorphism, HPV persistence, and cervical cancer risk. Int J STD AIDS. 2003;14:800–804. doi: 10.1258/095646203322556110. [DOI] [PubMed] [Google Scholar]
- 23.Comar M, Molin GD, Guaschino S, Campello C. p53 at codon 72 polymorphism, human papillomavirus infection and cervical lesions: a cross-sectional study from northeastern Italy. Eur J Obstet Gynecol Reprod Biol. 2004;114:210–214. doi: 10.1016/j.ejogrb.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Tanara G, Falugi C, Cesario A, Margaritora S, Russo P, Cosimi A. TP53 codon 72 polymorphism does not affect risk of cervical cancer in patients from The Gambia. Int J Biol Markers. 2003;18:280–283. doi: 10.1177/172460080301800405. [DOI] [PubMed] [Google Scholar]
- 25.Ngan HY, Liu VW, Liu SS, Cheng DK, Ng TY, Wong LC. Homozygous arginine at codon 72 of p53 has no prognostic significance in cervical cancer. Tumour Biol. 2000;21:135–138. doi: 10.1159/000030119. [DOI] [PubMed] [Google Scholar]
- 26.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 27.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 28.Scheffner M, Münger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA. 1991;88:5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hengstermann A, Linares LK, Ciechanover A, Whitaker NJ, Scheffner M. Complete switch from Mdm2 to human papillomavirus E6-mediated degradation of p53 in cervical cancer cells. Proc Natl Acad Sci USA. 2001;98:1218–1223. doi: 10.1073/pnas.031470698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vos M, Adams CH, Victor TC, van Helden PD. Polymorphisms and mutations found in the regions flanking exons 5 to 8 of the TP53 gene in a population at high risk for esophageal cancer in South Africa. Cancer Genet Cytogenet. 2003;140:23–30. doi: 10.1016/s0165-4608(02)00638-6. [DOI] [PubMed] [Google Scholar]


