Abstract
AIM: Cyclooxygenase (COX)-2 is over expressed in gastrointestinal neoplasm. Helicobacter pylori (H pylori) infection is causally linked to gastric cancer. However, the expression of COX-2 in various stages of H pylori-associated gastric carcinogenesis pathway has not been elucidated. Therefore, the aim of this study was to clarify the role of H pylori induced COX-2 expression during carcinogenesis in the stomach.
METHODS: Gastric biopsies from 138 subjects [30 cases of chronic superficial gastritis (CSG), 28 cases of gastric glandular atrophy (GA), 45 cases of gastric mucosal intestinal metaplasia (IM), 12 cases of moderate gastric epithelial dysplasia and 23 cases of gastric cancer] were enrolled. H pylori infection was assessed by a rapid urease test and histological examination (modified Giemsa staining). The expression of COX-1 and COX-2 in human gastric mucosa was detected by immunohistochemical staining.
RESULTS: H pylori infection rate was 64.3% in GA and 69.5% in gastric cancer, which was significantly higher than that (36.7%) in CSG (P < 0.05). The positive expression rates of COX-2 were 10.0%, 35.7%, 37.8%, 41.7% and 69.5% in CSG, GA, IM, dysplasia and gastric cancer, respectively. From CSG to GA, IM, dysplasia and finally to gastric cancer, expression of COX-2 showed an ascending tendency, whereas COX-1 expression did not change significantly in the gastric mucosa. The level of COX-2 expression in IM and dysplasia was significantly higher in H pylori-positive than in H pylori-negative subjects (P < 0.01).
CONCLUSION: COX-2 expression induced by H pylori infection is a relatively early event during carcinogenesis in the stomach.
INTRODUCTION
Gastric cancer remains the world’s second, and the Chinese first, commonest cause of cancer related deaths[1]. There is epidemiological evidence that Helicobacter pylori (H pylori) infection is causally linked to gastric cancer[2,3]. It has been classified as a class I biological carcinogen by the World Health Organization[4] . However, the exact mechanism responsible for the development of gastric cancer in H pylori-infected patients still remains obscure. According to Correa’s[5] model, gastric cancer develops in a multistep process from chronic active gastritis, gastric glandular atrophy (GA), intestinal metaplasia (IM), dysplasia, and finally to gastric cancer. Recent studies have shown that H pylori infection induces cyclooxygenase-2 (COX-2) expression in human gastric mucosa[6-8]. COX-2, an inducible isoform of cyclooxygenase enzyme, which converts arachidonic acid to prostanoids, is strongly expressed in colorectal cancer[9,10], pancreatic cancer[11], hepatocellular carcinoma[12,13], esophageal cancer[14,15], and gastric cancer[16,17]. Several studies have also shown that COX-2 expression is increased in premalignant lesions including colonic adenoma[9], Barrett’s esophagus[18,19], and gastric adenomas[20], indicating that this enzyme may be involved in the early process of carcinogenesis.
It is well known that H pylori infection causes inflammation, and COX-2 is involved in inflammatory responses and also related to carcinogenesis. However, COX-2 expression in various stages of H pylori-associated gastric carcinogenesis pathway has not been elucidated. To clarify the role of H pylori induced COX-2 expression during carcinogenesis in the stomach, COX-1 and COX-2 expression at different stages of gastric carcinogenesis from inflammation, premalignant lesions, to gastric cancer was investigated by using immunohistochemical analysis in the present study.
MATERIALS AND METHODS
Patients
A total of 138 patients were studied. Of these, 78 were males and 60 were females. The mean age was 52 years (range, 19-74). Endoscopies with biopsy were performed in all patients. Patients who took non-steroidal anti-inflammatory drugs (NSAIDs), H2 receptor antagonists, proton pump inhibitors, antimicrobials, bismuth compounds, over last 4 wk prior to the examination, were excluded. The Medical Ethics Committee of Nanjing Medical University approved this study and written informed consent was obtained from all patients.
Endoscopy and histological assessment
All endoscopic examinations were performed under local anesthesia with lidocaine. Four biopsy specimens, 2 from the antrum within 2 cm of the pyloric channel and 2 from the corpus, were taken during the procedure. When lesions suspected to be cancerous were noted, additional biopsies were taken from the site of lesions. Of these specimens, 2 (each from antrum and corpus) were submitted to a rapid urease test (RUT), and the others were processed for hematoxylin and eosin (H&E) stain and modified Giemsa stain. The pathologic assessment was performed by one pathologist according to the updated Sydney system[21]. GA was defined as loss of glandular tissue and fibrous replacement of lamina propria. IM or exchange of crypts by intestinal epithelium was recognized by the presence of goblet cells and absorptive cells.
Detection of H pylori infection
H pylori infection was identified by histological examination using modified Giemsa stain and RUT (CLO test, Delta West, Bentley, Australia). Patients were classified as H pylori positive if any of the two examinations yielded a positive result. Subjects were considered to be H pylori negative only when both assays were negative for the organism.
Immunohistochemistry
Immunohistochemical staining for COX-1 and COX-2 was performed by the avidin-biotin-peroxidase complex (ABC) method using a Vectastain kit (Vector Laboratories, Burlingame, CA). In brief, paraffin-embedded blocks were sectioned at about 4-μm thickness, deparaffinized, and rehydrated. After microwave pretreatment in citrate buffer (pH6.0) for antigen retrieval, slides were immersed in 3 mL/L H2O2 in methanol for 30 min to block the endogenous peroxidase activity. Nonspecific binding was blocked with 50 mL/L rabbit serum (DAKO, Glostrup, Denmark) in phosphate-buffered saline (PBS), and the tissues were then incubated with goat polyclonal antibody against COX-1 or COX-2 (1:200, Santa Cruz Biotechnology, Inc. Santa Cruz, CA) in PBS containing 20 mL/L rabbit serum and 1 mL/L Triton 100 overnight at 4 °C in a humidity chamber. After being rinsed with PBS, the sections were subsequently incubated with biotinylated secondary rabbit anti-goat immunoglobulins (1:400) for 45 min and then with avidin-biotin-peroxidase complex for another 45 min. The color was developed in 3,3’-diaminobenzidine tetrahydrochloride (Sigma Chemical Co., St. Louis, MO) solution containing 0.3 mL/L H2O2. Nuclei were counterstained with Mayer’s hematoxylin (Merck, Darmstadt, Germany). Tissues of part sections were incubated with PBS containing 20 mL/L rabbit serum and 1 mL/L Triton 100 without the primary antibody as a negative control.
Evaluation of COX-1 and COX-2 immunostaining
In each section, 5 high-power fields were selected, and a total of at least 1000 cells were calculated. The percentage of positive staining cells was graded semiquantitatively, and each sample was assigned to one of the following categories: - (negative, 0% to 4%); + (weak, 5% to 29%); + + (moderate, 30% to 59%); or + + + (strong, more than 60%). All immunostained sections were evaluated independently by two investigators who were blind to the pathological and clinical data. Evaluations were similar among assessors, with less than 10% disagreement. A final consensus was achieved between the 2 assessors using a multihead microscope.
Statistical analysis
Expressions of COX-1 and COX-2 between the 5 study groups (CSG, GA, IM, dysplasia and gastric cancer) were compared by Kruskal-Wallis nonparametric analysis of variance test, using Dunn’s multiple comparison tests for post hoc comparison. The association between COX-2 expression and H pylori infection was analyzed using Fisher’s exact test. Statistical significance was taken at P < 0.05.
RESULTS
Histopathologic characteristics and the prevalence of H pylori infection
Of the 138 patients, 63 were H pylori-positive and 75 were H pylori-negative. Table 1 shows the histopathologic characteristics and H pylori status. Histopathologic diagnosis in this population included CSG (n = 30), GA (n = 28), IM (n = 45), moderate dysplasia (n = 12) and gastric cancer (n = 23). The rates of H pylori infection in GA and gastric cancer were significantly higher than that in CSG.
Table 1.
Rates of H pylori infection in various gastric mucosal lesions
| Pathological diagnosis | n | H pylori rate (%) |
| CSG | 30 | 36.7 |
| GA | 28 | 64.3a |
| IM | 45 | 31.1 |
| Dysplasia | 12 | 33.3 |
| Gastric cancer | 23 | 69.5a |
P < 0.05 vs CSG.
Expression of COX-1 and COX-2 in human gastric mucosa with various lesions
COX-1 was clearly detected in the gastric foveolar and glandular epithelium including parietal cells. Patchy cytoplasmic staining for COX-1 was also seen in the inflammatory mononuclear cells and macrophages, myofibroblasts, as well as endothelial cells in the lamina propria. Spotty cytoplasmic staining for COX-1 was seen in gastric cancer cells (Figure 1). Perinuclear and cytoplasmic staining for COX-2 was mainly seen in the foveolar and glandular epithelium, and mild staining in mononuclear inflammatory cells and macrophages in the lamina propria. Strong expression of COX-2 was also found on glandular epithelium of IM and dysplasia. Immunoreactivity of COX-2 protein showed diffuse staining in the cytoplasm of gastric cancer cells (Figure 2). Furthermore, scattered expression for COX-2 was detected in interstitial cells such as vascular endothelial cells and myofibroblasts. Table 2 shows the positive rates of COX-1 and COX-2 expression in the gastric mucosa with various lesions. From CSG to GA, IM, dysplasia and finally to gastric cancer, the expression of COX-2 showed an ascending tendency, whereas COX-1 expression did not change significantly in the gastric mucosa with various lesions.
Figure 1.
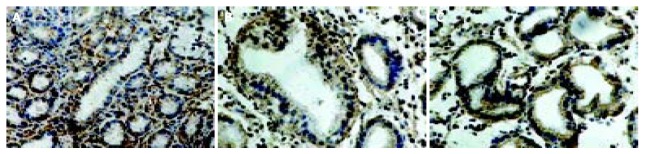
Immunostaining of cyclooxygenase-1 in the gastric mucosa with CSG (A), dysplasia (B) and gastric cancer (C) as shown by immunostaining. In the gastric biopsies of cases of CSG, GA, IM and dysplasia, COX-1 immunostaining was detected in the foveolar and glandular epithelium including parietal cells, and in the subepithelial mononuclear inflammatory cells. Spotty cytoplasmic staining for COX-1 was seen in the gastric cancer cells.
Figure 2.
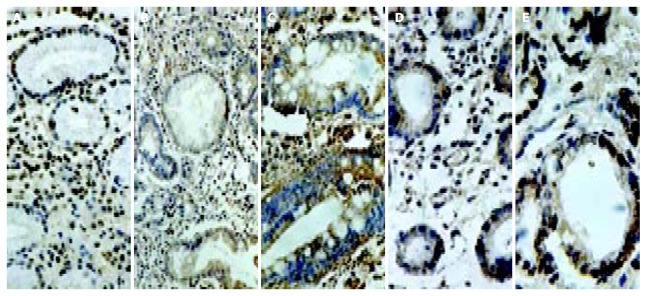
Immunostaining of cyclooxygenase-2 in the gastric mucosa with CSG (A), GA (B), IM (C), dysplasia (D) and gastric cancer (E). CSG showing strong expression in foveolar and glandular epithelium and weak expression in mononuclear inflam-matory cells, myofibroblasts, and endothelial cells in the lamina propria; GA showing strong expression in the atrophic glands of the gastric mucosa; IM showing strong expression in intestinal epithelium and goblet cells; and gastric cancer showing strong expression in cancer cells.
Table 2.
Expression of COX-1 and COX-2 in gastric mucosa with various lesions
| Pathological diagnosis | n | COX-1 expression (%) | COX-2 expression (%) |
| CSG | 30 | 56.6 | 10.0 |
| GA | 28 | 53.8 | 35.7a |
| IM | 45 | 53.3 | 37.8b |
| Dysplasia | 12 | 41.7 | 41.7a |
| Gastric cancer | 23 | 43.4 | 69.5bc |
P < 0.05;
P < 0.01 vs CSG;
P < 0.05 vs GA.
Relationship between H pylori infection and COX-2 expression in the gastric mucosa
COX-2 expression was found in 57% (36/63) H pylori-infected patients, including one case of CSG, eight cases of GA, twelve cases of IM, four cases of dysplasia, and eleven cases of gastric cancer, with intensity scoring ranged from + to +++ (Table 3). H pylori-associated gastritis exhibited strong expression of COX-2 in foveolar and glandular epithelium but with a lower intensity in mononuclear inflammatory cells. On the contrary, only 20% (15/75) of non-infected patients expressed COX-2 protein in the gastric biopsy. The intensity of COX-2 expression in IM and dysplasia was significantly higher in H pylori-positive than in H pylori-negative subjects (P < 0.01). However, there was no significant difference in COX-2 expression in CSG, GA and gastric cancer patients with or without H pylori infection (Table 3).
Table 3.
Relationship between H pylori infection and COX-1/COX-2 expression in the gastric mucosa with various lesions
| Pathological diagnosis | n |
COX-1 |
COX-2 |
|||||||
| - | + | ++ | +++ | - | + | ++ | +++ | |||
| CSG | H pylori (+) | 11 | 5 | 1 | 3 | 2 | 10 | 0 | 0 | 1 |
| H pylori (-) | 19 | 8 | 3 | 4 | 4 | 17 | 1 | 1 | 0 | |
| GA | H pylori (+) | 18 | 9 | 2 | 4 | 3 | 10 | 1 | 3 | 4 |
| H pylori (-) | 10 | 4 | 2 | 2 | 2 | 8 | 1 | 1 | 0 | |
| IM | H pylori (+) | 14 | 6 | 3 | 2 | 3 | 2 | 2 | 4 | 6b |
| H pylori (-) | 31 | 15 | 5 | 5 | 6 | 26 | 3 | 2 | 0 | |
| Dysplasia | H pylori (+) | 4 | 2 | 0 | 1 | 1 | 0 | 0 | 2 | 2b |
| H pylori (-) | 8 | 5 | 0 | 1 | 2 | 7 | 0 | 1 | 0 | |
| Gastric cancer | H pylori (+) | 16 | 9 | 2 | 2 | 3 | 5 | 1 | 3 | 7 |
| H pylori (-) | 7 | 4 | 2 | 0 | 1 | 2 | 1 | 2 | 2 | |
P < 0.01 vs H pylori (-).
DISCUSSION
Epidemiological studies have shown that long-term use of NSAIDs reduces the risk of colon cancer development by 40%[22,23] and the risk of esophageal cancer development by up to 90%[24,25]. In addition, NSAIDs could induce regression of adenomatous polyps in patients with familial adenomatous polyposis[26,27], as well as in an Apc Min mouse model[28]. Although the exact mechanisms of NSAIDs on cancer prevention have not been clarified, one possible role of NSAIDs is via the inhibition of COX enzymes, leading to chemopreventive effect. COX exists in two isoforms, of which COX-1 is constitutively expressed in many tissues, including the stomach, and COX-2 showing 61% homology with COX-1 is expressed at low concentrations or is even undetectable in unstimulated cells or tissues, but is readily induced by various stimuli including mitogens, cytokines, growth factors, and tumor promoters in inflammatory and certain cell types, such as fibroblasts, macrophages and endothelial cells[29,30]. It is well known that COX-2 is strongly expressed in gastric cancer[17,20]. More than 90% of gastric cancers are adenocarcinomas, which are divided into intestinal and diffuse histological types. Pathogenesis of the intestinal-type gastric cancer has been connected to precursor changes such as GA, IM, and dysplasia. The present study clearly showed that COX-2 protein was expressed not only in gastric cancer cells but also in the glandular epithelium of IM and dysplasia as detected by immunohistochemistry. From CSG, GA to IM and dysplasia and finally to gastric cancer, the expression of COX-2 showed an ascending tendency, whereas COX-1 expression did not change significantly in premalignant and malignant gastric lesions. These results provided evidence that COX-2 might contribute to an early event in gastric carcinogenesis[31], and suggesting the possibility that the use of selective COX-2 inhibitors may provide a chemopreventive strategy against gastric carcinogenesis.
H pylori infection is an important risk factor for adenocarcinoma of the distal stomach in humans[2-5], but the mechanism whereby H pylori infection contributes to gastric carcinogenesis is still hypothetical. Recent studies have shown that H pylori infection induces COX-2 expression in human gastric mucosa[6-8]. Furthermore, McCarthy et al[32] showed that COX-2 expression in antral mucosa was reduced but not eliminated in the epithelium after successful eradication of H pylori. Kimura et al[33] reported that immunoreactivity of COX-2 was observed in all cases of IM even after the cure of H pylori infection. Thus, cure of H pylori infection may decrease the risk of gastric carcinogenesis due to COX-2-related compounds in gastric mucosa but not in those patients with IM. In this study, we demonstrated that COX-2 was expressed in epithelial lining of the stomach in the H pylori-associated gastric carcinogenesis pathway from CSG, to GA and IM and dysplasia, and finally to gastric cancer. The level of COX-2 expression in patients with IM and dysplasia was significantly higher in H pylori-positive than in H pylori-negative subjects. In contrast, COX-1 protein was constitutively expressed in different gastric mucosal lesions with or without H pylori infection. Thus, COX-1 is considered as a housekeeping gene, and prostanoids synthesized via the COX-1 pathway are thought to be responsible for cytoprotection of the stomach, for vasodilatation in the kidney, and for production of a proaggregative prostanoid, thromboxane, by the platelets. In contrast, COX-2 is an inducible immediate-early gene, and its role has been connected to inflammation, reproduction, and carcinogenesis. Although the exact mechanism of COX-2 expression in carcinogenesis is still unclear, some studies have suggested that overexpression of COX-2 is associated with cellular resistance to apoptosis whereas treatment with specific COX-2 inhibitors could suppress cell proliferation and induces apoptosis[14,34-36]. It has been hypothesized that the alteration of the balance between apoptosis and proliferation of gastric epithelial cells, induced by H pylori infection, contributes to either gastric injury or carcinogenesis of the stomach. Although H pylori could decrease epithelial cell proliferation and induce apoptosis in vitro[40-43], H pylori infection in vivo was associated with both increased apoptosis and proliferation[44-46]. These differences between the in vivo and in vitro results suggest that in vivo factors including inflammatory cells, extracellular matrix proteins, cytokines, and adhesion molecules may also contribute to epithelial cell turnover. On the other hand, COX-2 expression has been reported to correlate with invasion of the lymphatic vessels, lymph node metastasis, and advanced tumor stage in gastric cancer[47,48].
In conclusion, COX-2 overex-pression plays an important role in the initiation of gastric carcinogenesis. The use of selective COX-2 inhibitors may provide a chemopreventive strategy against gastric carcinogenesis.
Footnotes
Supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, No. 9247342057
Edited by Xia HHX and Wang XL Proofread by Xu FM
References
- 1.Stadtländer CT, Waterbor JW. Molecular epidemiology, pathogenesis and prevention of gastric cancer. Carcinogenesis. 1999;20:2195–2208. doi: 10.1093/carcin/20.12.2195. [DOI] [PubMed] [Google Scholar]
- 2.Wang RT, Wang T, Chen K, Wang JY, Zhang JP, Lin SR, Zhu YM, Zhang WM, Cao YX, Zhu CW, et al. Helicobacter pylori infection and gastric cancer: evidence from a retrospective cohort study and nested case-control study in China. World J Gastroenterol. 2002;8:1103–1107. doi: 10.3748/wjg.v8.i6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welin M, Holmgren NM, Nilsson P, Enroth H. Statistical model of the interactions between Helicobacter pylori infection and gastric cancer development. Helicobacter. 2003;8:72–78. doi: 10.1046/j.1523-5378.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 4.Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 5.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37–S43. [PubMed] [Google Scholar]
- 6.Fu S, Ramanujam KS, Wong A, Fantry GT, Drachenberg CB, James SP, Meltzer SJ, Wilson KT. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116:1319–1329. doi: 10.1016/s0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- 7.Tatsuguchi A, Sakamoto C, Wada K, Akamatsu T, Tsukui T, Miyake K, Futagami S, Kishida T, Fukuda Y, Yamanaka N, et al. Localisation of cyclooxygenase 1 and cyclooxygenase 2 in Helicobacter pylori related gastritis and gastric ulcer tissues in humans. Gut. 2000;46:782–789. doi: 10.1136/gut.46.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan FK, To KF, Ng YP, Lee TL, Cheng AS, Leung WK, Sung JJ. Expression and cellular localization of COX-1 and -2 in Helicobacter pylori gastritis. Aliment Pharmacol Ther. 2001;15:187–193. doi: 10.1046/j.1365-2036.2001.00918.x. [DOI] [PubMed] [Google Scholar]
- 9.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 10.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 11.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 12.Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R, et al. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688–696. doi: 10.1002/hep.510290355. [DOI] [PubMed] [Google Scholar]
- 13.Shiota G, Okubo M, Noumi T, Noguchi N, Oyama K, Takano Y, Yashima K, Kishimoto Y, Kawasaki H. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999;46:407–412. [PubMed] [Google Scholar]
- 14.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 15.Ratnasinghe D, Tangrea J, Roth MJ, Dawsey S, Hu N, Anver M, Wang QH, Taylor PR. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res. 1999;19:171–174. [PubMed] [Google Scholar]
- 16.Sung JJ, Leung WK, Go MY, To KF, Cheng AS, Ng EK, Chan FK. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am J Pathol. 2000;157:729–735. doi: 10.1016/S0002-9440(10)64586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uefuji K, Ichikura T, Mochizuki H, Shinomiya N. Expression of cyclooxygenase-2 protein in gastric adenocarcinoma. J Surg Oncol. 1998;69:168–172. doi: 10.1002/(sici)1096-9098(199811)69:3<168::aid-jso9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929–2934. [PubMed] [Google Scholar]
- 19.Shirvani VN, Ouatu-Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487–496. doi: 10.1016/s0016-5085(00)70254-x. [DOI] [PubMed] [Google Scholar]
- 20.Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Oncol. 2001;76:26–30. doi: 10.1002/1096-9098(200101)76:1<26::aid-jso1005>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Thun MJ, Namboodiri MM, Heath CW. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg L, Palmer JR, Zauber AG, Warshauer ME, Stolley PD, Shapiro S. A hypothesis: nonsteroidal anti-inflammatory drugs reduce the incidence of large-bowel cancer. J Natl Cancer Inst. 1991;83:355–358. doi: 10.1093/jnci/83.5.355. [DOI] [PubMed] [Google Scholar]
- 24.Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW. Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 25.Funkhouser EM, Sharp GB. Aspirin and reduced risk of esophageal carcinoma. Cancer. 1995;76:1116–1119. doi: 10.1002/1097-0142(19951001)76:7<1116::aid-cncr2820760703>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 27.Waddell WR, Loughry RW. Sulindac for polyposis of the colon. J Surg Oncol. 1983;24:83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- 28.Jacoby RF, Marshall DJ, Newton MA, Novakovic K, Tutsch K, Cole CE, Lubet RA, Kelloff GJ, Verma A, Moser AR, et al. Chemoprevention of spontaneous intestinal adenomas in the Apc Min mouse model by the nonsteroidal anti-inflammatory drug piroxicam. Cancer Res. 1996;56:710–714. [PubMed] [Google Scholar]
- 29.Lee SH, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H, Liou S, Simmons D, Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992;267:25934–25938. [PubMed] [Google Scholar]
- 30.Mitchell JA, Larkin S, Williams TJ. Cyclooxygenase-2: regulation and relevance in inflammation. Biochem Pharmacol. 1995;50:1535–1542. doi: 10.1016/0006-2952(95)00212-x. [DOI] [PubMed] [Google Scholar]
- 31.Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, Kim HS, Nam DK, Lee KB, Kim HC. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000;6:519–525. [PubMed] [Google Scholar]
- 32.McCarthy CJ, Crofford LJ, Greenson J, Scheiman JM. Cyclooxygenase-2 expression in gastric antral mucosa before and after eradication of Helicobacter pylori infection. Am J Gastroenterol. 1999;94:1218–1223. doi: 10.1111/j.1572-0241.1999.01070.x. [DOI] [PubMed] [Google Scholar]
- 33.Kimura A, Tsuji S, Tsujii M, Sawaoka H, Iijima H, Kawai N, Yasumaru M, Kakiuchi Y, Okuda Y, Ali Z, et al. Expression of cyclooxygenase-2 and nitrotyrosine in human gastric mucosa before and after Helicobacter pylori eradication. Prostaglandins Leukot Essent Fatty Acids. 2000;63:315–322. doi: 10.1054/plef.2000.0220. [DOI] [PubMed] [Google Scholar]
- 34.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 35.Sawaoka H, Kawano S, Tsuji S, Tsujii M, Gunawan ES, Takei Y, Nagano K, Hori M. Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol. 1998;274:G1061–G1067. doi: 10.1152/ajpgi.1998.274.6.G1061. [DOI] [PubMed] [Google Scholar]
- 36.Erickson BA, Longo WE, Panesar N, Mazuski JE, Kaminski DL. The effect of selective cyclooxygenase inhibitors on intestinal epithelial cell mitogenesis. J Surg Res. 1999;81:101–107. doi: 10.1006/jsre.1998.5511. [DOI] [PubMed] [Google Scholar]
- 37.Anti M, Armuzzi A, Gasbarrini A, Gasbarrini G. Importance of changes in epithelial cell turnover during Helicobacter pylori infection in gastric carcinogenesis. Gut. 1998;43 Suppl 1:S27–S32. doi: 10.1136/gut.43.2008.s27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Correa P, Miller MJ. Carcinogenesis, apoptosis and cell proliferation. Br Med Bull. 1998;54:151–162. doi: 10.1093/oxfordjournals.bmb.a011665. [DOI] [PubMed] [Google Scholar]
- 39.Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, Fontham ET, Mera R, Miller MJ, Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- 40.Chen G, Sordillo EM, Ramey WG, Reidy J, Holt PR, Krajewski S, Reed JC, Blaser MJ, Moss SF. Apoptosis in gastric epithelial cells is induced by Helicobacter pylori and accompanied by increased expression of BAK. Biochem Biophys Res Commun. 1997;239:626–632. doi: 10.1006/bbrc.1997.7485. [DOI] [PubMed] [Google Scholar]
- 41.Ricci V, Ciacci C, Zarrilli R, Sommi P, Tummuru MK, Del Vecchio Blanco C, Bruni CB, Cover TL, Blaser MJ, Romano M. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infect Immun. 1996;64:2829–2833. doi: 10.1128/iai.64.7.2829-2833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudi J, Kuck D, Strand S, von Herbay A, Mariani SM, Krammer PH, Galle PR, Stremmel W. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Invest. 1998;102:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner S, Beil W, Westermann J, Logan RP, Bock CT, Trautwein C, Bleck JS, Manns MP. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- 44.Fraser AG, Sim R, Sankey EA, Dhillon AP, Pounder RE. Effect of eradication of Helicobacter pylori on gastric epithelial cell proliferation. Aliment Pharmacol Ther. 1994;8:167–173. doi: 10.1111/j.1365-2036.1994.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 45.Peek RM, Wirth HP, Moss SF, Yang M, Abdalla AM, Tham KT, Zhang T, Tang LH, Modlin IM, Blaser MJ. Helicobacter pylori alters gastric epithelial cell cycle events and gastrin secretion in Mongolian gerbils. Gastroenterology. 2000;118:48–59. doi: 10.1016/s0016-5085(00)70413-6. [DOI] [PubMed] [Google Scholar]
- 46.Peek RM, Moss SF, Tham KT, Pérez-Pérez GI, Wang S, Miller GG, Atherton JC, Holt PR, Blaser MJ. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst. 1997;89:863–868. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 47.Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, Shiozaki H, Hori M. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451–455. doi: 10.1111/j.1572-0241.1999.876_e.x. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto H, Itoh F, Fukushima H, Hinoda Y, Imai K. Overexpression of cyclooxygenase-2 protein is less frequent in gastric cancers with microsatellite instability. Int J Cancer. 1999;84:400–403. doi: 10.1002/(sici)1097-0215(19990820)84:4<400::aid-ijc12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]


