Abstract
AIM: The pathogenesis of hypogonadism in liver cirrhosis is not well understood. Previous results from our laboratory showed that IGF-1 deficiency might play a pathogenetic role in hypogonadism of cirrhosis. The administration of IGF-1 for a short period of time reverted the testicular atrophy associated with advanced experimental cirrhosis. The aim of this study was to establish the historical progression of the described alterations in the testes, explore testicular morphology, histopathology, cellular proliferation, integrity of testicular barrier and hypophyso-gonadal axis in rats with no ascitic cirrhosis.
METHODS: Male Wistar rats with histologically-proven cirrhosis induced with carbon tetrachloride (CCl4) for 11 wk, were allocated into two groups (n = 12, each) to receive recombinant IGF-1 (2 μg/100 g.d, sc) for two weeks or vehicle. Healthy rats receiving vehicle were used as control group (n = 12).
RESULTS: Compared to controls, rats with compensated cirrhosis showed a normal testicular size and weight and very few histopathological testicular abnormalities. However, these animals showed a significant diminution of cellular proliferation and a reduction of testicular transferrin expression. In addition, pituitary-gonadal axis was altered, with significant higher levels of FSH (P < 0.001 vs controls) and increased levels of LH in untreated cirrhotic animals. Interestingly, IGF-1 treatment normalized testicular transferrin expression and cellular proliferation and reduced serum levels of LH (P = ns vs controls, and P < 0.01 vs untreated cirrhotic group).
CONCLUSION: The testicular barrier is altered from an early stage of cirrhosis, shown by a reduction of transferrin expression in Sertoli cells, a diminished cellular proliferation and an altered gonadal axis. The treatment with IGF-1 could be also useful in this initial stage of testicular disorder associated with compensated cirrhosis.
INTRODUCTION
Hypogonadism (characterized by low testosterone levels and relative hyperestrogenism, loss of libido, sexual impotence and feminine body features in men) is a common complication of advanced liver cirrhosis[1]. Previous data demonstrated that rats with advanced cirrhosis showed reduced testicular size and weight and severe histopathological testicular abnormalities, including reduced tubular diameters, loss of the germinal line, and diminutions in cellular proliferation and spermatogenesis and testicular transferrin expression, a good marker of the integrity of blood-testis barrier[2]. In addition, low serum testosterone and high serum LH were present in cirrhotic animals, as well as decreased levels of serum IGF-1. Interestingly, the administration of IGF-1 at low doses for a short period of time reverted the testicular atrophy and improved the altered pituitary-testicular axis in these animals[2]. In cirrhotic patients, hypogonadism has been attributed to a variety of mechanisms including gonadal toxicities of alcohol, malnutrition and increased production of estrogens from androgens in peripheral tissues due to the existence of portal systemic shunting[3-9] .
Insulin-like growth factor-1 (IGF-1) is an anabolic hormone produced in different tissues although the liver accounts for 90% of the circulating hormone, which is synthesized in response to growth hormone (GH) stimulation[10,11]. In cirrhosis the reduction of receptors for GH in hepatocytes and the diminished synthesizing ability of the liver parenchyma caused a progressive fall of serum IGF-1 levels[11-14]. Although in an early stage of cirrhosis serum levels of IGF-1 were normal, its bio-availability seemed to be diminished[15]. The clinical impact of the reduced production or availability of IGF-1 in cirrhosis was largely unknown[11-14]. Recent studies from our laboratory have demonstrated that short courses of treatment with low doses of IGF-1 were able to induce marked improvements in nutritional state[15], nitrogen retention[16], intestinal absorption[17-19], osteopenia[20,21], liver function reducing fibrogenesis[22,23], and restore the reduced somatostatinergic tone[24] in rats with experimental cirrhosis. These data suggest that IGF-1 deficiency plays a pathogenetic role in several systemic complications occurring in cirrhosis.
It is well known that IGF-1 stimulates testosterone synthesis and spermatogenesis[25-32]. Its deficiency could contribute to the development of hypogonadism associated with cirrhosis as the previous data supported[2]. Since the pathogenesis of hypogonadism in cirrhosis is not yet well understood, the aim of the present study was to study the chronological progression of the described alterations in testes[2] in advanced cirrhosis, explore testicular morphology, histopathology, cellular proliferation, integrity of testicular barrier and hypophyso-gonadal axis in rats with cirrhosis in an early stage, without ascites.
MATERIALS AND METHODS
Induction of liver cirrhosis
All experimental procedures were performed in conformity with The Guiding Principles for Research Involving Animals[33]. Cirrhosis was induced as previously described[15,16]. Briefly, male Wistar rats (3-week-old, 130-150 g) were subjected to CCl4 inhalation (Merck, Darmstadt, Germany) twice a week for 11 wk with a progressively increasing exposure time from 1 to 5 min. During the whole period of cirrhosis induction animals received Phenobarbital (Luminal, Bayer, Leverkusen, Germany) in the drinking water (400 mg/L). Both food (standard semipurified diet for rodents; B.K. Universal, Sant Vicent del Horts, Spain) and water were given ad libitum. Healthy, age and sex-matched control rats were maintained under the same conditions but receiving neither CCl4 nor Phenobarbital.
Study design
The treatment was administrated for 2 wk after stopping of CCl4 exposure. In the morning of d 0, animals were weighed and blood samples were drawn from the retroocular venous plexus from all rats with capillary tubes (Marienfeld, Germany) and stored at -20 °C until used for analytical purposes. Cirrhotic rats were randomly assigned to receive either vehicle (saline) (group CI, n = 12) or recombinant human IGF-1 (Pharmacia-Uppsala, Sweden) (2 μg/100 g.d in two divided doses, subcutaneously) (group CI + IGF, n = 12) for two weeks. Control rats (group CO, n = 10) received saline during the same period.
In the morning of the 15th d, rats were weighed, blood was obtained from the retroocular plexus and animals were killed by decapitation. After the abdominal cavity was opened, the liver and testes were dissected and weighed. Samples from the left major liver lobe and testes were processed for histological examination (fixed in Bouin’s solution). The testicular diameters (AP and LM) were measured using a precision calliper, Mituyoto® (± 0.05 mm). Ascites was ruled out by direct exploration of abdominal cavity in all cirrhotic animals.
Liver histopathology
Bouin-fixed tissues were processed and sections (4-mm) were stained with Haematoxylin and Eosin and Masson’s trichrome. Livers were scored (1 to 4) as previously reported[15,22]. Preestablished criteria for inclusion of cirrhotic (CI) animals in the final analysis were the presence of: (1) altered baseline biochemical data of liver function; and (2) in retrospect, histologically proven liver cirrhosis (scores 3 or 4 of the classification) in CCl4-treated-animals. All animals had compensated cirrhosis without ascites.
Testicular histopathology and PCNA and transferrin immuno-histochemistry
For histopathological evaluation of testes, 30 seminiferous tubules from each rat of the three groups were blindly evaluated by two observers and the arithmetic mean of the scores was taken as the final result. Transverse sections of seminiferous tubuli were examined and evaluation of histological changes was made using a light projection microscope (Micro Promar Leitz GMBH, Wetzlar, Germany) at 150 × magnification. The following parameters were studied: tubular diameter, quantitation of the presence of different types of cells in tubuli, presence of peritubular fibrosis, and the number of proliferating cells. For general purposes haematoxylin & eosin stain and Masson’s trichrome stain were used. Specific techniques for other purposes are specified in the corresponding paragraphs.
Changes in tubuli were classified into 5 categories (Category I: highest damage to Category V: full normality). Category I: presence of only Sertoli cells; category II: Sertoli cells plus spermatids; category III: Sertoli cells, plus spermatides, and spermatocytes; category IV: presence of all kinds of cells but showing some morphological alterations (i.e.: severe vacuolization, aberrant cells); category V: presence of all kinds of cells without morphological alterations. The presence of peritubular fibrosis was evaluated in Masson’s trichrome preparations according to the thickness of the staining of collagen deposition surrounding tubuli. Proliferating cells were identified by immunostaining of proliferating cellular nuclear antigen (PCNA) using an avidin-biotin peroxidase method[34] with retrieval of antigen by means of microwave irradiation. Specific anti-PCNA antibody (mouse anti-PCNA, clone PC 10, DAKO, Denmark) biotinylated rabbit anti-mouse IgG (DAKO, Denmark) were used and the avidin-biotin complex technique (ABC, DAKO kit) was performed. The bound antibodies were visualized by means of 3,3’-diaminobenzidine tetrahydrochloride (Sigma Chemical Company, St. Louis, MO) with nickel enhancement[34]. Finally, samples were slightly counterstained (10 s) in hematoxylin, dehydrated, and mounted in DPX. Controls were performed by substitution of the primary antibody by TBS. The number of PCNA positive cells was recorded. The result was expressed as stained cells per tubuli (arithmetic mean of 30 screened tubuli).
In addition, the expression of transferrin[35] in tubuli was evaluated by immunostaining using similar technique as for PCNA with specific anti-transferrin antibody (obtained from rabbit, RARa/TRf, Nordic Immunological Laboratories, Teknovas, The Netherland). Transferrin expression was scored from 0 to 4 points. If 30 tubuli expressed transferrin normally all over the germinal epithelium, it was scored 0 points. The remaining scores were obtained according to the following formula: (30-tubuli showing expression of transferrin all over the germinal epithelium) × 0.075.
Analytical methods
Serum levels of albumin, total proteins, glucose, cholesterol, bilirubin, alkaline phosphatase, transferrin and aminotransferases (AST and ALT) were determined by routine laboratory methods using a Hitachi 747 autoanalyzer (Boerhringer-Mannheim, Germany). Serum levels of the different hormones were assessed by RIA in a GammaChen 9612 Plus (Serono Diagnostics, Roma, Italy) using specific commercial assay systems: total and free testosterone and estradiol-6, Coat-a-Count, DPC (Diagnostic Products Corporation, Los Angeles, CA); rat luteinizing hormone (rLH) and rat follicle stimulating hormone (rFSH) from Amersham International Plc (Little Chalfont Buckinghamshire, England HP79NA); IGF-1 by extraction (Nichols Institute Diagnostics, San Juan Capistrano, CA, USA).
Lipid peroxidation assessment of testicular homogenates
Malondyaldehyde (MDA) was assessed after heating samples at 45 °C for 60 min in acid medium. It was quantitated by a colorimetric assay using LPO-586 (Bioxytech; OXIS International Inc., Portland, OR, USA), which after reacting with MDA, generated a stable chromophore that could be measured at 586 nm (Hitachi U2000 Spectro; Roche).
Statistical analysis
Data are expressed as mean ± SE. To assess the homogeneity between the three groups of rats a Kruskall-Wallis test was used, followed by multiple post-hoc comparisons using Mann-Withney U tests (two tailed) with Bonferroni adjustment. A regression model was fitted considering histopathological score, PCNA or transferrin expression scores and IGF-1 plasma concentration as the dependent and independent variables respectively. Within group differences between pre-and post-treatment values were assessed by means of Wilcoxon matched pairs signed rank sum test. Any P value less than 0.05 was considered to be statistically significant. Calculations were performed by SPSS Win v.6.0. program.
RESULTS
At baseline, CI groups showed abnormal values compared to controls (CO) in serum levels of alanine aminotransferase (CO = 26 ± 2; CI = 273 ± 49 IU/L, P < 0.01), aspartate aminotransferase (CO = 55 ± 5; CI = 297 ± 49 IU/L, P < 0.001), cholesterol (CO = 82 ± 4; CI = 115 ± 5 mg/dL, P < 0.05), alkaline phosphatase (CO = 310 ± 43; CI = 701 ± 146 IU/L, P < 0.05), bilirubin (CO = 0.4 ± 0.0; CI = 1.2 ± 0.3 mg/dL, P < 0.05), total proteins (CO = 6.9 ± 0.1; CI = 6.4 ± 0.2 g/dL, P < 0.05) and albumin (CO = 3.6 ± 0.1; CI = 3.1 ± 0.2 g/dL, P < 0.05). No differences were found between cirrhotic groups.
At the end of the experimental period, all rats from groups CI and CI + IGF showed established cirrhosis (mixed micro-macronodular cirrhosis in liver histopathology) with some signs of portal hypertension (spleen weight, g, CO = 0.8 ± 0.0; CI = 1.4 ± 0.2; CI + IGF = 1.4 ± 0.1, P < 0.001 both cirrhotic groups vs controls). Ascites was absent in all of the cirrhotic animals.
Testicular morphology and morphometry
The testicular size and volume were normal in the cirrhotic groups as compared to controls. Morphometric study showed reduction in testicular weight (in absolute values but not if it was corrected by body mass) in untreated cirrhotic group as compared to controls and CI + IGF group. Findings regarding longitudinal and transverse testicular diameters were similar to those in testicular weight. Morphometric data in the three groups are summarized in Table 1.
Table 1.
Body mass and parameters of testicular size and weight in the three experimental groups (d 15)
| Healthy control rats (CO, n = 12) | Untreated cirrhotic rats (CI, n = 12) | Cirrhotic rats treated with IGF-1 (CI + IGF, n = 12) | |
| Body mass (g) | 545.0 ± 1.0 | 460.0 ± 1.0d | 458.0 ± 9.0d |
| Right testis (g) | 2.0 ± 0.0 | 1.6 ± 0.1d | 1.7 ± 0.1d |
| (× 100 g/bm) | (0.4 ± 0.0) | (0.3 ± 0.0) | (0.4 ± 0.0) |
| External testicular diameters (mm) | |||
| • Longitudina | 20.4 ± 0.26 | 18.56 ± 0.22d | 18.37 ± 0.34d |
| • Transversal | 10.3 ± 0.24 | 9.41 ± 0.23b | 9.64 ± 0.14a |
P < 0.05;
P < 0.01;
P < 0.001 vs CO group.
Testicular histopathology
Figure 1 shows testicular morphology in the three experimental groups. Testicular histological section of normal rat (CO) demonstrated active spermatogenesis in normal-size seminiferous tubuli with thin basement membranes and minimal peritubular fibrosis. Leydig cells were scarce, being widely separated by seminiferous tubuli. No evidence of peritubular fibrosis and other alterations was found in cirrhotic groups. Cellular analyses (see Methods) in 30 seminiferous tubuli were performed in each preparation and summarized in Table 2. No relevant findings were obtained in this morphologic study.
Figure 1.
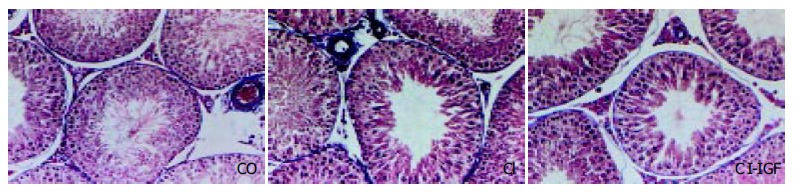
Microscopy of testes (× 150 magnification, Masson’s stain). Testicular histological sections of normal rat (CO) demonstrated active spermatogenesis in normal-size seminiferous tubuli with thin basement membranes and minimal peritubular fibrosis. Leydig cells were scarce, being widely separated by seminiferous tubuli. No evidence of peritubular fibrosis and other alterations were found in testes from cirrhotic animals.
Table 2.
Cellular analysis: Thirty seminiferous tubuli were examined in each preparation. The table summarizes the number of tubuli in each category: category I, only Sertoli s cells; category II, I + spermatids; category III, II + spermatocytes; category IV, all types of cells but with some alterations; category V, all types of cells with normal features
| Category | I | II | III | IV | V |
| Controls (CO) (n = 10) × 30 tubuli | 0 | 0 | 0 | 2 | 298 |
| Untreated cirrhotic rats (CI) (n = 10) × 30 tubuli | 0 | 0 | 5 | 20 | 275 |
| IGF-treated cirrhotic rats (CI + IGF) (n = 10) × 30 tubuli | 0 | 0 | 0 | 8 | 292 |
| Statistical analysis (P) | ns | ns | ns | aP < 0.05 | ns |
P < 0.05, CI vs CO group.
Testicular cellular proliferation, as evaluated by PCNA[35], was significantly reduced in CI rats while CI + IGF rats showed values similar to controls (CO: 66 ± 2, CI: 57 ± 1, CI + IGF: 61 ± 1, P < 0.001 untreated cirrhotic group vs controls and P < 0.05 CI + IGF vs controls and CI group). Figure 2 shows PCNA immunohistochemistry (PCNA + cells) in the three groups.
Figure 2.
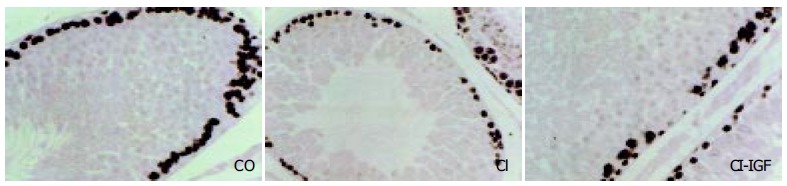
Study of proliferative activity, assessed by PCNA immunostaining. A significant reduction of cellular proliferation were observed in rats with compensated cirrhosis. This reduction was normalized in IGF-1 treated cirrhotic group (CI + IGF). (× 200 magnification, in the three pictures).
Testicular transferrin, a marker of the integrity of hemato-testicular barrier[36-38], was evaluated from 0 to 4 points by immunohistochemistry in testicular slices (see Methods). Transferrin expression was decreased in CI rats (3.40 ± 0.03 points, P < 0.001 vs CO and CI + IGF groups) as compared to controls (3.98 ± 0.04 points) and to cirrhotic rats receiving IGF-1 (3.76 ± 0.05 points). Figure 3 shows transferrin expression in the three experimental groups.
Figure 3.
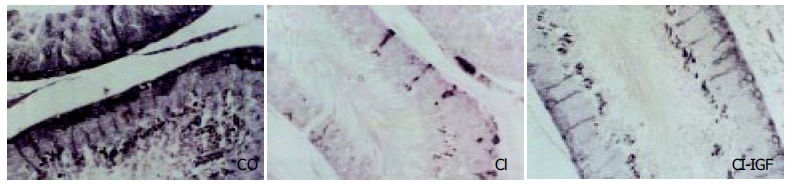
Immunohistochemistry for testicular transferrin in seminiferous tubuli. Transferrin immunostaining was observed at the level of Sertoli cells and in germ cells in normal rats (CO) and in cirrhotic rats treated with IGF-1 (CI + IGF) but a lower or absent transferrin immunostaining was observed in several tubuli of untreated cirrhotic rats (CI) (score, CI: 3.40 ± 0.03, CO: 3.98 ± 0.04, CI + IGF-1: 3.76 ± 0.05).
Testicular levels of MDA in order to study the likely direct damage of CCl4 to testes, MDA levels, an index of lipid peroxidation[39], were assessed in testicular homogenates. No differences were found between the three experimental groups in testicular MDA content (nmoL/mg protein, CO = 9.1 ± 0.8, CI = 13.0 ± 3.2, CI + IGF = 8.9 ± 1.6).
Pituitary-gonadal axis
Serum levels of sexual hormones are summarized in Table 3. No significant differences were found between groups, either in total and free testosterone, or estradiol, or in the ratio of estradiol/testosterone. However, a significant increase of FSH (P < 0.001 vs controls) and also high levels of LH (P = 0.06 vs controls) were observed in untreated rats with compensated cirrhosis, suggesting an altered negative feed-back since this early stage of the cirrhosis. On the other hand, LH concentrations were moved towards normal values in IGF-1 treated cirrhotic group (P = ns vs controls, and P < 0.01 vs untreated cirrhotic rats).
Table 3.
The pituitary-gonadal axis (on d 15) in the three experimental groups
| Healthy control rats (CO, n = 12) | Untreated cirrhotic rats (CI, n = 12) | IGF-1-treated cirrhotic rats (CI + IGF, n = 12) | |
| Total testosterone (ng/dL) | 62 ± 13 | 77 ± 17 | 64 ± 7 |
| Free testosterone (pg/mL) | 0.36 ± 0.09 | 0.36 ± 0.10 | 0.41 ± 0.05 |
| Estradiol (pg/mL) | 31 ± 2 | 33 ± 2 | 29 ± 1 |
| Estradiol/Total testosterone | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| LH (ng/mL) | 4.8 ± 0.6 | 6.0 ± 0.5 | 3.8 ± 0.7d |
| FSH (ng/mL) | 22 ± 8 | 27 ± 1f | 26 ± 4b |
P < 0.01 CI + IGF vs CO group;
P < 0.01 CI + IGF vs CI group;
P < 0.001 CI vs CO group.
Serum levels of IGF-1
At the time of animal sacrifice (d 15), no significant differences between the three groups were found in serum levels of IGF-1 (CO: 1030 ± 67, CI: 1165 ± 58, CI + IGF: 1030 ± 38 ng/mL), similar to those described previously in early stage of cirrhosis[16-18,24].
DISCUSSION
This study demonstrates that rats with compensated CCl4-induced cirrhosis show some testicular alterations and gonadal dysfunction, from early stages of liver cirrhosis. The main finding of this study is that there is an altered hemato-testicular barrier, probably responsible for the reduction of cellular proliferation, as well as a paradoxical response of pituitary-testicular axis.
The occurrence of testicular atrophy and gonadal dysfunction in advanced cirrhosis is a well known clinical event[1,3-9]. Both testicular histopathological abnormalities and low levels of serum testosterone have been described in patients with alcoholic and nonalcoholic cirrhosis several years ago[3-9]. Previous experimental data[2] showed that severe testicular atrophy and gonadal insufficiency treated with low doses of IGF-1 recovered to normal in a very short time (21 d). Data regarding experimentally induced cirrhosis are, however, scarce. Our previous data showed a severe testicular damage[2] as manifested by macroscopic testicular atrophy and a variety of histopathological abnormalities including a reduction in tubular diameters, presence of aberrant cells in tubular lumen, peritubular fibrosis, loss of the germinal line and a marked reduction in cellular proliferation. These alterations resembled those found in necropsic studies in alcoholic cirrhotics[1] and those reported in experimental models of testicular damage such as chronic testicular ischemia[35].
The mechanisms responsible for the described alterations are not fully understood, although the relationship between IGF-1 deficiency and testicular damage in cirrhosis was demonstrated in the mentioned work[2]. In cirrhotic rats included in the current work, no IGF-1 deficiency was present but there was a reduced availability of this hormone as it has been suggested previously[16-18,22]. Moreover, we have previously demonstrated that the changes induced by cirrhosis in the serum profile of IGF-1 binding proteins further reduce bio-availability of IGF-1 in cirrhotic rats[16]. In fact, exogenous administration of IGF-1 was able to reverse several abnormalities (decreased food utility and intestinal absorption of nutrients, and somatostatinergic tone and osteopenia) associated with cirrhosis in animals with normal serum levels of this hormone[16-20,22-24].
On the other hand, a direct effect of IGF-1 on testes seemed to be the most important factor to explain previous findings[2]. This idea is supported by the existence of receptors for IGF-1 in Sertoli cells, germ cells and Leydig cells[25-27] and by findings demonstrating a direct effect of IGF-1 on testes[27-31]. Since IGF-1 is a well recognized trophic factor for testis, its deficiency could be a contributing factor to testicular damage in cirrhosis.
The present work was designed in order to gain more insights into the mechanisms involved in the pathogenesis of hypogonadism associated with liver cirrhosis. In fact, many factors that have been involved in this process, such as portal systemic shunting[1,39,40] or undernutrition[1,41] were minimized in this early stage of the liver disease. Specifically, this study was targeted to establish the historical progression of the described testicular alterations[2].
The blood-testis barrier is considered nearly as specific as the blood-brain barrier[42]. Although all cells require irons from serum transferrin produced by hepatocytes, cells that create a blood barrier such as Sertoli cells in the testis and choroid plexus epithelium in the brain also express the transferrin gene to provide irons to cells sequestered within the serum-free environment. Testicular transferrin expression was a good marker of the integrity of the hemato-testicular barrier[36,37]. A major finding of this work was that transferrin expression by Sertoli cells was reduced in untreated cirrhotic rats. The medical bioavailability of IGF-1 could be due to the mechanism of testicular transferrin reduction. IGF-1 treatment increased the expression of this protein in Sertoli cells of cirrhotic rats (Figure 3). This possibility seemed to be plausible since several metabolic functions of Sertoli cells were also influenced by IGF-1[32,43-45]. Interestingly, the recovery of transferrin expression in Sertoli cells observed in our study suggested a role for IGF-1 in maintaining the integrity of the hematotesticular barrier[2,33-38].
The first step of the pathogenesis of testicular atrophy occurring in advanced cirrhosis seems to be the decreased expression of transferrin, showing a dysfunction of Sertoli cells and consequently the disruption in blood-testis barrier integrity. Therefore, the observed reduction of cellular proliferation finally affecting spermatogenesis[2,46] would be its logical consequence.
A question arises as to whether direct toxicity of CCl4 on testicular tissue could contribute to testicular injury. Alcohol was known to produce oxidative damage and to be able to pass across testicular barrier[47,48]. This toxic possibility has been reasonably ruled out by the presence of similar levels of MDA, a marker of lipid peroxidation[39], in testicular homogenates from the three experimental groups. Certainly, a slight increase of testicular MDA was found in untreated cirrhotic rats, but this did not reach statistical significance.
In the early stage of cirrhosis, testosterone levels were normal. However, both FSH and LH were increased in untreated cirrhotic animals. This abnormal response of the negative feedback could be an initial hormonal reaction of primary hypogonadism. In advanced stages of cirrhosis, we found increased levels of serum LH associated with a significant reduction of total and free serum testosterone defining a picture of primary hypogonadism, thus ruling out hypothalamic-pituitary dysfunction as the responsible mechanism[2]. Interestingly, LH levels were reduced by IGF-1 treatment in this series with compensated cirrhosis and an incipient gonadal dysfunction. The significant increase of FSH could be related to the observed reduction of cellular proliferation in cirrhotic rats. Since a close relationship has been reported between IGF-1 and gonadotropins[49-51], our findings require further investigation.
In summary, this study shows an altered hemato-testicular barrier from an early stage of cirrhosis and suggests that the reduction of IGF-1 bioavailability may play a critical role in the beginning stage of testicular damage and hypogonadism associated with liver cirrhosis. In addition, these results support the conclusion that the exogenous administration of IGF-1 may be useful for the treatment of testicular alterations in cirrhotic patients.
ACKNOWLEDGEMENTS
The authors wish to express their gratitude to Dr. Bruce Scharschmidt, Chiron, for generously granting the rhIGF-1 used in this study. We are as well deeply indebted to the "Real Academia de Medicina de Cataluña" (Barcelona) and Mrs.C.Alonso-Borrás and Mr. J. Celaya for financial collaboration.
Footnotes
Supported by the Spanish Program I + D, SAF 99/0072 and SAF 2001/1672
Edited by Zhu LH and Xu FM
References
- 1.Van Thiel DH. Endocrine function. In The liver: biology and pathobiology. Raven Press; 1982. pp. 717–912. [Google Scholar]
- 2.Castilla-Cortazar I, Garcia M, Quiroga J, Diez N, Diez-Caballero F, Calvo A, Diaz M, Prieto J. Insulin-like growth factor-I reverts testicular atrophy in rats with advanced cirrhosis. Hepatology. 2000;31:592–600. doi: 10.1002/hep.510310308. [DOI] [PubMed] [Google Scholar]
- 3.Van Thiel DH, Gavaler JS, Slone FL, Cobb CF, Smith WI, Bron KM, Lester R. Is feminization in alcoholic men due in part to portal hypertension: a rat model. Gastroenterology. 1980;78:81–91. [PubMed] [Google Scholar]
- 4.BENNETT HS, BAGGENSTOSS AH, BUTT HR. The testis, breast and prostate of men who die of cirrhosis of the liver. Am J Clin Pathol. 1950;20:814–828. doi: 10.1093/ajcp/20.9.814. [DOI] [PubMed] [Google Scholar]
- 5.Van Steenbergen W. [Alcohol, liver cirrhosis and disorders in sex hormone metabolism] Acta Clin Belg. 1993;48:269–283. doi: 10.1080/17843286.1993.11718318. [DOI] [PubMed] [Google Scholar]
- 6.Galvaõ-Teles A, Monteiro E, Gavaler JS, Van Thiel DH. Gonadal consequences of alcohol abuse: lessons from the liver. Hepatology. 1986;6:135–140. doi: 10.1002/hep.1840060126. [DOI] [PubMed] [Google Scholar]
- 7.Guechot J, Vaubourdolle M, Ballet F, Giboudeau J, Darnis F, Poupon R. Hepatic uptake of sex steroids in men with alcoholic cirrhosis. Gastroenterology. 1987;92:203–207. doi: 10.1016/0016-5085(87)90860-2. [DOI] [PubMed] [Google Scholar]
- 8.Bannister P, Handley T, Chapman C, Losowsky MS. Hypogonadism in chronic liver disease: impaired release of luteinising hormone. Br Med J (Clin Res Ed) 1986;293:1191–1193. doi: 10.1136/bmj.293.6556.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pajarinen JT, Karhunen PJ. Spermatogenic arrest and 'Sertoli cell-only' syndrome--common alcohol-induced disorders of the human testis. Int J Androl. 1994;17:292–299. doi: 10.1111/j.1365-2605.1994.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 10.Sara VR, Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990;70:591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- 11.Schimpff RM, Lebrec D, Donnadieu M. Somatomedin production in normal adults and cirrhotic patients. Acta Endocrinol (Copenh) 1977;86:355–362. doi: 10.1530/acta.0.0860355. [DOI] [PubMed] [Google Scholar]
- 12.Hattori N, Kurahachi H, Ikekubo K, Ishihara T, Moridera K, Hino M, Saiki Y, Imura H. Serum growth hormone-binding protein, insulin-like growth factor-I, and growth hormone in patients with liver cirrhosis. Metabolism. 1992;41:377–381. doi: 10.1016/0026-0495(92)90071-h. [DOI] [PubMed] [Google Scholar]
- 13.Møller S, Becker U, Juul A, Skakkebaek NE, Christensen E. Prognostic value of insulinlike growth factor I and its binding protein in patients with alcohol-induced liver disease. EMALD group. Hepatology. 1996;23:1073–1078. doi: 10.1002/hep.510230521. [DOI] [PubMed] [Google Scholar]
- 14.Møller S, Grønbaek M, Main K, Becker U, Skakkebaek NE. Urinary growth hormone (U-GH) excretion and serum insulin-like growth factor 1 (IGF-1) in patients with alcoholic cirrhosis. J Hepatol. 1993;17:315–320. doi: 10.1016/s0168-8278(05)80211-9. [DOI] [PubMed] [Google Scholar]
- 15.Caufriez A, Reding P, Urbain D, Golstein J, Copinschi G. Insulin-like growth factor I: a good indicator of functional hepatocellular capacity in alcoholic liver cirrhosis. J Endocrinol Invest. 1991;14:317–321. doi: 10.1007/BF03346823. [DOI] [PubMed] [Google Scholar]
- 16.Picardi A, de Oliveira AC, Muguerza B, Tosar A, Quiroga J, Castilla-Cortázar I, Santidrián S, Prieto J. Low doses of insulin-like growth factor-I improve nitrogen retention and food efficiency in rats with early cirrhosis. J Hepatol. 1997;26:191–202. doi: 10.1016/s0168-8278(97)80026-8. [DOI] [PubMed] [Google Scholar]
- 17.Castilla-Cortazar I, Prieto J, Urdaneta E, Pascual M, Nuñez M, Zudaire E, Garcia M, Quiroga J, Santidrian S. Impaired intestinal sugar transport in cirrhotic rats: correction by low doses of insulin-like growth factor I. Gastroenterology. 1997;113:1180–1187. doi: 10.1053/gast.1997.v113.pm9322513. [DOI] [PubMed] [Google Scholar]
- 18.Castilla-Cortázar I, Picardi A, Tosar A, Ainzúa J, Urdaneta E, García M, Pascual M, Quiroga J, Prieto J. Effect of insulin-like growth factor I on in vivo intestinal absorption of D-galactose in cirrhotic rats. Am J Physiol. 1999;276:G37–G42. doi: 10.1152/ajpgi.1999.276.1.G37. [DOI] [PubMed] [Google Scholar]
- 19.Pascual M, Castilla-Cortazar I, Urdaneta E, Quiroga J, Garcia M, Picardi A, Prieto J. Altered intestinal transport of amino acids in cirrhotic rats: the effect of insulin-like growth factor-I. Am J Physiol Gastrointest Liver Physiol. 2000;279:G319–G324. doi: 10.1152/ajpgi.2000.279.2.G319. [DOI] [PubMed] [Google Scholar]
- 20.Cemborain A, Castilla-Cortázar I, García M, Quiroga J, Muguerza B, Picardi A, Santidrián S, Prieto J. Osteopenia in rats with liver cirrhosis: beneficial effects of IGF-I treatment. J Hepatol. 1998;28:122–131. doi: 10.1016/s0168-8278(98)80211-0. [DOI] [PubMed] [Google Scholar]
- 21.Cemborain A, Castilla-Cortázar I, García M, Muguerza B, Delgado G, Díaz-Sánchez M, Picardi A. Effects of IGF-I treatment on osteopenia in rats with advanced liver cirrhosis. J Physiol Biochem. 2000;56:91–99. doi: 10.1007/BF03179904. [DOI] [PubMed] [Google Scholar]
- 22.Castilla-Cortazar I, Garcia M, Muguerza B, Quiroga J, Perez R, Santidrian S, Prieto J. Hepatoprotective effects of insulin-like growth factor I in rats with carbon tetrachloride-induced cirrhosis. Gastroenterology. 1997;113:1682–1691. doi: 10.1053/gast.1997.v113.pm9352873. [DOI] [PubMed] [Google Scholar]
- 23.Muguerza B, Castilla-Cortázar I, García M, Quiroga J, Santidrián S, Prieto J. Antifibrogenic effect in vivo of low doses of insulin-like growth factor-I in cirrhotic rats. Biochim Biophys Acta. 2001;1536:185–195. doi: 10.1016/s0925-4439(01)00045-x. [DOI] [PubMed] [Google Scholar]
- 24.Castilla-Cortázar I, Aliaga-Montilla MA, Salvador J, García M, Delgado G, González-Barón S, Quiroga J, Prieto J. Insulin-like growth factor-I restores the reduced somatostatinergic tone controlling growth hormone secretion in cirrhotic rats. Liver. 2001;21:405–409. doi: 10.1034/j.1600-0676.2001.210607.x. [DOI] [PubMed] [Google Scholar]
- 25.Tajima Y, Watanabe D, Koshimizu U, Matsuzawa T, Nishimune Y. Insulin-like growth factor-I and transforming growth factor-alpha stimulate differentiation of type A spermatogonia in organ culture of adult mouse cryptorchid testes. Int J Androl. 1995;18:8–12. doi: 10.1111/j.1365-2605.1995.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 26.Spiteri-Grech J, Weinbauer GF, Bolze P, Chandolia RK, Bartlett JM, Nieschlag E. Effects of FSH and testosterone on intratesticular insulin-like growth factor-I and specific germ cell populations in rats treated with gonadotrophin-releasing hormone antagonist. J Endocrinol. 1993;137:81–89. doi: 10.1677/joe.0.1370081. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Bondy C. Anatomy of the insulin-like growth factor system in the human testis. Fertil Steril. 1993;60:897–904. doi: 10.1016/s0015-0282(16)56294-3. [DOI] [PubMed] [Google Scholar]
- 28.Dubois W, Callard GV. Culture of intact Sertoli/germ cell units and isolated Sertoli cells from Squalus testis. II. Stimulatory effects of insulin and IGF-I on DNA synthesis in premeiotic stages. J Exp Zool. 1993;267:233–244. doi: 10.1002/jez.1402670217. [DOI] [PubMed] [Google Scholar]
- 29.Moore A, Morris ID. The involvement of insulin-like growth factor-I in local control of steroidogenesis and DNA synthesis of Leydig and non-Leydig cells in the rat testicular interstitium. J Endocrinol. 1993;138:107–114. doi: 10.1677/joe.0.1380107. [DOI] [PubMed] [Google Scholar]
- 30.Grizard G. [IGF (s) and testicular functions. Secretion and action of IGF-1 on Leydig cells] Contracept Fertil Sex. 1994;22:551–555. [PubMed] [Google Scholar]
- 31.Lin T, Wang D, Nagpal ML, Shimasaki S, Ling N. Expression and regulation of insulin-like growth factor-binding protein-1, -2, -3, and -4 messenger ribonucleic acids in purified rat Leydig cells and their biological effects. Endocrinology. 1993;132:1898–1904. doi: 10.1210/endo.132.5.7682935. [DOI] [PubMed] [Google Scholar]
- 32.Rappaport MS, Smith EP. Insulin-like growth factor (IGF) binding protein 3 in the rat testis: follicle-stimulating hormone dependence of mRNA expression and inhibition of IGF-I action on cultured Sertoli cells. Biol Reprod. 1995;52:419–425. doi: 10.1095/biolreprod52.2.419. [DOI] [PubMed] [Google Scholar]
- 33.The Guiding Principles for Research Involving Animals. National Academy of Sciences and published by the National Institute of Health -NIH-, revised in 1991 [Google Scholar]
- 34.Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- 35.Santamaría L, Martín R, Codesal J, Paniagua R. Myoid cell proliferation in rat seminiferous tubules after ischaemic testicular atrophy induced by epinephrine. Morphometric and immunohistochemical (bromo-deoxyuridine and PCNA) studies. Int J Androl. 1995;18:13–22. doi: 10.1111/j.1365-2605.1995.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 36.Skinner MK, Cosand WL, Griswold MD. Purification and characterization of testicular transferrin secreted by rat Sertoli cells. Biochem J. 1984;218:313–320. doi: 10.1042/bj2180313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhary J, Skinner MK. Comparative sequence analysis of the mouse and human transferrin promoters: hormonal regulation of the transferrin promoter in Sertoli cells. Mol Reprod Dev. 1998;50:273–283. doi: 10.1002/(SICI)1098-2795(199807)50:3<273::AID-MRD3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 38.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- 39.Zaitoun AM, Apelqvist G, Wikell C, Al-Mardini H, Bengtsson F, Record CO. Quantitative studies of testicular atrophy following portacaval shunt in rats. Hepatology. 1998;28:1461–1466. doi: 10.1002/hep.510280603. [DOI] [PubMed] [Google Scholar]
- 40.Smanik EJ, Mullen KD, Giroski WG, McCullough AJ. The influence of portacaval anastomosis on gonadal and anterior pituitary hormones in a rat model standardized for gender, food intake, and time after surgery. Steroids. 1991;56:237–241. doi: 10.1016/0039-128x(91)90040-3. [DOI] [PubMed] [Google Scholar]
- 41.Mezey E. Liver disease and nutrition. Gastroenterology. 1978;74:770–783. [PubMed] [Google Scholar]
- 42.Ghabriel MN, Lu JJ, Hermanis G, Zhu C, Setchell BP. Expression of a blood-brain barrier-specific antigen in the reproductive tract of the male rat. Reproduction. 2002;123:389–397. doi: 10.1530/rep.0.1230389. [DOI] [PubMed] [Google Scholar]
- 43.Itoh N, Nanbu A, Tachiki H, Akagashi K, Nitta T, Mikuma N, Tsukamoto T, Kumamoto Y. Restoration of testicular transferrin, insulin-like growth factor-1 (IGF-1), and spermatogenesis by exogenously administered purified FSH and testosterone in medically hypophysectomized rats. Arch Androl. 1994;33:169–177. doi: 10.3109/01485019408987821. [DOI] [PubMed] [Google Scholar]
- 44.Rappaport MS, Smith EP. Insulin-like growth factor I inhibits aromatization induced by follice-stimulating hormone in rat sertoli cell culture. Biol Reprod. 1996;54:446–452. doi: 10.1095/biolreprod54.2.446. [DOI] [PubMed] [Google Scholar]
- 45.Prati M, Palmero S, De Marco P, Trucchi P, Fugassa E. [Effect of insulin-like growth factor I (IGF-I) on Sertoli cell metabolism in the pubescent rat] Boll Soc Ital Biol Sper. 1992;68:121–128. [PubMed] [Google Scholar]
- 46.Waites GM, Gladwell RT. Physiological significance of fluid secretion in the testis and blood-testis barrier. Physiol Rev. 1982;62:624–671. doi: 10.1152/physrev.1982.62.2.624. [DOI] [PubMed] [Google Scholar]
- 47.Farghali H, Williams DS, Gavaler J, Van Thiel DH. Effect of short-term ethanol feeding on rat testes as assessed by 31P NMR spectroscopy, 1H NMR imaging, and biochemical methods. Alcohol Clin Exp Res. 1991;15:1018–1023. doi: 10.1111/j.1530-0277.1991.tb05204.x. [DOI] [PubMed] [Google Scholar]
- 48.Salonen I, Eriksson CJ. Penetration of ethanol into the male reproductive tract. Alcohol Clin Exp Res. 1989;13:746–751. doi: 10.1111/j.1530-0277.1989.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 49.Adam CL, Gadd TS, Findlay PA, Wathes DC. IGF-I stimulation of luteinizing hormone secretion, IGF-binding proteins (IGFBPs) and expression of mRNAs for IGFs, IGF receptors and IGFBPs in the ovine pituitary gland. J Endocrinol. 2000;166:247–254. doi: 10.1677/joe.0.1660247. [DOI] [PubMed] [Google Scholar]
- 50.Carneiro G, Lorenzo P, Pimentel C, Pegoraro L, Bertolini M, Ball B, Anderson G, Liu I. Influence of insulin-like growth factor-I and its interaction with gonadotropins, estradiol, and fetal calf serum on in vitro maturation and parthenogenic development in equine oocytes. Biol Reprod. 2001;65:899–905. doi: 10.1095/biolreprod65.3.899. [DOI] [PubMed] [Google Scholar]
- 51.Xia YX, Weiss JM, Polack S, Diedrich K, Ortmann O. Interactions of insulin-like growth factor-I, insulin and estradiol with GnRH-stimulated luteinizing hormone release from female rat gonadotrophs. Eur J Endocrinol. 2001;144:73–79. doi: 10.1530/eje.0.1440073. [DOI] [PubMed] [Google Scholar]


