Abstract
AIM: To investigate the protective effect of lansoprazole on ischemia and reperfusion (I/R)-induced rat intestinal mucosal injury in vivo.
METHODS: Intestinal damage was induced by clamping both the superior mesenteric artery and the celiac trunk for 30 min followed by reperfusion in male Sprague-Dawley rats. Lansoprazole was given to rats intraperitoneally 1 h before vascular clamping.
RESULTS: Both the intraluminal hemoglobin and protein levels, as indices of mucosal damage, significantly increased in I/R-groups comparion with those of sham-operation groups. These increases in intraluminal hemoglobin and protein levels were significantly inhibited by the treatment with lansoprazole at a dose of 1 mg/kg. Small intestine exposed to I/R resulted in mucosal inflammation that was characterized by significant increases in thiobarbituric acid-reactive substances (TBARS), tissue-associated myeloperoxidase activity (MPO), and mucosal content of rat cytokine-induced neutrophil chemoattractant-1 (CINC-1). These increases in TBARS, MPO activities and CINC-1 content in the intestinal mucosa after I/R were all inhibited by pretreatment with lansoprazole at a dose of 1 mg/kg. Furthermore, the CINC-1 mRNA expression was increased during intestinal I/R, and this increase in mRNA expression was inhibited by treatment with lansoprazole.
CONCLUSION: Lansoprazole inhibits lipid peroxidation and reduces development of intestinal mucosal inflammation induced by I/R in rats, suggesting that lansoprazole may have a therapeutic potential for I/R injury.
INTRODUCTION
Reactive oxygen species such as superoxide radicals, hydrogen peroxide, and hydroxyl radicals, have been implicated in the pathogenesis of ischemia-reperfusion (I/R) injury in a variety of organs, including the brain[1], large intestine[2], and heart[3]. Intestinal I/R is an especially grave condition resulted from acute mesenteric ischemia, small bowel transplantation, abdominal aortic aneurysm, hemorrhagic, traumatic, or septic shock, and severe burns[4,5]. I/R injury of the small intestine is characterized by a number of microvascular and mucosal alterations, including endothelial cell swelling, capillary plugging, prolonged reduction in intestinal blood flow, and mucosal barrier dysfunction[6-8]. Previous studies have demonstrated that neutrophils are critically involved in I/R injury. This hypothesis based on experiments shows that neutrophil depletion[9,10] and inhibition of neutrophil-endothelial cell interactions[11] could protect against injury after I/R.
Proton-pump inhibitors (PPIs)[12] such as omeprazole and lansoprazole are extensively used for therapeutic control of acid-related disorders, including gastroesophageal reflux disease and peptic-ulcer diseases caused by stress, nonsteroidal anti-inflammatory drugs and Helicobacter pylori infection[13-16]. PPIs are strong antisecretory agents that act on gastric (H+/K+) ATPase of parietal cells[17]. Recently, it has been suggested that PPIs inhibit neutrophil functions such as chemotaxis, superoxide production and degradation[18]. We have already reported that PPIs can attenuate neutrophil adherence to endothelial cells via inhibition of the expression of adhesion molecules[19]. These results have indicated that PPIs have anti-inflammatory effects and can inhibit acid secretion.
The present study was to evaluate anti-inflammatory effects of lansoprazole, which has dramatically influenced the management of acid-peptic disorders in recent years, using I/R-induced rat intestinal mucosal injury model unrelated to acid secretion.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley (SD) rats weighing 190-210 g were obtained from Keari Co. Ltd. (Osaka, Japan). The rats were housed in stainless steel cages with wire bottoms and maintained on a 12-h light and dark cycle, with the temperature and relative humidity of the animal room controlled at 21-23 °C and 55%-65%, respectively. The rats were not fed for 18 h prior to the experiments, but allowed free access to water. All experimental procedures described below were approved by the Animal Care Committee of the Kyoto Prefectural University of Medicine (Kyoto, Japan).
Preparation of rats for intestinal ischemia-reperfusion
Intestinal ischemia was induced for 30 min by applying a small clamp to the superior mesenteric artery after ligating the celiac artery in rats given intraperitoneal urethane anesthesia (1000 mg/kg). Reoxygenation was produced by removal of the clamp. Sixty minutes after reperfusion, the rats were killed by exsanguinations via the abdominal aorta under urethane anesthesia (1000 mg/kg). 2-[[[3-methyl-4- (2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulfinyl] benzimidazole (Lansoprazole), a gift from Takeda Chemical Industries Ltd. Japan, was diluted with physiological saline at a dose of 0.3-1 mg/kg after dissolved in dimethylsulfoxide, and was given to rats intraperitoneally 1 h before the vascular clamping. In the control groups, rats received an equivalent volume of the vehicle.
Assessment of intestinal mucosal injury induced by ischemia-reperfusion
To estimate the severity of the intestinal mucosal damage by I/R, leakage of intraluminal protein and intraluminal hemoglobin levels were measured as mg/cm intestine.
The concentrations of thiobarbituric acid-reactive substances (TBARS) in the intestinal mucosa, an index of lipid peroxidation, were measured by the method of Ohkawa et al[20]. In brief, the small intestine was opened by a longitudinal incision, and the intestinal mucosa was scraped off using two glass slides. Mucosal tissue was then homogenized with 10 mmol/L potassium phosphate buffer (pH7.8) containing 30 mmol/L KCl in a Teflon Potter-Elvehjem homogenizer. The levels of TBARS in the mucosal homogenates were expressed as nmol of malondialdehyde per gram of wet weight using 1,1,3,3-tetramethoxypropane as the standard.
Myeloperoxidase (MPO) activity in the intestinal mucosa, an index of polymorphonuclear leukocyte accumulation, were determined by a modification of the method of Grisham et al[21]. Two milliliters of mucosal homogenate was centrifuged at 20000 g for 15 min at 4 °C to pellet the insoluble cellular debris. The pellet was then rehomogenized in an equivalent volume of 0.05 mol/L potassium phosphate buffer (pH5.4) containing 5 g/L hexadecyltrimethylammonium bromide. Samples were centrifuged at 20000 g for 15 min at 4 °C, and the supernatants were saved. MPO activity was assessed by measuring the H2O2-dependent oxidation of 3,3’,5,5’-tetramethylbenzidine. One unit of enzyme activity was defined as the amount of MPO that caused a change in the absorbance of 1.0/min at 655 nm and 25 °C.
The content of cytokine-induced neutrophil chemoattractant-1 (CINC-1) in the intestinal mucosal homogenates was determined by enzyme-linked immunosorbent assay (ELISA) using a kit (Immuno Biological Laboratories, Gunma, Japan) according to the manufacturer’s instructions. The absorbance of each well was read at 490 nm by a microplate reader (MPR-A4i; Tosoh, Tokyo, Japan).
The expression of intestinal CINC-1 was determined using the RT-PCR method. Samples of intestinal tissue for mRNA isolation were prepared from the whole intestine. Total RNA was isolated using the acid-guanidium-phenol-chloroform (AGPC) method with ISOGEN (NIPPON GENE). The concentration of RNA was determined by the absorbance at 260 nm in relation to that at 280 nm. The RNA was stored at -70 °C until used for RT-PCR. One microliter of RT-PCR products was added to 3 mmol/L of each primer, CINC-1 and β-actin (for internal standard purpose), and a solution of 0.5 U of Taq DNA polymerase (Takara Biochemicals) in a final volume of 50 L. The primers had the following sequences: for CINC-1, sense 5’-ACAGTGGCAGGGATTCACTT-3’, and antisense 5’-CTAGCA CAGTGGTTGACACT-3’; for β-actin, sense 5’-TCCTGTGG CATCCATGAAACT-3’, and antisense 5’-GAAGCATTTGCGG TGCACGAT-3’. The mixture was subjected to 30 cycles (1 min at 94 °C, 1 min at 54 °C, 1 min at 72 °C) of amplification. Then the reaction products were separated by electrophoresis on 25 g/L agarose gel and stained with ethidium bromide.
Statistical analysis
All values were expressed as mean ± SE. The data were compared by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. All analyses were performed using the Stat View 5.0-J program (Abacus Concepts Inc. Berkeley, CA). P < 0.05 was considered statistically significant.
RESULTS
Intraluminal protein and hemoglobin levels of the small intestine
Intraluminal hemoglobin and protein levels, the reflections of intestinal mucosal injury, were significantly increased above basal levels after 60 min of reperfusion. These increases in intraluminal protein and hemoglobin levels induced by I/R were significantly decreased by lansoprazole treatment at a dose of 1 mg/kg (Table 1).
Table 1.
Effect of lansoprazole on intraluminal hemoglobin and protein levels in small intestine of I/R-induced rats (mean ± SE )
| Sham-operation (Normal) | Ischemia-reperfusion (Control) |
Lansoprazole (mg/kg) |
||
| 0.3 | 1 | |||
| Intraluminal hemoglobin (mg/cm) | 0.000043 ± 0.000002 | 0.0043 ± 0.0006a | 0.0035 ± 0.0007 | 0.0010 ± 0.0004c |
| Intraluminal protein (mg/cm) | 0.065 ± 0.012 | 0.172 ± 0.016a | 0.156 ± 0.022 | 0.087 ± 0.010c |
P < 0.05 vs normal;
P < 0.05 vs control.
TBARS in the intestinal mucosa
TBARS in the intestinal mucosa, an index of lipid peroxidation, significantly increased 60 min after reperfusion (13.0 ± 2.5 nmoL/ 100 g wet wt). These levels were significantly decreased to 7.98 ± 1.98 nmol/100 g wet wt after lansoprazole treatment at a dose of 1 mg/kg (Figure 1A).
Figure 1.
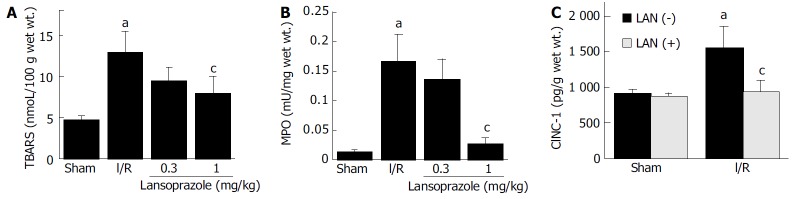
Effect of lansoprazole on the level of thiobarbituric-acid reactive substances (TBARS), mucosal mye-loperoxidase (MPO) activity, and cytolline induced meutrophil chemo-attracttant-1 (CINC-1) in intestinal mucosa of I/R-induced rats. A: Effect of lansoprazole on the level of TBARS in intestinal mucosa of I/R-induced rats. aP < 0.05 vs normal; cP < 0.05 vs control. Sham group and I/R group received an equivalent volume of vehicle instead of lansoprazole. B: Effect of lansoprazole on the level of mucosal myeloperoxidase (MPO) activities in intestinal mucosa of I/R-induced rats. aP < 0.05 vs normal; cP < 0.05 vs control. Sham group and I/R group received an equivalent volume of vehicle instead of lansoprazole. C: Effect of lansoprazole on cytokine induced neutrophil chemoattractant-1 (CINC-1) of intestinal mucosa of I/R-induced rats. aP < 0.05 vs normal; cP < 0.05 vs control. LAN(+): with lansoprazole; LAN(-): without lansoprazole.
MPO activity in the intestinal mucosa
The I/R group showed a significant increase in mucosal MPO activity (0.167 ± 0.046 mU/mg wet wt) compared with the sham-operation group (0.014 ± 0.001 mU/mg wet wt). However, this increase in MPO activity significantly decreased to 0.028 ± 0.008 mU/mg wet wt after lansoprazole treatment at a dose of 1 mg/kg (Figure 1B).
Inflammatory cytokine in the intestinal mucosa
The content of mucosal CINC-1 in the I/R groups was significantly increased compared with the levels in sham-operated groups. This increase in the levels of inflammatory cytokines was significantly inhibited by lansoprazole at a dose of 1 mg/kg (Figure 1C).
Intestinal expression of CINC-1 mRNA during ischemia-reperfusion
The expression of CINC-1 mRNA in the intestinal mucosa was up-regulated by I/R injury. This increase in the expression of CINC-1 mRNA was also inhibited by lansoprazole at a dose of 1 mg/kg (Figure 2).
Figure 2.
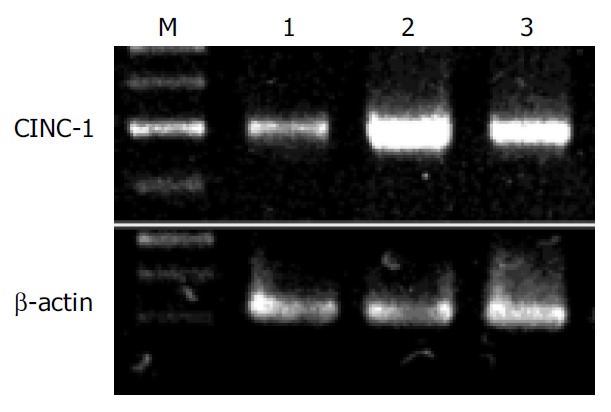
Intestinal CINC-1 mRNA expression during I/R in rats. M: Marker; Lane 1: Before ischemia; Lane 2: After I/R; Lane 3: I/R + lansoprazole.
DISCUSSION
Our results clearly showed that lansoprazole had a protective effect against reperfusion-induced intestinal mucosal injury in rats. In addition, increases in TBARS contents, MPO activity and CINC-1 contents were inhibited by the treatment of lansoprazole. In I/R-induced tissue injury, oxygen radicals have been suggested to be generated via several mechanisms, including the xanthine/xanthine oxidase reaction[22], NADPH oxidase and myeloperoxidase of activated leukocytes migrating into the previously ischemic area[23], and mitochondrial respiratory system[24]. We have already reported that oxygen-derived free radicals and lipid peroxidation played a role in the formation of gastrointestinal mucosal damage induced by I/R[25,26]. Furthermore, it is well known that neutrophils that adhere to post-capillary venules and subsequently emigrate into the interstitium are implicated in the I/R-related tissue injury[27,28]. Activated neutrophils release a variety of cytotoxic substances, including proteases, collagenases, cytokines, leukotrienes, and cationic proteins, thereby causing tissue damage. In addition, adhered and aggregated neutrophils can physically disturb capillary flow and induce a non-reflow phenomenon.
Little is known about the protective mechanism of lansoprazole against I/R injury. However, there have been some reports about the anti-inflammatory action of PPIs. We have shown that the expression of adhesion molecules on neutrophils and endothelial cells elicited by H pylori extract and IL-1 was inhibited by lansoprazole and omeprazole at clinical relevant doses[19]. Other reports have also revealed that PPIs could prevent the neutrophil-endothelial cell adhesion reaction[29,30]. Recently, some reports regarding the anti-oxidative effects of PPIs have also appeared. Suzuki et al[31] reported that PPIs inhibited the production of oxygen-derived free radicals from neutrophils activated by chemotactic peptides or opsonized zymosan. Other studies have concluded that PPIs block stress-induced increased reactive oxygen intermediates and associated lipid peroxidation and protein oxidation, indicating that its antioxidant properties play a major role in preventing oxidative damage[32-34].
Lansoprazole has been widely used in the treatment of acid-related diseases including reflux esophagitis. This drug is thought to be transformed into two active species which inhibit acid secretion by (H+, K+)-ATPase within the parietal cell canaliculi. However, acid secretion was not directly involved in the pathogenesis of I/R-induced intestinal injury model we used. In the present study, lansoprazole was found to prevent lipid peroxidation and to reduce the development of intestinal mucosal inflammation via inhibition of the production of inflammatory cytokines such as CINC-1. These results suggest that lansoprazole may protect against I/R injury via an anti-inflammatory effect but not inhibition of acid secretion.
It was previously reported that CINC levels were increased during small intestinal I/R injury, and that CINC-1 was related with the extent of mucosal damage[35]. It was reported that CINC could be produced by many types of cells, including macrophages, monocytes, and endothelial cells[36]. In the present study, lansoprazole inhibited both CINC-1 protein and mRNA in the small intestine after I/R injury. These results suggest that lansoplazole may prevent CINC-1 production by scavenging active oxygen species that are related to signal transduction for the promotion of CINC-1 synthesis. Further study needs to be done to determine the molecular mechanisms involved in the inhibition of CINC-1 production by lansoprazole.
In conclusion, lansoprazole protects against acid-unrelated intestinal injury induced by I/R via inhibition of neutrophil-dependent inflammation. We suggest that lansoprazole has potential as a new therapeutic agent for reperfusion injury.
Footnotes
Edited by Chen WW and Wang XL Proofread by Xu FM
References
- 1.Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 2.Panés J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066–1090. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- 3.Jolly SR, Kane WJ, Bailie MB, Abrams GD, Lucchesi BR. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res. 1984;54:277–285. doi: 10.1161/01.res.54.3.277. [DOI] [PubMed] [Google Scholar]
- 4.Cappell MS. Intestinal (mesenteric) vasculopathy. I. Acute superior mesenteric arteriopathy and venopathy. Gastroenterol Clin North Am. 1998;27:783–825, vi. doi: 10.1016/s0889-8553(05)70033-9. [DOI] [PubMed] [Google Scholar]
- 5.Homer-Vanniasinkam S, Crinnion JN, Gough MJ. Post-ischaemic organ dysfunction: a review. Eur J Vasc Endovasc Surg. 1997;14:195–203. doi: 10.1016/s1078-5884(97)80191-8. [DOI] [PubMed] [Google Scholar]
- 6.Schoenberg MH, Muhl E, Sellin D, Younes M, Schildberg FW, Haglund U. Posthypotensive generation of superoxide free radicals--possible role in the pathogenesis of the intestinal mucosal damage. Acta Chir Scand. 1984;150:301–309. [PubMed] [Google Scholar]
- 7.Granger DN, Höllwarth ME, Parks DA. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- 8.Haglund U, Bulkley GB, Granger DN. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand. 1987;153:321–324. [PubMed] [Google Scholar]
- 9.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 10.Sisley AC, Desai T, Harig JM, Gewertz BL. Neutrophil depletion attenuates human intestinal reperfusion injury. J Surg Res. 1994;57:192–196. doi: 10.1006/jsre.1994.1130. [DOI] [PubMed] [Google Scholar]
- 11.Kurose I, Anderson DC, Miyasaka M, Tamatani T, Paulson JC, Todd RF, Rusche JR, Granger DN. Molecular determinants of reperfusion-induced leukocyte adhesion and vascular protein leakage. Circ Res. 1994;74:336–343. doi: 10.1161/01.res.74.2.336. [DOI] [PubMed] [Google Scholar]
- 12.Horn J. The proton-pump inhibitors: similarities and differences. Clin Ther. 2000;22:266–280; discussion 265. doi: 10.1016/S0149-2918(00)80032-6. [DOI] [PubMed] [Google Scholar]
- 13.Langtry HD, Wilde MI. Omeprazole. A review of its use in Helicobacter pylori infection, gastro-oesophageal reflux disease and peptic ulcers induced by nonsteroidal anti-inflammatory drugs. Drugs. 1998;56:447–486. doi: 10.2165/00003495-199856030-00012. [DOI] [PubMed] [Google Scholar]
- 14.Garnett WR. Lansoprazole: a proton pump inhibitor. Ann Pharmacother. 1996;30:1425–1436. doi: 10.1177/106002809603001212. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann AE, Katona BG. Lansoprazole: a comprehensive review. Pharmacotherapy. 1997;17:308–326. [PubMed] [Google Scholar]
- 16.Wolfe MM, Sachs G. Acid suppression: optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Gastroenterology. 2000;118:S9–31. doi: 10.1016/s0016-5085(00)70004-7. [DOI] [PubMed] [Google Scholar]
- 17.Fellenius E, Berglindh T, Sachs G, Olbe L, Elander B, Sjöstrand SE, Wallmark B. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+)ATPase. Nature. 1981;290:159–161. doi: 10.1038/290159a0. [DOI] [PubMed] [Google Scholar]
- 18.Wandall JH. Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome b-245. Gut. 1992;33:617–621. doi: 10.1136/gut.33.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida N, Yoshikawa T, Tanaka Y, Fujita N, Kassai K, Naito Y, Kondo M. A new mechanism for anti-inflammatory actions of proton pump inhibitors--inhibitory effects on neutrophil-endothelial cell interactions. Aliment Pharmacol Ther. 2000;14 Suppl 1:74–81. doi: 10.1046/j.1365-2036.2000.014s1074.x. [DOI] [PubMed] [Google Scholar]
- 20.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- 22.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 23.Fantone JC, Ward PA. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982;107:395–418. [PMC free article] [PubMed] [Google Scholar]
- 24.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 25.Yoshikawa T, Naito Y, Ueda S, Ichikawa H, Takahashi S, Yasuda M, Kondo M. Ischemia-reperfusion injury and free radical involvement in gastric mucosal disorders. Adv Exp Med Biol. 1992;316:231–238. doi: 10.1007/978-1-4615-3404-4_27. [DOI] [PubMed] [Google Scholar]
- 26.Yoshikawa T, Ueda S, Naito Y, Takahashi S, Oyamada H, Morita Y, Yoneta T, Kondo M. Role of oxygen-derived free radicals in gastric mucosal injury induced by ischemia or ischemia-reperfusion in rats. Free Radic Res Commun. 1989;7:285–291. doi: 10.3109/10715768909087953. [DOI] [PubMed] [Google Scholar]
- 27.Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988;255:H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida N, Granger DN, Anderson DC, Rothlein R, Lane C, Kvietys PR. Anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Am J Physiol. 1992;262:H1891–H1898. doi: 10.1152/ajpheart.1992.262.6.H1891. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki M, Mori M, Fukumura D, Suzuki H, Miura S, Ishii H. Omeprazole attenuates neutrophil-endothelial cell adhesive interaction induced by extracts of Helicobacter pylori. J Gastroenterol Hepatol. 1999;14:27–31. doi: 10.1046/j.1440-1746.1999.01809.x. [DOI] [PubMed] [Google Scholar]
- 30.Ohara T, Arakawa T. Lansoprazole decreases peripheral blood monocytes and intercellular adhesion molecule-1-positive mononuclear cells. Dig Dis Sci. 1999;44:1710–1715. doi: 10.1023/a:1026604203237. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M, Mori M, Miura S, Suematsu M, Fukumura D, Kimura H, Ishii H. Omeprazole attenuates oxygen-derived free radical production from human neutrophils. Free Radic Biol Med. 1996;21:727–731. doi: 10.1016/0891-5849(96)00180-3. [DOI] [PubMed] [Google Scholar]
- 32.Biswas K, Bandyopadhyay U, Chattopadhyay I, Varadaraj A, Ali E, Banerjee RK. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J Biol Chem. 2003;278:10993–11001. doi: 10.1074/jbc.M210328200. [DOI] [PubMed] [Google Scholar]
- 33.Zedtwitz-Liebenstein K, Wenisch C, Patruta S, Parschalk B, Daxböck F, Graninger W. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit Care Med. 2002;30:1118–1122. doi: 10.1097/00003246-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 34.Noble DW. Proton pump inhibitors and stress ulcer prophylaxis: pause for thought? Crit Care Med. 2002;30:1175–1176. doi: 10.1097/00003246-200205000-00046. [DOI] [PubMed] [Google Scholar]
- 35.Tsuruma T, Yagihashi A, Tarumi K, Hirata K. Anti-rat IL-8 (CINC) monoclonal antibody administration reduces ischemia-reperfusion injury in small intestine. Transplant Proc. 1998;30:2644–2645. doi: 10.1016/s0041-1345(98)00765-9. [DOI] [PubMed] [Google Scholar]
- 36.Yagihashi A, Tsuruma T, Tarumi K, Kameshima T, Yajima T, Yanai Y, Watanabe N, Hirata K. Prevention of small intestinal ischemia-reperfusion injury in rat by anti-cytokine-induced neutrophil chemoattractant monoclonal antibody. J Surg Res. 1998;78:92–96. doi: 10.1006/jsre.1998.5367. [DOI] [PubMed] [Google Scholar]


