Abstract
Mammalian cells often exhibit a hyper-radiosensitivity (HRS) to radiation doses <20 cGy, followed by increased radioresistance (IRR) at slightly higher doses (∼20–30 cGy). Here, the influence of DNA double-strand break repair (DSBR) on IRR was examined. The failure of Ataxia telangiectasia (AT) cells to undergo IRR reported by others was confirmed. Flow cytometric analysis indicated that normal cells fail to show a measurable increase in serine 1981 phosphorylated AT-mutated (ATM) protein after 10 cGy up to 4 h post irradiation, but a two- to fourfold increase after 25 cGy. Similarly, more proficient reduction of phosphorylated histone H2AX was observed 24 h after 25 cGy than after 10 cGy, suggesting that DSBR is more efficient during IRR than HRS. A direct examination of the consequences of inefficient DNA repair per se (as opposed to ATM-mediated signal transduction/cell cycle responses), by determining the clonogenic survival of cells lacking the DNA repair enzyme polynucleotide kinase/phosphatase, indicated that these cells have a response similar to AT cells, i.e. HRS but no IRR, strongly linking IRR to DSBR.
INTRODUCTION
Many human and rodent cell lines exhibit hyper-radiosensitivity (HRS) after exposure to low doses of radiation (typically 10–50 cGy, depending on the cell line), which manifest as a significant deviation from the clonogenic survival response predicted by a linear quadratic fit to higher doses(1–4). Evidence suggests that this sensitivity is the result of a p53/caspase 3-dependent apoptotic process with G2-phase cells being particularly vulnerable(5, 6). At slightly higher doses (typically 20–60 cGy, depending on the cell line), HRS is followed by the induction of moderate radioresistance, termed increased radioresistance (IRR). IRR is only observed in cells that display HRS. The relationship between DNA repair and HRS/IRR still needs to be fully elucidated. There is some indication that IRR is associated with an increase in DNA repair, especially repair of DNA double-strand breaks (DSBs) by non-homologous end joining (NHEJ). For example, it is clear that IRR requires active Ataxia telangiectasia mutated (ATM(7)). Also cells with effective DNA-PK activity display IRR, while cells lacking DNA-PK do not(8). Wykes et al.(9) carefully examined a series of HRS-positive and HRS-negative cells for the appearance of phosphorylated histone H2AX (γH2AX) foci as a function of dose and time after irradiation. (Histone H2AX becomes phosphorylated in an ATM-dependent manner shortly after cell irradiation and serves as a marker of DNA DSBs.) The authors found no relationship between the initial or residual number of foci and HRS.
The purpose of the present study was to further clarify the relationship between DNA repair and IRR using a series of human cell lines with well-characterised HRS/IRR phenotypes. In their original observation of ATM autophosphorylation following DNA damage, Bakkenist and Kastan(10) noted a sharp diminution of ATM activity below 30 cGy in the primary human fibroblasts they were examining. In the present study, ATM phosphorylation was monitored at doses where HRS and IRR are each close to maximal, and similarly, the appearance and disappearance of H2AX phosphorylation at these HRS and IRR doses. Finally, the low-dose response of cells deficient in polynucleotide kinase/phosphatase (PNKP), a DNA modifying enzyme required for repair of radiation-induced DSBs, was examined.
MATERIALS AND METHODS
Reagents
Cell culture reagents including Dulbecco's MEM/Ham's F12 nutrient mix (DMEM/F12), l-glutamine, trypsin, penicillin–streptomycin, sodium pyruvate and foetal bovine serum (FBS) were purchased from Invitrogen (Grand Island, NY). General cell culture-grade chemicals were obtained from Sigma-Aldrich Chemicals (St. Louis, MO). Goat anti-mouse IgG conjugated to AlexaFluor 488 was supplied by Molecular Probes (Eugene, OR), the primary antibody to γH2AX by Upstate Cell Signaling Solutions (Charlottesville, VA) and the monoclonal antibody to ATM-pS1981 was from Cell Signaling Technologies (Beverly, MA). Cytofix/Cytoperm™ and Perm/Wash™ solutions were from BD Biosciences.
Cell culture
GM38 normal human skin fibroblasts (National Institute of General Medical Sciences Human Genetic Repository, Camden, NJ), A549 lung adenocarcinoma cells and MCF7 breast carcinoma cells (ATCC, Rockland, MD), and A549 cells stably depleted of PNKP by shRNA, A549δPNKP(11), were grown in DMEM/F12 with 10 % FCS at 37°C and 5 % CO2. Ataxia telangiectasia fibroblasts (AT5BI and AT2BE obtained from Coriell Cell Repository, Bethesda, MD) were also grown in DMEM/F12 but with 15 % FBS.
Radiation exposures
Cells were exposed to radiation at a dose rate of 21 cGy min−1 in a J.L. Shepherd Mark I 137Cs irradiator (San Fernando, CA) for appropriate times (up to 5 min) under ambient conditions.
Cell survival
Cells used for survival assays were from healthy non-confluent populations. About 100–300 cells were plated per dish 24 h prior to irradiation. After incubation for 7–10 days (for tumour cells) or 3 weeks (for fibroblasts), colonies were fixed and stained in 5 % crystal violet in 60 % methanol. Colonies with more than 30–50 cells were scored, and surviving fractions were normalised to sham-irradiated controls. For experiments requiring inhibition of protein phosphatase 2A (PP2A), cells were exposed to 10 nM calyculin A (Merck, Billerica, MA) 15 min prior to irradiation and removed 1 h after irradiation.
Flow cytometric assay for ATM phosphorylated at serine 1981 (ATM-pS1981)
A flow cytometric assay to determine the intracellular presence of ATM-pS1981 was performed by adapting the methods outlined by the manufacturer of Cytofix/Cytoperm™ and Perm/Wash™ solutions, which were used to fix, permeabilize and wash the cells in preparation for incubation with the primary antibody. Goat anti-mouse IgG conjugated to AlexaFluor 488 was used to detect ATM-pS1981 bound to its antibody.
After irradiation and subsequent incubations, cells were trypsinised and washed, resuspended in Cytofix/Cytoperm™ and incubated for 20 min on ice. After 2 washes in Perm/Wash™, the fixed and permeabilized cells were incubated for 30 min with the primary antibody at room temperature (RT). The cells were washed again and incubated with the secondary antibody for another 30 min at RT and washed again. Cells were resuspended in Perm/Wash™ solution in preparation for flow cytometry. Flow cytometry was performed on a FACSort instrument (BD Biosciences, Mississauga, ON). A minimum of 10 000 cells were examined to identify differences in the histogram curves occurring as a result of radiation.
Measurement of phosphorylated histone H2AX
A procedure developed by MacPhail et al.(12) was followed. Briefly, after irradiation and various incubation times, cells were trypsinised, washed and fixed in 1 ml 70 % ethanol at −20°C for between 1 and 16 h. Subsequently, fixed cells were washed in phosphate-buffered saline (PBS) and resuspended gently in PST buffer (4 % FBS and 0.1 % Triton X100 in PBS) for 10 min to rehydrate. Cells were then incubated with shaking at RT for 2 h with the antibody to γH2AX in PST, washed in PBS with 2 % FBS in PBS and then incubated for 1 h with a goat anti-mouse IgG conjugated to AlexaFluor 488 also in PST. After another wash, cells were counterstained with 5 μg ml−1 propidium iodide in 0.5 ml PBS with 2 μg ml−1 RNase. The number of cells with bound antibody to γH2AX was determined by flow cytometry.
RESULTS
Cell survival of ATM-positive and ATM-negative cells
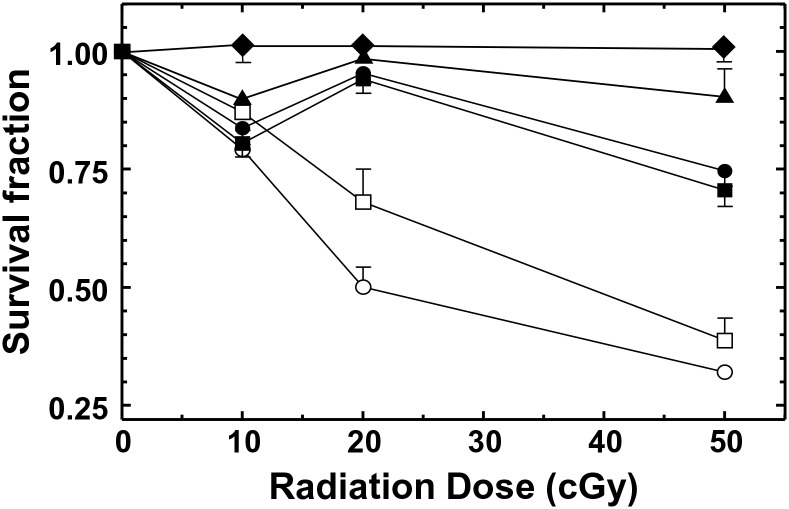
The clonogenic survival responses of several human cancer cell lines and fibroblasts subjected to low doses of 137Cs γ-radiation are shown in Figure 1. As previously reported, the lung carcinoma cell line, A549, and normal skin fibroblast cells, GM38, displayed both an HRS response, with a maximum sensitivity at ∼10 cGy, and an IRR response, with a maximum resistance at ∼20 cGy. On the other hand, cells lacking active caspase 3, such as MCF7 breast carcinoma cells, did not respond significantly to doses <20 cGy. In agreement with others(9), the AT fibroblasts (in this case AT5BI and AT2BE) displayed a similar response to normal fibroblasts at 10 cGy but failed to undergo IRR at higher doses and instead continued their decline in survival with increasing dose.
Figure 1.
Low-dose radiation survival response of normal, GM38 (▪) and CRL2522 (●), and AT, AT5BI (□) and AT2BE (○), fibroblasts and A549 (▴) and MCF7 (♦) cancer cells. All data points are based on three independent determinations. Error bars indicate SEM.
ATM phosphorylation
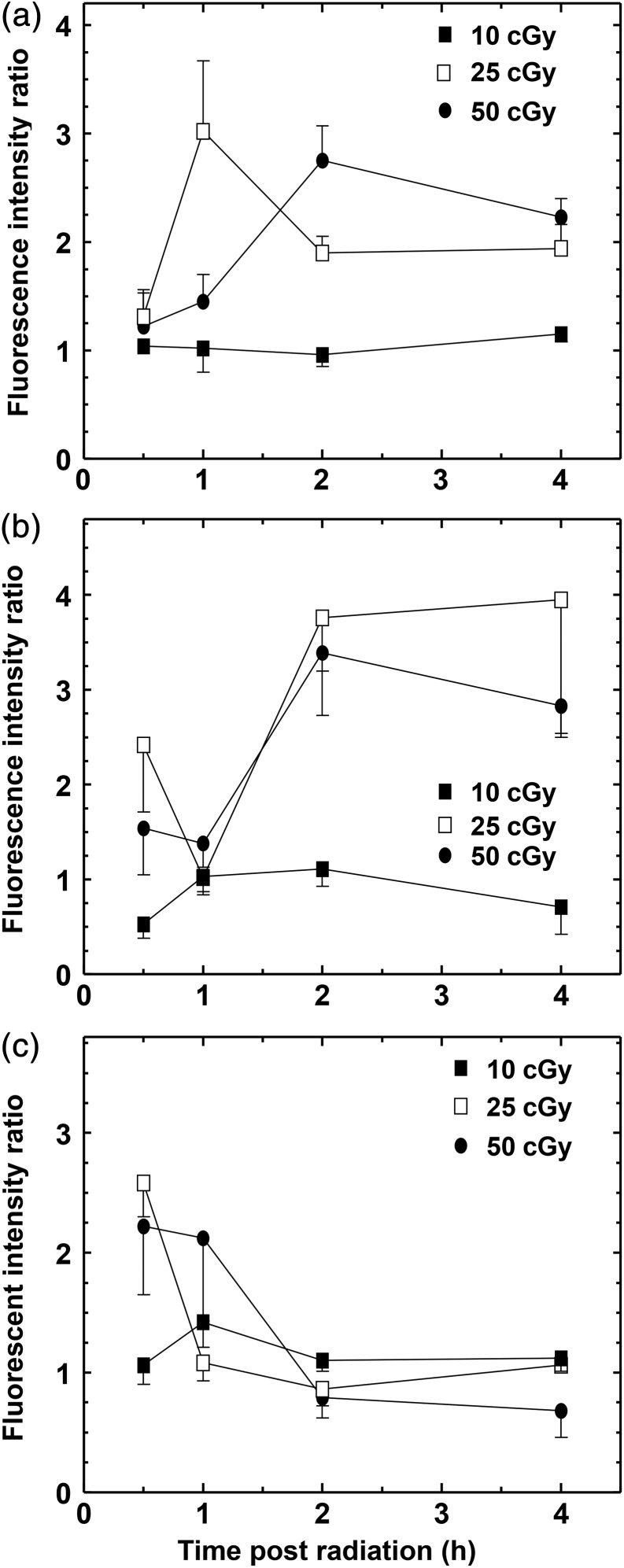
Phosphorylation of ATM serine 1981 was monitored in GM38, A549 and MCF7 cells up to 4 h after irradiation with 10, 25 and 50 cGy, where the first dose represents a dose close to the maximum for HRS in the normally responding cells, while 25 cGy was close to the optimal dose for IRR. Because only a small percentage of cells were expected to exhibit ATM phosphorylation, a flow cytometric approach was employed. The results (Figure 2) indicate that although each cell line displayed a different time/dose dependency, all the cell lines appeared to have a very limited, if any ATM phosphorylation at 10 cGy, and a much more pronounced signal at 25 and 50 cGy.
Figure 2.
Phosphorylation of ATM-S1981 following irradiation with 10, 25 and 50 cGy in (a) GM38 fibroblasts, (b) A549 lung cancer cells and (c) MCF7 breast cancer cells. Note the lack of increased phosphorylation after 10 cGy. The data are normalised to the non-irradiated controls. All data points are based on three independent determinations. Error bars indicate SEM.
Phosphorylation of histone H2AX
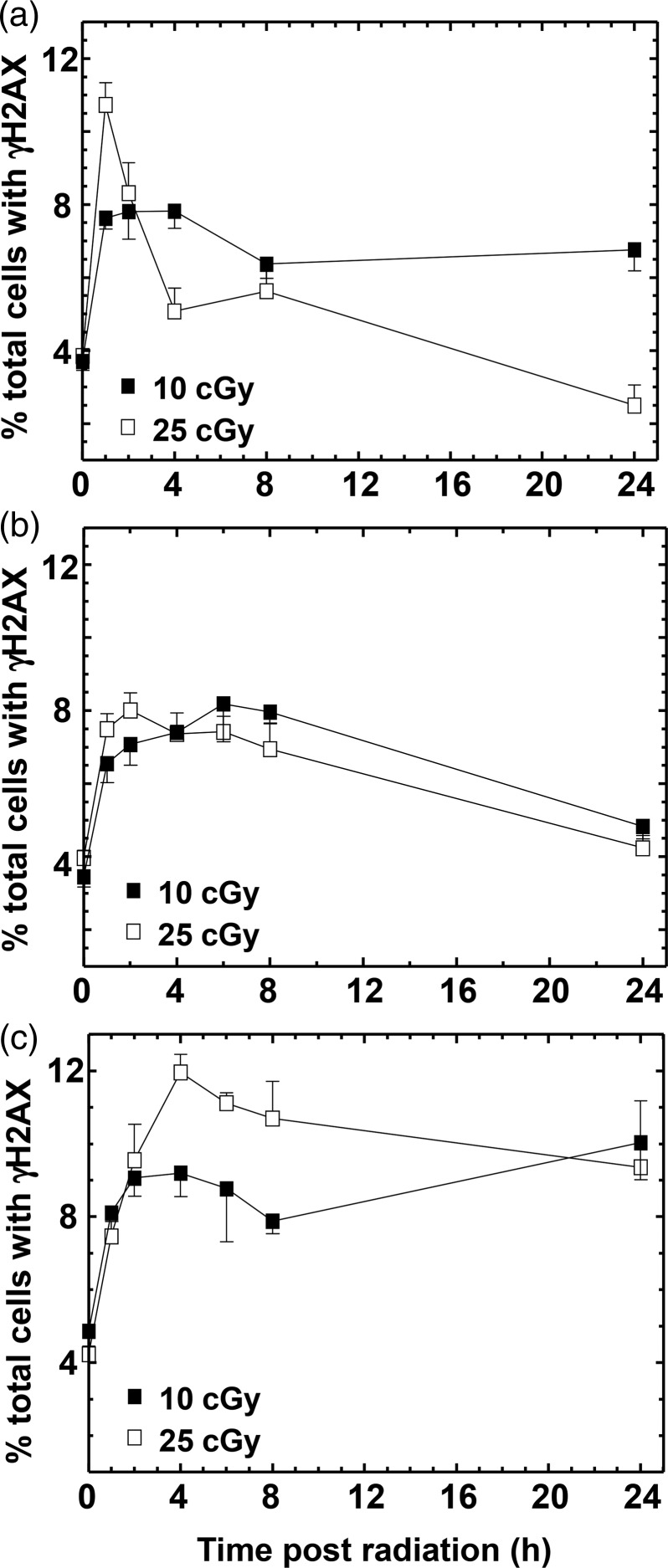
A flow cytometric approach was used to assay γH2AX in cells at various times post irradiation with 10 or 25 cGy (Figure 3). Low-dose radiation damage engendered rapid phosphorylation of H2AX (Figure 3), but the disappearance of γH2AX 24 h post irradiation was more proficient after 25 cGy than after 10 cGy in A549 cells, suggesting that repair is more efficient during IRR (Figure 3a). Normal human fibroblasts appeared to repair their DNA equally efficiently at both 10 and 25 cGy (Figure 3b), whereas the AT fibroblasts did not appreciably repair their DNA over the time examined (up to 24 h) (Figure 3c). This would indicate a requirement for the ATM gene product upstream of, or during, the repair process as well as for the IRR response.
Figure 3.
Phosphorylation and dephosphorylation of H2AX. Repair of DSB was followed over time as the number of cells with γH2AX remaining in (a) A549 lung cancer cells, (b) GM38 fibroblasts and (c) AT5BI fibroblasts. All data points are based on three independent determinations. Error bars indicate SEM. In general, repair is more efficient after 25 cGy than after 10 cGy. AT cells show no repair post 10 cGy and only minimal repair post 25 cGy.
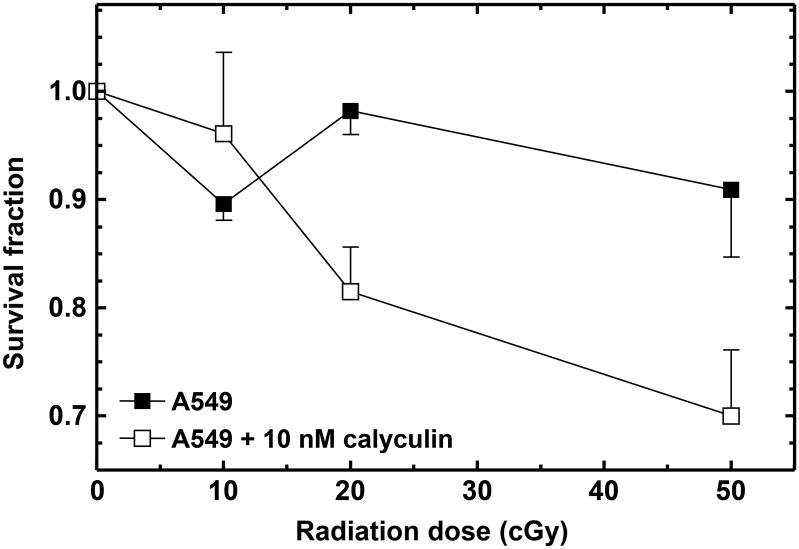
It has been shown that PP2A dephosphorylates γH2AX and this facilitates the repair of DSBs(13). Furthermore, calyculin A, a well-known inhibitor of PP2A, inhibits dephosphorylation of γH2AX and DSB rejoining(14) and sensitises cells to radiation(15). The latter study was carried out with doses ≥2 Gy, and it was, therefore, of interest to examine the influence of calyculin A on the low-dose response. The modulation of the A549 cell survival curve due to the presence of 10 nM calyculin A during radiation exposure and for 1 h post irradiation is shown in Figure 4. It is apparent that calyculin A under these exposure conditions abrogates IRR.
Figure 4.
Influence of 10 nM calyculin A on radiation response measured by clonogenic survival assay. Inhibition of phosphatase PP2A by calyculin A appears to abrogate IRR in A549 cells. All data points are based on three independent determinations. Error bars indicate SEM.
Influence of polynucleotide kinase/phosphatase (PNKP) on cellular response to low-dose radiation
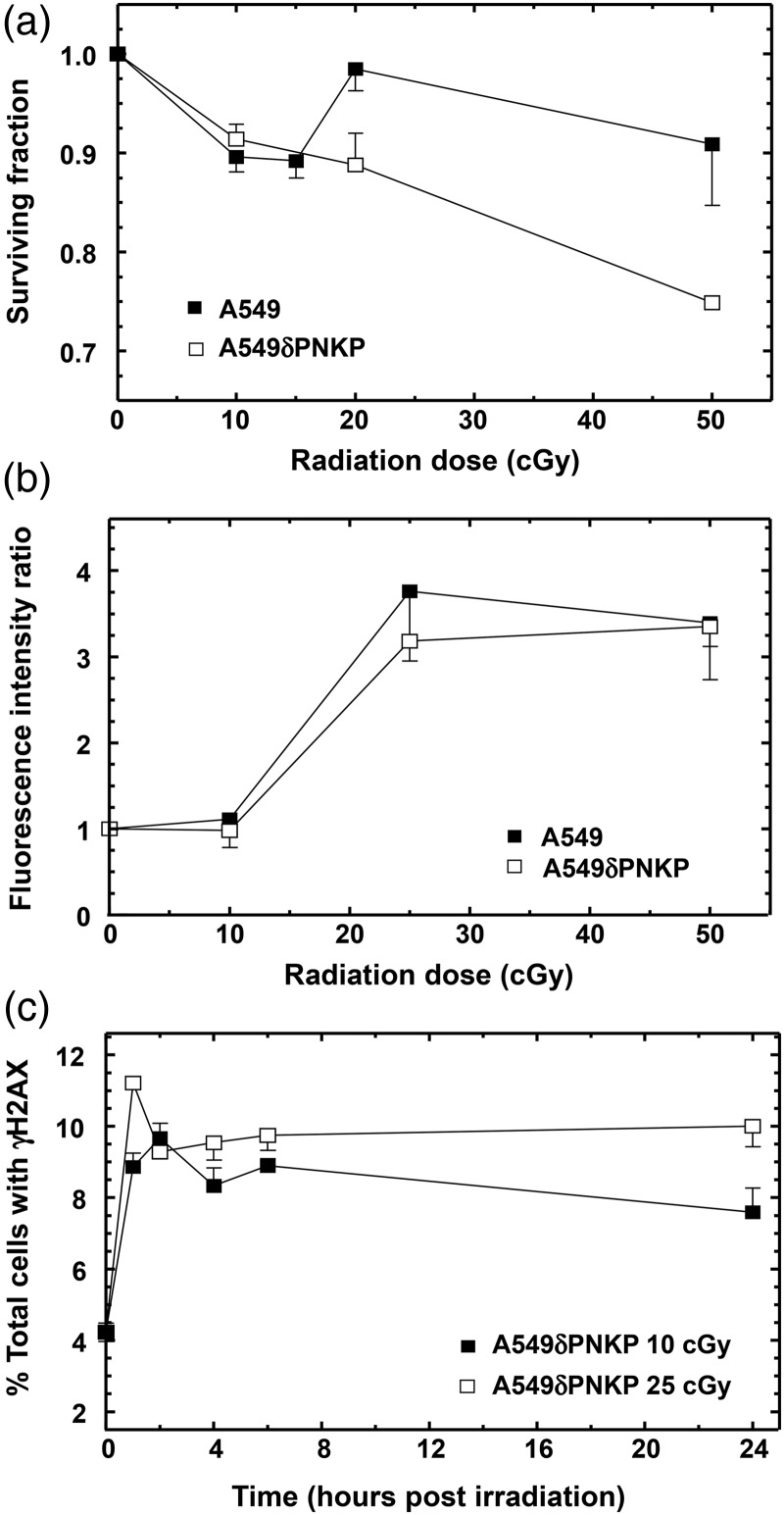
To date, several DNA repair enzymes have been investigated for their association with IRR, including ATM, DNA-PK and poly(ADP-ribose) polymerase (PARP)(7, 8). However, in addition to their direct roles in NHEJ (ATM and DNA-PK) and single-strand break (SSB) repair (PARP), all three of these enzymes have signalling/regulatory roles related to cell cycle and transcription(16–18). It is conceivable that the effects observed with cells deficient in these enzymes or with inhibitors may be the result of dysregulation of the signalling roles rather than disruption of DNA repair per se. Therefore, the low-dose response of cells lacking an enzyme that is considered to act purely on damaged DNA downstream of signalling events was examined. PNKP possesses both a DNA 5′-kinase and a 3′-phosphatase activity and renders DNA strand break termini suitable for the subsequent actions of DNA polymerases and ligases in the repair of SSB and DSB(19). Figure 5a indicates that A549 cells in which expression of PNKP had been stably down regulated (A549δPNKP) exhibited a similar HRS response as the wild-type A549 cells but lacked an IRR response. While the lack of PNKP had no influence on the dose response for ATM phosphorylation (Figure 5b), a significant decrease in γH2AX dephosphorylation was observed following 25 cGy irradiation, indicative of reduced DSB repair (compare Figure 5c).
Figure 5.
Influence of knockdown of the DNA repair protein PNKP on low-dose radiation response as measured by (a) clonogenic survival, (b) ATM phosphorylation and (c) H2AX phosphorylation. All data points are based on three independent determinations. Error bars indicate SEM. Clonogenic survival indicates no IRR in the PNKP-deficient cell line (A549δPNKP). Measuring γH2AX in these cells also shows that limited dephosphorylation, i.e. repair, is occurring. ATM phosphorylation, however, occurs in a similar fashion to that observed in the parental cell line.
DISCUSSION
Irradiation of cells elicits a complex response. While the consequences of irradiation with doses >1 Gy have been the subject of considerable attention, less is known about the response to low doses (<1 Gy), even though such low doses have relevance to both environmental and clinical exposures to radiation. The low-dose hypersensitivity and IRR seen in many cells indicate a response unique to the low-dose range. Marples et al.(2) have proposed a threshold dose model in which cells receiving a dose below ∼20 cGy fail to effectively recognise DNA damage and as a result undergo HRS; on the other hand, cells irradiated with a slightly higher dose cause sufficient conformational change to the DNA to activate ATM and promote repair and increased cell survival, i.e. IRR. It has been shown previously that HRS is associated with a p53-dependent apoptotic process(5), which may possibly be switched off by passage through the ATM-dependent early G2 checkpoint(2, 7). Thus, it would be predicted that AT cells would not display IRR, and indeed, Wykes et al. have shown that the SV40-transformed AT cell line AT22IJE-TJ EBS7 does not undergo IRR, while its ATM complemented line AT22IJE-TJ EBS7YZ5 does(9). In light of the influence that SV40 immortalisation has on p53-dependent radiation responses(20, 21), it was important to confirm the AT HRS/IRR phenotype with two non-transformed AT fibroblast strains.
The importance of ATM in the HRS/IRR phenotype led us to examine the kinetics for serine 1981 phosphorylation and dephosphorylation following irradiation with doses typical of HRS and IRR. While no appreciable increase above background levels of AT phosphorylation was detected after 10 cGy irradiation, a marked activation was observed after 25 and 50 cGy. In the case of the GM38 and A549 cells, the activation persisted for up to 4 h. The data of the present study are similar to that of Dai et al.(22) and Bakkenist and Kastan(10), who observed only a low degree of ATM activation below 20 cGy in A549 cells and primary human fibroblasts, respectively, and that the phosphorylation after a 0.5 Gy dose remained stable and detectable up to 24 h(10). On the other hand, Suzuki et al.(23) have reported the relatively rapid loss (>80 % after 6 h) of phosphorylated ATM foci following exposure to 0.1 or 0.5 Gy. G2 checkpoint activation at low doses requires H2AX and MDC1, which are central to the formation of foci surrounding DSBs(24, 25). The inefficient activation of the checkpoint at low doses is thought to reflect a higher dependence on local signal amplification when only small numbers of DSBs are present. The HRS and IRR phenotypes could relate to the efficiency of amplification of ATM activation in individual γH2AX foci, which is involved in pS1981 ATM generation. Further investigation will be required to reconcile these different data, but it is important to bear in mind that they were generated by three different assays: western blot, FACS and immunodetection of foci, each of which measures phosphorylated ATM in a different capacity.
The examination of ATM and H2AX phosphorylation in the present study supports the suggestion that IRR (doses ≥ 25 cGy) is associated with increased DNA repair, while repair at HRS doses is very limited. (A caveat to observations in the present study is the sensitivity of the flow cytometric approach used to examine protein phosphorylation in cells subjected to low-dose radiation. For example, a dose of 25 cGy would be anticipated to generate ∼5–10 DSBs per cell, yet the maximum percentage of γH2AX-positive cells detected was ∼12 %.) The AT cells appeared to show little to no DNA repair at either 10 or 25 cGy. However, these data do not prove that DNA repair is responsible for IRR, since it could be argued that DNA repair occurs as a result of IRR. Interestingly, the inhibition of PP2A phosphatase abrogated the IRR response. Although it is known that calyculin A can inhibit dephosphorylation of γH2AX and DNA DSB rejoining(14), another possible explanation for the observed effect on IRR could be its inhibition of dephosphorylation of p53 serine 46 and consequent promotion of apoptosis(26), i.e. an extension of the apoptosis associated with HRS. On the other hand, the abrogation of IRR observed with depletion of PNKP, an enzyme required for repair of radiation-induced SSBs and DSBs, strongly supports the notion that IRR is dependent on DNA repair.
FUNDING
This work was supported by the Canadian Institutes of Health Research (grant number MOP 115069 to MW).
REFERENCES
- 1.Marples B., Joiner M. C. The response of Chinese hamster V79 cells to low radiation doses: evidence of enhanced sensitivity of the whole cell population. Radiat. Res. 133, 41–51 (1993). [PubMed] [Google Scholar]
- 2.Marples B., Wouters B. G., Collis S. J., Chalmers A. J., Joiner M. C. Low-dose hyper-radiosensitivity: a consequence of ineffective cell cycle arrest of radiation-damaged G2-phase cells. Radiat. Res. 161, 247–255 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Marples B., Collis S. J. Low-dose hyper-radiosensitivity: past, present, and future. Int. J. Radiat. Oncol. Biol. Phys. 70, 1310–1318 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Martin L. M., Marples B., Lynch T. H., Hollywood D., Marignol L. Exposure to low dose ionising radiation: molecular and clinical consequences. Cancer Lett. 338, 209–218 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Enns L., Bogen K. T., Wizniak J., Murtha A. D., Weinfeld M. Low-dose radiation hypersensitivity is associated with p53-dependent apoptosis. Mol. Cancer Res. 2, 557–566 (2004). [PubMed] [Google Scholar]
- 6.Krueger S. A., Joiner M. C., Weinfeld M., Piasentin E., Marples B. Role of apoptosis in low-dose hyper-radiosensitivity. Radiat. Res. 167, 260–267 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Fernet M., Mégnin-Chanet F., Hall J., Favaudon V. Control of the G2/M checkpoints after exposure to low doses of ionising radiation: implications for hyper-radiosensitivity. DNA Repair (Amst.). 9, 48–57 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Marples B., Cann N. E., Mitchell C. R., Johnston P. J., Joiner M. C. Evidence for the involvement of DNA-dependent protein kinase in the phenomena of low dose hyper-radiosensitivity and increased radioresistance. Int. J. Radiat. Biol. 78, 1139–1147 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Wykes S. M., Piasentin E., Joiner M. C., Wilson G. D., Marples B. Low-dose hyper-radiosensitivity is not caused by a failure to recognize DNA double-strand breaks. Radiat. Res. 165, 516–524 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Bakkenist C. J., Kastan M. B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 421, 499–506 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Rasouli-Nia A., Karimi-Busheri F., Weinfeld M. Stable down-regulation of human polynucleotide kinase enhances spontaneous mutation frequency and sensitizes cells to genotoxic agents. Proc. Natl. Acad. Sci. USA. 101, 6905–6910 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacPhail S. H., Banáth J. P., Yu Y., Chu E., Olive P. L. Cell cycle-dependent expression of phosphorylated histone H2AX: reduced expression in unirradiated but not X-irradiated G1-phase cells. Radiat. Res. 159, 759–767 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury D., Keogh M. C., Ishii H., Peterson C. L., Buratowski S., Lieberman J. Gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol. Cell. 20, 801–809 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Nazarov I. B., et al. Dephosphorylation of histone gamma-H2AX during repair of DNA double-strand breaks in mammalian cells and its inhibition by calyculin A. Radiat. Res. 160, 309–317 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K., Antoku S. Enhancement of X-ray cell killing in cultured mammalian cells by the protein phosphatase inhibitor calyculin A. Cancer Res. 54, 2088–2090 (1994). [PubMed] [Google Scholar]
- 16.Shiloh Y., Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell. Biol. 14, 197–210 (2013). [PubMed] [Google Scholar]
- 17.Kong X., Shen Y., Jiang N., Fei X., Mi J. Emerging roles of DNA-PK besides DNA repair. Cell Signal. 23, 1273–1280 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Gibson B. A., Kraus W. L. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat. Rev. Mol. Cell. Biol. 13, 411–424 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Weinfeld M., Mani R. S., Abdou I., Aceytuno R. D., Glover J. N. Tidying up loose ends: the role of polynucleotide kinase/phosphatase in DNA strand break repair. Trends Biochem. Sci. 36, 262–271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziv Y., Jaspers N. G., Etkin S., Danieli T., Trakhtenbrot L., Amiel A., Ravia Y., Shiloh Y. Cellular and molecular characteristics of an immortalized ataxia-telangiectasia (group AB) cell line. Cancer Res. 49, 2495–2501 (1989). [PubMed] [Google Scholar]
- 21.Kohli M., Jorgensen T. J. The influence of SV40 immortalization of human fibroblasts on p53-dependent radiation responses. Biochem. Biophys. Res. Commun. 257, 168–176 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Dai X., Tao D., Wu H., Cheng J. Low dose hyper-radiosensitivity in human lung cancer cell line A549 and its possible mechanisms. J. Huazhong Univ. Sci. Technol. Med. Sci. 29, 101–106 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Suzuki K., Okada H., Yamauchi M., Oka Y., Kodama S., Watanabe M. Qualitative and quantitative analysis of phosphorylated ATM foci induced by low-dose ionizing radiation. Radiat. Res. 165, 499–504 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Capetillo O., et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat. Cell Biol. 4, 993–997 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Lou Z., et al. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol. Cell. 21, 187–200 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Mi J., Bolesta E., Brautigan D. L., Larner J. M. PP2A regulates ionizing radiation-induced apoptosis through Ser46 phosphorylation of p53. Mol. Cancer Ther. 8, 135–140 (2009). [DOI] [PubMed] [Google Scholar]