Abstract
AIM: To explore the expression of heparanase mRNA and point mutation in hepatocellular carcinoma (HCC).
METHODS: Reverse transcription polymerase chain reaction was used to measure the expression of heparanase mRNA in the primary tumor tissues and surrounding liver tissues of 33 HCC patients. T-A cloning and sequencing were used to detect whether there was any mutation in the amplified PCR products.
RESULTS: The expression of heparanase mRNA was positive in 16 primary tumor tissues of HCC, and the positive rate was 48.5%, which was significantly higher than that in the surrounding liver parenchyma (P < 0.01). The positive rate for heparanase gene in high-tendency to metastatic recurrence group (71.4%, 10/14) was obviously higher than that in low-tendency to metastatic recurrence group (31.6%, 6/19) (P = 0.023). The positive rate for heparanase gene in patients with metastatic recurrence during postoperative follow-up (78.6%, 11/14) was also significantly higher than that in those without metastatic recurrence (21.4%, 3/14) (P = 0.003). Sequence analysis of the HPA PCR products was made in 7 patients, and 2-point mutations were found in 4 patients, one of which was sense mutation, neither base insertion nor deletion was detected. The mutation rate was 57.1% (4/7).
CONCLUSION: The expression rate of heparanase mRNA increases in HCC, and HPA mRNA may be one of the reliable markers for the metastatic activity gained by the liver tumor cells and could be used clinically in predicting metastatic recurrence of HCC. Point mutation may be one of the causes for enhanced heparanase mRNA expression.
INTRODUCTION
Primary hepatocellular carcinoma (HCC) is a common malignant tumor, especially in China and southeast Asia. Although the resection rate of HCC has improved in recent 20 years, the general therapeutic efficacy is still not satisfactory yet. The death rate of HCC ranks second in all malignant tumors in China, due to postoperative metastatic recurrence as the main cause. Tumor invasion and metastasis can break through the tissue barriers, which are formed of extracellular matrix (ECM) and basement membranes (BMs) and are composed of structural proteins, including collagen, laminin and vitronectin,etc. and glycosaminoglycans (GSGs). The chief components of GSG are heparan sulfate proteoglycans (HSPGs) that are principally composed of a core protein covalently linked to several heparan sulfate side chains[1]. Over the past ten years, most studies about neoplasm metastasis including HCC, focused on some proteases, such as matrix metalloproteinases (MMPs)[2-4], u-PA[5,6], serine and cysteine protease[7,8] whose substrates were structural proteins, but heparanase (HPA), whose substrate was GSGs, was ignored. In recent years, mammalian HPA gene has successfully been cloned and sequenced in Israel and U.S.A[9-13], and HPA was found to play an important role in tumor invasion and metastasis. El-Assal et al[14] found that HPA mRNA expression in HCC patients was related to the tumor sizes, staging, classification, infection of hepatitis C virus (HCV), vascularization, postoperative metastasis and prognosis. However, in their studies, over half of HCC patients (52.7%, 29/55) were accompanied with HCV infection, and few were associated with hepatitis B virus (HBV). Ikeguchi et al[15] found that relative heparanase mRNA expression level in HCC was significantly lower than that in noncancerous liver tissues (P < 0.001), and that tumor heparanase expression did not correlate to tumor differentiation, tumor stage, or patient prognosis. They concluded that enhanced heparanase mRNA expression might not be a good biological marker for HCC. In addition, the possible mechanism of HPA expression has not been explored in both studies. It is well known that most HCCs in China are HBV-associated, which is different from the HCCs reported in Japan, but whether there is some difference of HPA expression in HCCs between in China and Japan or other regions is still unknown. In the present study, we tried to find out whether HPA mRNA expression was related to the clinicopathological indexes, including infection of HBV and postoperative metastatic recurrence of Chinese HCC patients, whether there was mutation in HPA gene, and whether the mutation of HPA gene was associated with HPA mRNA expression.
MATERIALS AND METHODS
Patients
Thirty-three patients (28 men and 5 women) undergoing curative hepatic resection for HCC between October 2000 and April 2001, were included in the present study. None of the patients received preoperative chemo- or embolic therapy. The patients’ ages ranged from 27 to 73 years (49 ± 7 years, mean ± SD). Among the 33 patients, the biggest diameter of tumors was > 5 cm in 25 cases and ≤ 5 cm in 8. Tumor capsules were integrated in 14 and disintegrated in 19 cases. Serum AFP was positive in 21 and negative in 12; HBsAg was positive in 24 and negative in 9. Liver cirrhosis was detected in 18 cases. Thirteen patients belonged to Edmondson grade I or II, and the remaining 20 patients to Edmondson grade III or IV. Eighteen patients belonged to TNM staging I or II,and 15 to TNM staging III or IV. According to the operative records and postoperative pathologic data, 14 HCC patients with cancer emboli, intrahepatic dissemination (satellite foci or multiple nodules) and/or lymph node metastasis were demarcated to high-tendency to metastatic recurrence group, and the other 19 patients without emboli, dissemination and/or metastasis belonged to low-tendency to metastatic recurrence group. A total of 28 patients were followed up 6-16 mo after operation, during which neoplasm metastasis or recurrence was found in 14 patients.
Tissue selection
After the neoplasm was resected, HCC tissues from all the patients were selected from the most viable areas of the tumors immediately. This aimed at excluding areas of tissue necrosis and hemorrhages, which might influence the quality and the quantity of the extracted RNA. For selection of surrounding non-tumor liver tissues, specimens were obtained from tissues at a clear distance from the edge of tumors (> 1cm), if there was no evidence of nearby tumor invasion. Tissues were at once preserved in liquid nitrogen after the resection and kept at -80 °C until the experiment began.
RNA extraction and cDNA synthesis
About 100 mg tumor or liver tissue was used for total RNA isolation using TRIzol reagent (Gibco-BRL), according to the instructions of the manufacturer. First-strand cDNA was synthesized using 5 L total RNA with oligo (dT)16 primer in a 50-L reverse transcription mixture containing 10 L of 5 × first-strand buffer, 2.5 L dNTP mixture containing 25 mmoL/L each deoxynucleotide triphosphate base (Pharmacia Biotech, Tokyo, Japan), 2.5 L ribonuclease inhibitor (TaKaRa Biochemicals, Ohotsu, Japan), 25 L ddH2O (managed with DEPC in advance), and 2.5 L avian myeloblastosis virus reverse transciptase (TaKaRa Biochemicals, Ohotsu, Japan).
PCR amplification of HPA and β-actin genes
The resulting cDNA was used for PCR amplification using Taq polymerse (TaKaRa Biochemicals, Ohotsu, Japan). The primers for HPA PCR amplification were designed according to the literature[9]. The sequences of the oligonucleotides were: forward,5’-TTCGATCCCAAGAAGGAATCAAC-3’; and reverse, 5’-GATTCAGTTACATGGCATCACTAC-3’. The first and final bases of the amplified HPA segment were at the 409th and 993rd positions of the HPA cDNA, respectively, and the amplified segment should be 585 bp in length. The primers used for β-actin had the following sequences: forward, 5’-TTCCAGCCTTCCTTCCTGG-3’,and reverse, 5’-ATTGCTCCTCCTGAGCGCAA-3’, as generated by Oligo 4.0 S computer software. The amplified β-actin segment was 224 bp in length. The PCR conditions included initial denaturation at 94 °C for 4 min, followed by 35 cycles of amplification with subsequent denaturation at 94 °C for 30 s, annealing at 57 °C for 45 s, and extension for 1 min at 72 °C. Ten μL PCR products underwent electrophoresis using 12 g/L agarose and was visualized by UV absorption and ethidium bromide.
T-A cloning and sequencing
By using Escherichia coli, competent cell DH5α was routinely prepared and stored at -80 °C. One hundred μL liquid HPA PCR products was used for purification. Five liters of purified preparation, 1 L pGEM-Teasy vector (3015 bp in length) and 1 L T4-DNA ligase (Promega, New York, U.S.A) were mixed and incubated overnight at 4 °C for ligation reaction. After 200 L competent cell suspension was thawed at room temperature, 5 L ligated products was added for transformation test. The recombinant plasmid was screened, and the plasmid DNA was extracted by the alkaline lysis method. Five liters plasmid DNA was digested with 0.5 L restriction endonuclease EcoR I for 2-3 h at 37 °C, then the enzyme digestion products were identified by electrophoresis. Results of enzyme digestion analysis were essentially identical with expected ones. Using the recombinant plasmid DNA as a template, DNA sequencing for both strands was performed on an ALF express DNA automatic sequencer (Pharmacia Co.) by the dideoxy terminal termination method. The sequenced HPA segment was 585 bp in length. The sequence of amplified HPA segment was compared with the gene bank database and analyzed for homogeneity using BLAST program at NCBI.
Statistical analysis
The significance of difference between two groups was tested with Chi-square analysis or exact probabilities in fourfold table. A P value less than 0.05 was considered statistically significant.
RESULTS
Expression of HPA mRNA in HCC
HPA mRNA was amplified in the tumor tissues from 16 patients. Electrophoretic analysis showed a bright band about 550-600 bp in length in these patients. No amplification strand was found in the other 17 patients (Figure 1). The HPA mRNA expression rate in the tumor tissues of HCC was 48.5% (16/33) and significantly higher than that in the surrounding non-tumor liver tissues (P < 0.01) in which HPA mRNA was positive in only one patient.
Figure 1.
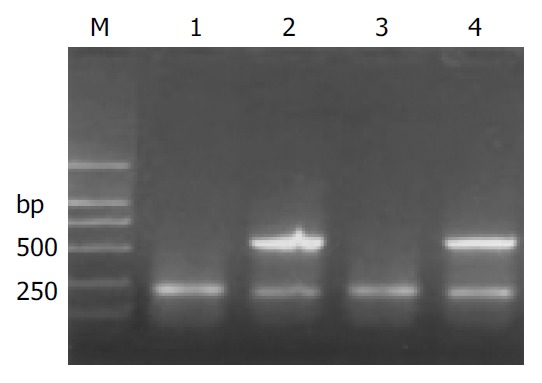
Expression of HPA mRNA in HCC. M: molecular mass markers (DL2000); lanes 1 and 3: the noncancerous liver tissue; lanes 2 and 4: the HPA positive samples of cancer tissues both with a bright band at 585 bp.
Relationship between HPA expression and clinicopathological indexes
By statistical analysis, no significant difference in HPA mRNA expression was found among the tumor size, capsule, AFP, HBsAg and liver cirrhosis groups (P > 0.05) (Table 1). HPA expression rate in Edmondson grade I or II group was significantly lower than that in Edmondson grade III or IV group (P = 0.019), and the rate in TNM staging I or II group was also obviously lower than that in TNM staging III or IV group (P = 0.047) (Table 1).
Table 1.
Relationship between the HPA expression and the clinicopathological parameters of HCC
| Items | Number | HPA positive | HPA negative | P value |
| Size of tumor | ||||
| > 5 cm | 25 | 13 | 12 | 0.250 |
| ≤ 5 cm | 8 | 3 | 5 | |
| Tumor capsule | ||||
| Integrated | 14 | 5 | 9 | 0.130 |
| Disintegrated | 19 | 11 | 8 | |
| AFP | ||||
| Positive | 21 | 12 | 9 | 0.125 |
| Negative | 12 | 4 | 8 | |
| HBsAg | ||||
| Positive | 24 | 12 | 12 | 0.292 |
| Negative | 9 | 4 | 5 | |
| Liver cirrhosis | ||||
| Yes | 18 | 8 | 10 | 0.241 |
| No | 15 | 8 | 7 | |
| Edmondson grade | ||||
| I, II | 13 | 3 | 10 | 0.019 |
| III, IV | 20 | 13 | 7 | |
| TNM staging | ||||
| I, II | 18 | 6 | 12 | 0.047 |
| III, IV | 15 | 10 | 5 |
Relationship between HPA expression and metastatic recurrence of HCC
HPA mRNA expression rate in high-tendency to metastatic recurrence group was obviously higher than that in low-tendency to metastatic recurrence group (P = 0.023), and the rate in metastatic recurrence group was also significantly higher than that in non-metastatic recurrence group (P = 0.003) (Table 2).
Table 2.
Relationship between HPA expression and metastatic recurrence of HCC
| Items | Number | HPA positive | HPA negative | P value |
| Tendency to metastatic recurrence | ||||
| High | 14 | 10 | 4 | 0.023 |
| Low | 19 | 6 | 13 | |
| Metastatic recurrence | ||||
| Yes | 14 | 11 | 3 | 0.003 |
| No | 14 | 3 | 11 |
Point mutation
After the recombinant plasmid DNA was digested by EcoR I and run in 10 g/L agarose gel electrophoresis, 2 bright bands could be seen, which were about 750 and 2900 bp in length, respectively, according to the markers. The recombinant plasmid that was not digested by the enzyme showed only one band about 3600 bp in length, and the pure HPA RT-PCR products only produced one strip about 550-600 bp in length in the same agarose gel. These results proved that it was successful to purify HPA RT-PCR products, ligate them with plasmid DNA to be digested by restriction endonuclease (Figure 2). Seven samples of HPA mRNA positive PCR products were cDNA sequenced, and the results confirmed that the target gene segment in all the 7 samples of PCR products was human HPA cDNA. Two point mutations (at the 513th and 878th base of the HPA cDNA, respectively) were observed in 4 samples, and no insertion or deletion was found. The mutation rate was 57.1% (4/7). One of the point mutations of G to A transversion was at the third base position of codon 138. Because the varied codon was still translated to glutamic acid resulting in no alternation of amino acid residues, the mutation belonged to nonsense mutation. The other point mutation of A to G transversion at nucleotide 878 corresponded to the second base position of codon 260. After transversion, the codon became AGU from AAU, resulting in alternation of amino acid residues (asparamide to serine), it was therefore sense mutation (Table 3, Figure 3).
Figure 2.
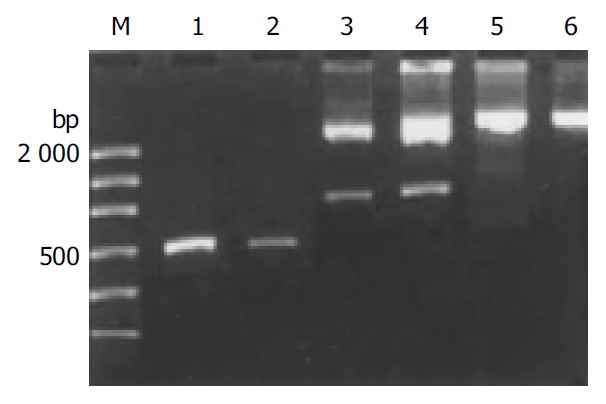
Electrophoretogram of the recombinant plasmid DNA digested by EcoRI. M: molecular mass markers (DL2000); lanes 1 and 2: the pure HPA RT-PCR products; lanes 3 and 4: the products of digestion; lanes 5 and 6: the products without digestion.
Table 3.
Sequencing and corresponding series number
| Number | Nucleotide sequence | Remarks | |||||
| TTGG | GCCCGACGTC | GCATGCTCCC | GGCCGCCATG | GCGGCCGCGG | GAATTCGATT | Plasmid DNA | |
| 409-468 | TTCGATCCCA | AGAAGGAATC | AA1CCTTTGAA | GAGAGAAGTT | ACTGGCAATC | TCAAGTCAAC | HPA cDNA |
| 469-528 | CAGGATATTT | GCAAATATGG | ATCCATCCCT | CCTGATGTGG | AGGAA3,5AAGTT | ACGGTTGGAA | HPA cDNA |
| 529-588 | TGGCCCTACC | AGGAGCAATT | GCTACTCCGA | GAACACTACC | AGAAAAAGTT | CAAGAACAGC | HPA cDNA |
| 589-648 | ACCTACTCAA | GAAGCTCTGT | AGATGTGCTA | TACACTTTTG | CAAACTGCTC | AGGACTGGAC | HPA cDNA |
| 649-708 | TTGATCTTTG | GCCTAAATGC | GTTATTAAGA | ACAGCAGATT | TGCAGTGGAA | CAGTTCTAAT | HPA cDNA |
| 709-768 | GCTCAGTTGC | TCCTGGACTA | CTGCTCTTCC | AAGGGGTATA | ACATTTCTTG | GGAACTAGGC | HPA cDNA |
| 769-828 | AATGAACCTA | ACAGTTTCCT | TAAGAAGGCT | GATATTTTCA | TCAATGGGTC | GCAGTTAGGA | HPA cDNA |
| 829-888 | GAAGATTTTA | TTCAATTGCA | TAAACTTCTA | AGAAAGTCCA | CCTTCAAAAG4 | T6GCAAAACTC | HPA cDNA |
| 889-948 | TATGGTCCTG | ATGTTGGTCA | GCCTCGAAGA | AAGACGGCTA | AGATGCTGAA | GAGCTTCCTG | HPA cDNA |
| 949-993 | AAGGCTGGTG | GAGAAGTGAT | TGATTCAGTT | ACATGGCATC | ACTAC2 | HPA cDNA | |
| AATCACTAGT | GAATTCGCGG | CCGCCTGCAG | GTCGACCATA | TGGGAGAGCT | CCCAACGCGT | Plasmid DNA | |
| TGAATCACTA | GTGAATTCGC | GGCCGCCTGC | AGGTCGACCA | TATGGGAGAG | CTCCCAACGC | Plasmid DNA | |
| GTTG | Plasmid DNA | ||||||
1,2The segments with italics and boldface corresponded to the primers used in RT-PCR. 3,4The letters represented the mutated bases. 5,6The parts with underline represented the codons.
Figure 3.
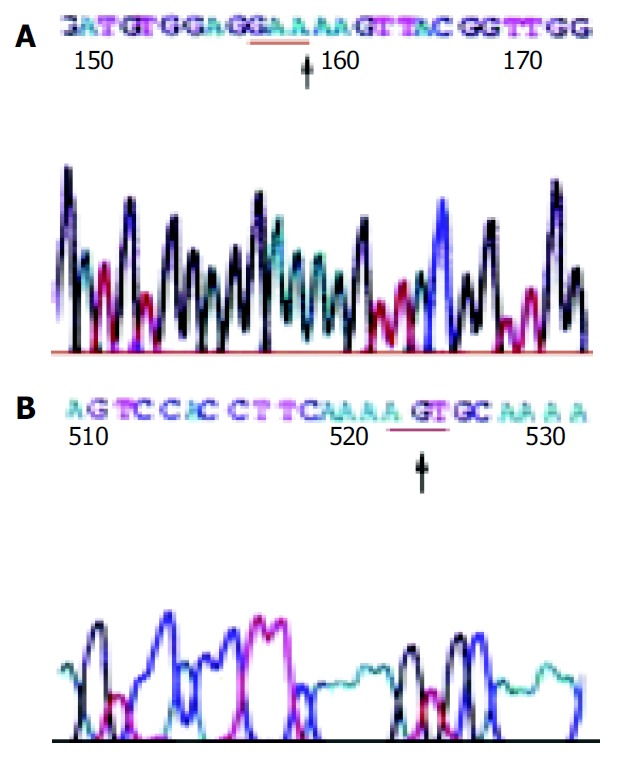
The sequencing map 1 (A) and map 2 (B) ↑, the mu-tated base; the parts with underline, the codons.
DISCUSSION
Mammalian genes including human HPA gene have been cloned and identified in recent years[9-13]. It is known that the gene is on chromosome 4q22[16] and includes 14 exons separated by 13 introns. The complete cDNA of HPA is 1758[9] or 1629 bp in length[10], and contains an open reading frame encoding a polypeptide of 543 amino acids, with a calculated molecular weight of 61 192 daltons. The HPA enzymes of about 50 ku isolated from human placenta and hepatoma cell line (SK-hep-1) may represent a processed or mature form of the native protein. In normal state, HPA is mainly identified in placenta, fetus liver, thymus gland, spleen, platelets, neutrophils, and activated T-and B-lymphocytes. HPA plays important physiological roles in embryonic morphogenesis, wound healing, inflammatory and autoimmune conditions by degrading GAGs. Many tumors and their stroma cells also produce or secrete HPA and use the same molecular machinery to induce neoplasm metastasis. RT-PCR analysis showed that most highly metastatic tumor cells expressed higher levels of HPA mRNA or HPA activity, but nonmetastatic carcinoma cells and low metastatic cells expressed no or only very weak HPA activity. HPA expression correlates with the metastatic potential of breast cancers[17], and antisense-mediated suppression of human heparanase gene expression may inhibit pleural dissemination of human cancer cells[18]. It has been found that high levels of HPA can be detected in a variety of malignant tissues or cells with high malignance or powerfully metastatic potential, among which are lymphoma, fibrosarcoma[9,19],oral cancer cell lines and oral cancer[20], esophageal carcinomas[21], lung cancer[18,22],malignant melanoma[19,23,24],breast cancer[9,25],gastric carcinoma and carcinoma of colon[26,27], pancreatic carcinoma[28,29], prostate cancer[30],bladder carcinoma[31], pheochromocytoma and ovarian cancer, and that no or only very weak HPA could be observed in some tumor cells with low or no metastatic potential. We detected the HPA mRNA expression of cancerous tissues in 33 HCC patients, and found that it was positive in 16 HCC samples (48.5%, 16/33). The expression rate of HPA mRNA in our study was similar to that of El-Assal et al[14] (47%). In addition, we detected the HPA mRNA expression in the surrounding non-tumor liver tissues, and found that it was positive in only one sample. These results showed that about half of HCC tissues could synthesize or secrete HPA, and the surrounding non-tumor liver tissues could hardly produce the enzyme on the whole.
HPA could degrade HSPG by cleaving the glycosidic linkages[24] with a hydrolase mechanism, destroy and degrade the ECM and BM barricade in coordination with other proteases to promote the invasion and metastasis of tumor cells[9,24]. Moreover, HPA could not only activate plasminogen and MMPs by means of promoting the release of urokinase-type plasminogen activator (u-PA) and tissue-type plasminogen activator (t-PA) but also facilitate the release of HS-banding active basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) to deliver its effect of enhancing cell metastasis and angiogenesis. It has been proved that HPA in cancerous tissues is closely related to tumor invasion, metastasis and angiogenesis[9,10]. Both metastatic recurrence and tumor microvessel density (MVD) in tumors might significantly increase with high levels of HPA. By analyzing statistically the relationship between HPA mRNA and clinicopathological parameters in HCC patients, El-Assal et al[14] found that HPA mRNA expression was related to the tumor size, staging, classification, infection of HCV, vascularization, postoperative metastasis and prognosis, but was not related to other clinicopathological parameters. In this study, the expression rate in HCC was 48.5%, and HPA expression was associated with the pathological classification and TNM staging, which were similar to the conclusions drawn by El-Assal et al[14]. The obvious difference in HPA expression between low- and high-tendency to metastatic recurrence groups in our study preliminarily indicated that HPA was associated with the invasion and metastasis of HCC. The significant difference between metastatic recurrence group and nonmetastatic recurrence group in our study was also similar to that of El-Assal[14], and further proved that HPA expression was associated with metastatic recurrence, and that there were stronger invasiveness and tendency of postoperative recurrence in patients with positive HPA compared with HPA negative patients, and that HPA might provide a potential and valuable index to predict clinically postoperative metastatic recurrence.
No relationship was found between HPA expression and the integrity of tumor size or capsule in this study, which was different from the study of El-Assal et al[14]. We speculate that the probable cause is that there were relatively fewer patients with tumors ≤ 5 cm in diameter (only 8 cases). In addition, HPA expression was not found to be associated with AFP, HBsAg and liver cirrhosis in this study perhaps because the types of causative viruses were different. Patients in the study of El-Assal et al[14] were mainly infected with HCV, but most HCCs in China were related to HBV infection and posthepatitic cirrhosis, few were infected with HCV. In addition, our conclusions are different from those of Ikeguchi et al[15], in which relative heparanase mRNA expression level in HCC was significantly lower than that in noncancerous liver tissues, and tumor heparanase expression did not correlate with tumor differentiation, tumor stage, or patient prognosis. Both the discrepancy of hepatitis virus and experimental methods could contribute to the different results. The surrounding non-tumor liver tissues in our study were at a clear distance from the edge of tumors (> 1 cm), but they could be close to the tumor tissues in the study of keguchi et al.
Both the positive rates of HPA mRNA in the study of El-Assal et al[14] and ours were close to 50% (47% and 48.5%, respectively), but very few HPAs were expressed in the surrounding non-tumor liver tissues. To our knowledge, no concrete mechanism of enhanced HPA mRNA expression in tumor tissues has been evaluated so far. To explore the probable mechanism of enhanced HPA expression in cancerous tissues, the positive PCR products of 7 patients were randomly selected and sequenced by T-A cloning. Two-point mutations including a G to A transversion at nucleotide 513 and a A to G at nucleotide 878 of the HPA cDNA were found in 4 cases. By sequence analysis, we did not find base insertion and deletion, and the mutation rate was 57.1%. The point mutation of G to A transversion at nucleotide 513 resulting in GAG to GAA at condon 138 was a nonsense mutation because the amino acid residue was still glutamic acid after translation. The other point mutation of A to G transversion at nucleotide 878 leading to AAU to AGU at condon 260 belonged to sense mutation because it resulted in the alteration of amino acid residue (asparamide to serine), which may cause HPA structure to change and HPA activity to increase, resulting in the acceleration of ECM and BM barrier degrading and the final metastasis of tumor cells. Therefore, we guess that the point mutation at HPA gene might be one of the important mechanisms of increased HPA expression and enhanced metastasis of carcinoma cells, and it deserves further study.
Footnotes
Edited by Ma JY and Wang XL Proofread by Xu FM
References
- 1.Eccles SA. Heparanase: breaking down barriers in tumors. Nat Med. 1999;5:735–736. doi: 10.1038/10455. [DOI] [PubMed] [Google Scholar]
- 2.Airola K, Karonen T, Vaalamo M, Lehti K, Lohi J, Kariniemi AL, Keski-Oja J, Saarialho-Kere UK. Expression of collagenases-1 and -3 and their inhibitors TIMP-1 and -3 correlates with the level of invasion in malignant melanomas. Br J Cancer. 1999;80:733–743. doi: 10.1038/sj.bjc.6690417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan YZ, Zhang JT, Yang HC, Yang YQ. Expression of MMP-2,TIMP-2 protein and the ratio of MMP-2/TIMP-2 in gallbladder carcinoma and their significance. World J Gastroenterol. 2002;8:1138–1143. doi: 10.3748/wjg.v8.i6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Z, Zhou X, Lin Z, Yang B, Ma Z, Ye S, Wu Z, Fan J, Liu Y, Liu K, et al. Surgical treatment of hepatocellular carcinoma and related basic research with special reference to recurrence and metastasis. Chin Med J (Engl) 1999;112:887–891. [PubMed] [Google Scholar]
- 5.Zheng Q, Tang ZY, Xue Q, Shi DR, Song HY, Tang HB. Invasion and metastasis of hepatocellular carcinoma in relation to urokinase-type plasminogen activator, its receptor and inhibitor. J Cancer Res Clin Oncol. 2000;126:641–646. doi: 10.1007/s004320000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita Y, Hayashi Y, Wang Y, Kanamaru T, Suzuki S, Kawasaki K, Ohta K, Yamamoto M, Saitoh Y, Itoh H, et al. Expression of urokinase-type plasminogen activator receptor in hepatocellular carcinoma. Hepatology. 1997;25:856–861. doi: 10.1002/hep.510250412. [DOI] [PubMed] [Google Scholar]
- 7.Herszènyi L, Plebani M, Carraro P, De Paoli M, Roveroni G, Cardin R, Tulassay Z, Naccarato R, Farinati F. The role of cysteine and serine proteases in colorectal carcinoma. Cancer. 1999;86:1135–1142. doi: 10.1002/(sici)1097-0142(19991001)86:7<1135::aid-cncr6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Xiao S, Shi Y, Wang L, Ren W, Sloane BF. Cathepsin B on invasion and metastasis of gastric carcinoma. Chin Med J (Engl) 1998;111:784–788. [PubMed] [Google Scholar]
- 9.Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, et al. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 10.Hulett MD, Freeman C, Hamdorf BJ, Baker RT, Harris MJ, Parish CR. Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nat Med. 1999;5:803–809. doi: 10.1038/10525. [DOI] [PubMed] [Google Scholar]
- 11.Toyoshima M, Nakajima M. Human heparanase. Purification, characterization, cloning, and expression. J Biol Chem. 1999;274:24153–24160. doi: 10.1074/jbc.274.34.24153. [DOI] [PubMed] [Google Scholar]
- 12.Kussie PH, Hulmes JD, Ludwig DL, Patel S, Navarro EC, Seddon AP, Giorgio NA, Bohlen P. Cloning and functional expression of a human heparanase gene. Biochem Biophys Res Commun. 1999;261:183–187. doi: 10.1006/bbrc.1999.0962. [DOI] [PubMed] [Google Scholar]
- 13.Miao HQ, Navarro E, Patel S, Sargent D, Koo H, Wan H, Plata A, Zhou Q, Ludwig D, Bohlen P, et al. Cloning, expression, and purification of mouse heparanase. Protein Expr Purif. 2002;26:425–431. doi: 10.1016/s1046-5928(02)00558-2. [DOI] [PubMed] [Google Scholar]
- 14.El-Assal ON, Yamanoi A, Ono T, Kohno H, Nagasue N. The clinicopathological significance of heparanase and basic fibroblast growth factor expressions in hepatocellular carcinoma. Clin Cancer Res. 2001;7:1299–1305. [PubMed] [Google Scholar]
- 15.Ikeguchi M, Ueta T, Yamane Y, Hirooka Y, Kaibara N. Quantitative analysis of heparanase messenger RNA expression in hepatocellular carcinoma. J Surg Oncol. 2002;81:148–154; disscusion 154. doi: 10.1002/jso.10163. [DOI] [PubMed] [Google Scholar]
- 16.Dong J, Kukula AK, Toyoshima M, Nakajima M. Genomic organization and chromosome localization of the newly identified human heparanase gene. Gene. 2000;253:171–178. doi: 10.1016/s0378-1119(00)00251-1. [DOI] [PubMed] [Google Scholar]
- 17.Maxhimer JB, Quiros RM, Stewart R, Dowlatshahi K, Gattuso P, Fan M, Prinz RA, Xu X. Heparanase-1 expression is associated with the metastatic potential of breast cancer. Surgery. 2002;132:326–333. doi: 10.1067/msy.2002.125719. [DOI] [PubMed] [Google Scholar]
- 18.Uno F, Fujiwara T, Takata Y, Ohtani S, Katsuda K, Takaoka M, Ohkawa T, Naomoto Y, Nakajima M, Tanaka N. Antisense-mediated suppression of human heparanase gene expression inhibits pleural dissemination of human cancer cells. Cancer Res. 2001;61:7855–7860. [PubMed] [Google Scholar]
- 19.Nakajima M, Irimura T, Di Ferrante D, Di Ferrante N, Nicolson GL. Heparan sulfate degradation: relation to tumor invasive and metastatic properties of mouse B16 melanoma sublines. Science. 1983;220:611–613. doi: 10.1126/science.6220468. [DOI] [PubMed] [Google Scholar]
- 20.Ikuta M, Podyma KA, Maruyama K, Enomoto S, Yanagishita M. Expression of heparanase in oral cancer cell lines and oral cancer tissues. Oral Oncol. 2001;37:177–184. doi: 10.1016/s1368-8375(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 21.Mikami S, Ohashi K, Usui Y, Nemoto T, Katsube K, Yanagishita M, Nakajima M, Nakamura K, Koike M. Loss of syndecan-1 and increased expression of heparanase in invasive esophageal carcinomas. Jpn J Cancer Res. 2001;92:1062–1073. doi: 10.1111/j.1349-7006.2001.tb01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki M, Ito T, Kashima M, Fukui S, Izumiyama N, Watanabe A, Sano M, Fujiwara Y, Miura M. Erythromycin and clarithromycin modulation of growth factor-induced expression of heparanase mRNA on human lung cancer cells in vitro. Mediators Inflamm. 2001;10:259–267. doi: 10.1080/09629350120093731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staquicini FI, Moreira CR, Nascimento FD, Tersariol IL, Nader HB, Dietrich CP, Lopes JD. Enzyme and integrin expression by high and low metastatic melanoma cell lines. Melanoma Res. 2003;13:11–18. doi: 10.1097/00008390-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Marchetti D, Li J, Shen R. Astrocytes contribute to the brain-metastatic specificity of melanoma cells by producing heparanase. Cancer Res. 2000;60:4767–4770. [PubMed] [Google Scholar]
- 25.Zcharia E, Metzger S, Chajek-Shaul T, Friedmann Y, Pappo O, Aviv A, Elkin M, Pecker I, Peretz T, Vlodavsky I. Molecular properties and involvement of heparanase in cancer progression and mammary gland morphogenesis. J Mammary Gland Biol Neoplasia. 2001;6:311–322. doi: 10.1023/a:1011375624902. [DOI] [PubMed] [Google Scholar]
- 26.Endo K, Maejara U, Baba H, Tokunaga E, Koga T, Ikeda Y, Toh Y, Kohnoe S, Okamura T, Nakajima M, et al. Heparanase gene expression and metastatic potential in human gastric cancer. Anticancer Res. 2001;21:3365–3369. [PubMed] [Google Scholar]
- 27.Friedmann Y, Vlodavsky I, Aingorn H, Aviv A, Peretz T, Pecker I, Pappo O. Expression of heparanase in normal, dysplastic, and neoplastic human colonic mucosa and stroma. Evidence for its role in colonic tumorigenesis. Am J Pathol. 2000;157:1167–1175. doi: 10.1016/S0002-9440(10)64632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koliopanos A, Friess H, Kleeff J, Shi X, Liao Q, Pecker I, Vlodavsky I, Zimmermann A, Büchler MW. Heparanase expression in primary and metastatic pancreatic cancer. Cancer Res. 2001;61:4655–4659. [PubMed] [Google Scholar]
- 29.Rohloff J, Zinke J, Schoppmeyer K, Tannapfel A, Witzigmann H, Mössner J, Wittekind C, Caca K. Heparanase expression is a prognostic indicator for postoperative survival in pancreatic adenocarcinoma. Br J Cancer. 2002;86:1270–1275. doi: 10.1038/sj.bjc.6600232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosir MA, Wang W, Zukowski KL, Tromp G, Barber J. Degradation of basement membrane by prostate tumor heparanase. J Surg Res. 1999;81:42–47. doi: 10.1006/jsre.1998.5519. [DOI] [PubMed] [Google Scholar]
- 31.Gohji K, Okamoto M, Kitazawa S, Toyoshima M, Dong J, Katsuoka Y, Nakajima M. Heparanase protein and gene expression in bladder cancer. J Urol. 2001;166:1286–1290. [PubMed] [Google Scholar]


