Abstract
AIM: To sum up the clinical characteristics of patients with central pontine myelinolysis (CPM) after orthotopic liver transplantation (OLT) and to document the possible causes of CPM.
METHODS: Data of 142 patients undergoing OLT between January 1999 to May 2003 were analyzed retrospectively. Following risk factors during perioperation were analyzed in patients with and without CPM: primary liver disease, preoperative serum sodium level, magnesium level and plasma osmolality, fluctuation degree of serum sodium concentration, and immunosuppressive drug level, etc.
RESULTS: A total of 13 (9.2%) neurologic symptoms appeared in 142 patients post-operation including 5 cases (3.5%) with CPM and 8 cases (5.6%) with cerebral hemorrhage or infarct. Two patients developing CPM after OLT had a hyponatremia history before operation (serum sodium < 130 mmol/L), their mean serum sodium level was 130.6 ± 5.54 mmol/L. The serum sodium level was significantly lower in CPM patients than in patients without neurologic complications or with cerebral hemorrhage/infarct (P < 0.05).The increase in serum sodiumduring perioperative 48 h after OLT in patients with CPM was significantly greater than that in patients with cerebral hemorrhage/infarct but without neurologic complications (19.5 ± 6.54 mmol/L, 10.1 ± 6.43 mmol/L, 4.5 ± 4.34 mmol/L, respectively, P < 0.05). Plasma osmolality was greatly increased postoperation in patients with CPM. Hypomagnesemia was noted in all patients perioperation, but there were no significant differences between groups. The duration of operation on patients with CPM was longer than that on others (492 ± 190.05 min, P < 0.05). Cyclosporin A (CsA) levels were normal in all patients, but there were significant differences between patients with or without neurologic complications (P < 0.05).
CONCLUSION: CPM may be more prevalent following liver transplantation. Although the diagnosis of CPM after OLT can be made by overall neurologic evaluations including magnetic resonance imaging (MRI) of the head, the mortality is still very high. The occurance of CPM may be associated with such factors as hyponatremia, rapid rise of serum sodium concentration, plasma osmolality increase postoperation, the duration of operation, and high CsA levels.
INTRODUCTION
The morbidity and mortality of central nervous system (CNS) complications after orthotopic liver transplantation (OLT) was 19% and 47% respectively[1]. Central pontine myelinolysis (CPM)[2] is the most serious CNS complication that could be seen after OLT, and represents an important source of mortality early after OLT[3]. CPM following liver transplantation was reported more and more in foreign literatures[4,5], but it was rarely reported in our country[6,7].
In this paper, we studied retrospectively 142 patients undergoing OLT in our center. Fifteen patients had CNS complications after OLT including 5 patients with CPM. The clinical features and possible causes of CPM after liver transplantation were analyzed.
MATERIALS AND METHODS
Clinical data
Between January 1999 to May 2003, consecutive OLT was performed on 142 patients at our center. Medical records and clinical data of the patients were retrospectively investigated. Of them 117 were males and 25 females, age from 19 to 65 years, their age was 45 ± 8.9 years. Indications for OLT included severe hepatitis 28 cases, hepatic cirrhosis (32 cases), liver carcinoma (53 cases), polycystic liver (3 cases), and others (24 cases). Operative procedures were performed with the standard technique and UW preservation solution. All patients had similar perioperative intensive care and received cyclosporin A (CsA) and methylprednisolone-based induction immunosuppression.
Observational indicators
Patients who developed posttransplant abnormal neurological symptoms underwent overall neurological evaluation. Magnetic resonance imaging (MRI) of head was performed in selected cases. CPM was diagnosed based on: (1) patients who had a variety of signs including mental status changes, quadriparesis, pseudobulbar palsy, and drowsiness, etc; (2) MRI showed a hypointense signal in T1-weighted images and a hyperintense signal in T2-weighted images in the central pontine. The signal of T2 was slightly increased after contrast administration.
Following risk factors during perioperation were analyzed between patients with and without CPM: age, gender, primary liver disease, pretransplant serum sodium , magnesium levels, plasma osmolality, fluctuation degree of serum sodium and plasma osmolality 48 h after transplantation, duration of operation, and CsA level. Hyponatremia was defined as serum sodium < 130 mmol/L. Hypomagnesium was defined as serum magnesium < 1.5 mg/L.
Statistical analysis
Data were expressed as mean ± SD. Comparisons of group means were made with ANOVA for unpaired data, and Chi-Square test for enumeration data. P < 0.05 and P < 0.01 were considered statistically significant.
RESULTS
Clinical features of patients with CNS complications
Fifty-eight of 142 patients undergoing OLT developed abnormal neurological symptoms such as mania, tremor, confusion, drowsiness, and diminished state of responsiveness. Thirteen patients were diagnosed having CNS complications based on clinical features and MRI. The demographic characteristics of the patients are outlined in Table 1. There were 10 males and 3 females aged 40-55 years. Primary diseases included severe hepatitis in 6 cases; hepatic cirrhosis in 4 cases; liver carcinoma, drug liver failure, IgA nephropathy, and polycystic liver in 1 case, respectively. Patients developed neurological complications in the early postoperative period including cerebral hemorrhage/infarct in 8 cases, CPM in 5 cases. The incidence of cerebral hemorrhage/infarct and CPM was 5.6% (8/142) and 3.5% (5/142), respectively. The extent of CPM on MRI was variable, showing a hypointensity signal of T1-weighted images in the pontine without space occupying sign (Figure 1A), and increased signal intensity of T2-weighted images in central pontine (Figure 2A, 2B). None of the patients showed extrapontine myelinolysis. CNS complications occurred two weeks after liver transplantation, ranged from 2 to 18 d. The median time of survival of patients with CPM after OLT was 24 ± 16.1 d, ranged 7 to 48 d. The patients who were complicated with cerebral hemorrhage or infarct survived 2 to 96 d after transplantation, the mean time was 33 ± 30.7 d.
Table 1.
Clinical characteristics of patients with CNS complications
| Patient NO. | Hospital NO. | Age (yr) | Gender | Primary disease | CNS complication | Timing of onset (d) | Timing of survival (d) |
| 1 | 287206 | 40 | Male | Severe hepatitis | Cerebral hemorrhage | 6 | 11 |
| 2 | 286967 | 48 | Male | Severe hepatitis | CPM | 10 | 15 |
| 3 | 291172 | 41 | Female | Severe hepatitis | Cerebral hemorrhage | 8 | 26 |
| 4 | 299921 | 54 | Male | Severe hepatitis | CPM | 6 | 23 |
| 5 | 306331 | 53 | Female | polycystic liver | Cerebral infarct | 4 | 28 |
| 6 | 312075 | 51 | Male | Hepatic cirrhosis | Cerebral hemorrhage | 2 | 9 |
| 7 | 314112 | 53 | Male | Severe hepatitis | CPM | 18 | 48 |
| 8 | 325113 | 49 | Male | Liver carcinoma | Cerebral hemorrhage | 2 | 2 |
| 9 | 315349 | 41 | Male | Hepatic cirrhosis | CPM | 3 | 20 |
| 10 | 328762 | 46 | Male | Drug liver failure IgA nephropathy | CPM | 5 | 7 |
| 11 | 351480 | 55 | Female | Hepatic cirrhosis | Cerebral hemorrhage | 14 | 56 |
| 12 | 362960 | 52 | Male | Hepatic cirrhosis | Cerebral hemorrhage | 3 | 36 |
| 13 | 363980 | 46 | Male | Severe hepatitis | Cerebral hemorrhage | 14 | 96 |
Figure 1.
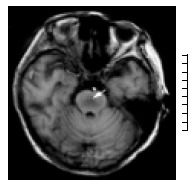
T1-Weighted axial magnetic resonance imaging scan shows symmetric area of hypointensity in the pons. (arrow).
Figure 2.
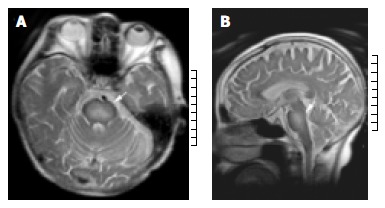
A: T2-Weighted axial magnetic resonance imaging shows bilaterally symmetric high-signal intensity is seen in the central pons; (arrow) B: T2-Weighted sagittal magnetic resonance imaging demonstrates hyperintensity area in central pons. (arrow).
Comparison of clinical characteristics in patients with and without CPM (Table 2)
Table 2.
Comparison of Clinical characteristics in patients with and without CPM
| Index |
CNS Complications |
NO CNS Complication | |
| CPM | Cerebral hemorrhage/cerebral infarct | ||
| Preoperative serum sodium (mmol/L) | 130.6 ± 5.54bc | 135.9 ± 2.61 | 137.4 ± 3.83 |
| Preoperative serum magnesium (mg/L) | 0.8 ± 0.19 | 0.9 ± 0.20 | 1.0 ± 0.21 |
| Preoperative sosm (mOsm/kg H2O) | 274.3 ± 33.09 | 290.2 ± 22.12 | 292.5 ± 26.05 |
| Change in serum sodium After 48 h (mmol/L) | 19.5 ± 6.54bd | 10.1 ± 6.43b | 4.5 ± 4.34 |
| Postoperative sosm (mOsm/kg H2O) | 341.6 ± 14.99bc | 317.9 ± 29.76 | 308.8 ± 16.89 |
| Surgery time (min) | 492.0 ± 190.05a | 450.0 ± 93.50 | 399.9 ± 76.07 |
| CsA level (ng/dL) | 301.3 ± 9.23b | 273.8 ± 28.55a | 247.2 ± 35.44 |
Abbreviations: Sosm: Serum Osmolarity
P < 0.05 (0.016, 0.037),
P < 0.01 (0.000, 0.001) compared with no CNS complications;
P < 0.05 (0.017, 0.02),
P < 0.01 (0.000) compared with cerebral hemorrhage/infarct.
Pretransplant hyponatremia was present in all symptomatic patients with CPM. Two patients developing CPM after OLT had a severe hyponatremia history before operation (serum sodium level < 130 mmol/L). The average serum sodium level was 130.6 ± 5.54 mmol/L. The serum sodium level was significantly lower in patients with CPM than in patients without neurologic complications or with cerebral hemorrhage/infarct (P < 0.05). The average increase of perioperative serum sodium 48 h after OLT in patients with CPM, cerebral hemorrhage/infarct, and without CNS complications was 19.5 ± 6.54 mmol/L, 10.1 ± 6.43 mmol/L, 4.5 ± 4.34 mmol/L, respectively (P < 0.01). Plasma osmolarity was greatly increased 48 h postoperation in patients with CPM. Despite this, the plasma osmolality was normal in patients without CPM, but no significant difference was noted between patient who had complication of cerebral hemorrhage/infarct and patients who had no CNS complication. Hypomagnesemia was noted in all patients perioperation, but there were no significant differences between groups. The operation time for patients with CPM was longer than that for others (P < 0.05). But no significant difference was found in operation time between patients who had complication of cerebral hemorrhage/infarct and those who had no CNS complication. Following transplantation, all patients received CsA-based induction immunosuppression. Though the mean CsA level in all patients was normal, the average CsA level was greatly higher in patients with CNS complications than in patients without CNS complications. Age, gender, primary liver disease were similar among three groups.
DISCUSSION
CPM was first described in 1959 by Adams. CPM after OLT was first reported by Starzl[8] in 1978. CPM[9,10] was characterized by symmetrical loss of myelin in the pontine basalis, with relatively well-preserved axons and neuronal cell bodies. Acute CPM often occurred and had a variety of signs including quadriparesis, pseudobulbar palsy, and locked-in syndrome. As a severe neurological complication, the progress of CPM was usually dismal, with a high mortality rate[11]. Most cases of CPM were diagnosed post-morten. With the recognition of CPM and the development in MRI technology, more cases of CPM could be diagnosed while patients were alive. Liver transplant recipients constituted a high risk group for developing CPM. The incidence of CPM after OLT varied from 5%-10%[12-14], which was higher than that in other patients (0.16%-5.8%)[15]. The exact etiology of CPM is uncertain, the rapid correction of hyponatremia might be an important factor. However, controversy has been going on[16-20].
Pathogenesis of CPM after OLT
End-stage hepatic insufficiency was a common feature of pretransplanted patients. Advanced liver failure was always associated with some degree of renal insufficiency. So almost all hyponatremic states associated with liver disease were chronic and difficult to be corrected[21]. Input for patients after OLT was considerably increased as a consequence of bleeding during operation. The sodium content for fresh frozen plasma was around 165 mmol/L, 150 mmol/L for albumin solution, and 140 mmol/L for red blood cells, significantly higher than the serum sodium of patients with hyponatremia. Correction of blood loss would thus inevitably lead to a rapid rise in serum sodium concentration. A multicenter study showed[22] that rapid correction of hyponatremia exceeding 18 mmol/L in the first 48 h was significantly associated with CPM. The present study demonstrated that not all patients with hyponatremia developed CPM. But increase of serum sodium 48 h after OLT in patients with CPM was 19.5 ± 6.54 mmol/L, which was significantly higher than that in patients without CPM. Plasma osmolality was also greatly increased 48 h postoperation. The results of our study suggested that rapid correction of hyponatremia and abrupt change of plasma osmolality might account for the development of CPM. Liver failure might lead to disruption of astrocyte metabolism with resulting abnormalities of blood-brain barrier function and a decreased ability to generate new intracellular osmoles in response to osmotic changes. Thus, patients with liver transplantation were particularly susceptible to CPM[23,24]. Lien et al[25] suggested that rapid correction of hyponatremia might produce acute dehydration of edematous brain, leading to high ion to organic osmolyte ratio, osmotic endothelial injury, and endothelial cell shrinkage with loosening of tight junctions. Subsequently, transvascular transport increased and myelinotoxic factors released. Because of an extensive gray-white matter admixture in the pontine, this anatomic arrangement could provide a suitable environment wherein myelinotoxic factors, chiefly derived from the richly vascular gray matter, could interact with surrounding bundles of myelin-containing white matter, then lead to demyelination viz development of CPM.
Dunn et al[26] demonstrated the neurotoxicity of CsA in transplanted patients. Three cases developing CsA associated akinetic mutism after liver transplantation were reported by Bird et al[27], two of three were identified by MRI. The CsA level in all patients was monitored and controlled in normal range. Although the mean in-hospital CsA level in CPM group was not different with that in cerebral hemorrhage/infarct group, the CsA leves in patients with CNS complications was higher than that in patients without CNS complications. In this study, pretransplant hypomagnesemia was noted in all patients after OLT. Previous study suggested[28] that CsA neurotoxicity could lead to massive white matter lesions. Hypomagnesemia might contribute to CsA neurotoxicity and was associated with development of CPM after OLT, although the mechanism of CPM is unclear.
The results of our study also suggested that operation time in patients with CPM was significantly longer than that in patients without CNS complications. But there were no differences in operation time between patients with cerebral hemorrhage/infarct and without CNS complications, indicating that CNS lesions in liver transplant recipients may be related with intraoperative bleeding, prolonged low blood perfusion.
Prevention and therapy of CPM after OLT
To our knowledge, at present there is no definitive therapy for CPM. Therefore, prevention of this condition has become crucial[29]. Slower correction of perioperative hyponatremia may be critical for patients undergoing OLT. The rate of correction should not exceed 15 mmol/L/24 h or 18 mmol/L/48 h. Major fluctuations in serum sodium during surgery should be avoided. Plasma osmolality level should be remained within normal reference, and aggressive magnesium replacement should be initiated for hypomagnesemia. To decrease the duration of operation and intraoperative bleeding, no veno-venous bypass should be recommended. Immunosuppressive agent concentrations should be carefully monitored and controlled to avoid neurotoxicity[30]. MRI of head should be performed when patients occurred neurologic and psychiatric symptoms after OLT[31,32]. The best way of preventing CPM was to perform transplantation at an early stage of the disease. More recently, subclinical CPM after OLT was reported[33], the patients were nearly asymptomatic, but MRI showed marked lesions in the pontine. According to these we suggest that the diagnosis of CPM should be considered in patients undergoing OLT with major electrolyte fluctuations, and high immunosuppressive agent levels. MRI is currently the best modality available to identify CPM. Support treatment is very important for patients with CPM. It was reported that cortical hormonel, vitamin, and serum replacement were used in CPM[34]. Whether these therapies play a role in CPM remains to be determined.
Prognosis of CPM after OLT
A previous study showed[35] that the occurrence of CPM after OLT varied from 2 to 11 d, average 7 d. The prognosis of CPM is usually dismal, with a high mortality rate. The results of the present study demonstrated that CPM occurred 3 to 18 d OLT. The median time of survival after OLT was 24.6 ± 16.13 d, ranged 7 to 48 d, the mortality was 100%. These liver transplant recipients with CPM were associated with the following aspects. (1) Patients with CPM often had other complications[36], such as infection, hemorrhage of digestive tract, and multi-organ failure. (2) Inadequate water intake and electrolyte derangements were not corrected due to CPM. (3) Venovenous bypass was used in part of transplantations[37,38], which led to the increase in operation time, interoperative bleeding, time of low perfusion. The limited number of patients in this study may account for the high mortality of CPM after OLT.
Footnotes
Edited by Wang XL and Xu FM
References
- 1.Bonham CA, Dominguez EA, Fukui MB, Paterson DL, Pankey GA, Wagener MM, Fung JJ, Singh N. Central nervous system lesions in liver transplant recipients: prospective assessment of indications for biopsy and implications for management. Transplantation. 1998;66:1596–1604. doi: 10.1097/00007890-199812270-00005. [DOI] [PubMed] [Google Scholar]
- 2.Kajs-Wyllie M. Central pontine myelinolysis: a multidisciplinary approach to care. J Neurosci Nurs. 1994;26:36–41. doi: 10.1097/01376517-199402000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Lampl C, Yazdi K. Central pontine myelinolysis. Eur Neurol. 2002;47:3–10. doi: 10.1159/000047939. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Yu VL, Gayowski T. Central nervous system lesions in adult liver transplant recipients: clinical review with implications for management. Medicine (Baltimore) 1994;73:110–118. doi: 10.1097/00005792-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Fryer JP, Fortier MV, Metrakos P, Verran DJ, Asfar SK, Pelz DM, Wall WJ, Grant DR, Ghent CN. Central pontine myelinolysis and cyclosporine neurotoxicity following liver transplantation. Transplantation. 1996;61:658–661. doi: 10.1097/00007890-199602270-00026. [DOI] [PubMed] [Google Scholar]
- 6.Xia SS. Current status of liver transplantation in China. Shijie Huaren Xiaohua Zazhi. 1999;7:645–646. [Google Scholar]
- 7.Qian YB, Cheng GH, Huang JF. Multivariate regression analysis on early mortality after orthotopic liver transplantation. World J Gastroenterol. 2002;8:128–130. doi: 10.3748/wjg.v8.i1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Schneck SA, Mazzoni G, Aldrete JA, Porter KA, Schröter GP, Koep LJ, Putnam CW. Acute neurological complications after liver transplantation with particular reference to intraoperative cerebral air embolus. Ann Surg. 1978;187:236–240. doi: 10.1097/00000658-197803000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreiro JA, Robert MA, Townsend J, Vinters HV. Neuropathologic findings after liver transplantation. Acta Neuropathol. 1992;84:1–14. doi: 10.1007/BF00427209. [DOI] [PubMed] [Google Scholar]
- 10.Wijdicks EF, Blue PR, Steers JL, Wiesner RH. Central pontine myelinolysis with stupor alone after orthotopic liver transplantation. Liver Transpl Surg. 1996;2:14–16. doi: 10.1002/lt.500020104. [DOI] [PubMed] [Google Scholar]
- 11.Menger H, Jörg J. Outcome of central pontine and extrapontine myelinolysis (n = 44) J Neurol. 1999;246:700–705. doi: 10.1007/s004150050435. [DOI] [PubMed] [Google Scholar]
- 12.Boon AP, Carey MP, Adams DH, Buckels J, McMaster P. Central pontine myelinolysis in liver transplantation. J Clin Pathol. 1991;44:909–914. doi: 10.1136/jcp.44.11.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estol CJ, Faris AA, Martinez AJ, Ahdab-Barmada M. Central pontine myelinolysis after liver transplantation. Neurology. 1989;39:493–498. doi: 10.1212/wnl.39.4.493. [DOI] [PubMed] [Google Scholar]
- 14.Bramhall SR, Minford E, Gunson B, Buckels JA. Liver transplantation in the UK. World J Gastroenterol. 2001;7:602–611. doi: 10.3748/wjg.v7.i5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinschmidt-DeMasters BK, Norenberg MD. Rapid correction of hyponatremia causes demyelination: relation to central pontine myelinolysis. Science. 1981;211:1068–1070. doi: 10.1126/science.7466381. [DOI] [PubMed] [Google Scholar]
- 16.Tien R, Arieff AI, Kucharczyk W, Wasik A, Kucharczyk J. Hyponatremic encephalopathy: is central pontine myelinolysis a component. Am J Med. 1992;92:513–522. doi: 10.1016/0002-9343(92)90748-z. [DOI] [PubMed] [Google Scholar]
- 17.Mast H, Gordon PH, Mohr JP, Tatemichi TK. Central pontine myelinolysis: clinical syndrome with normal serum sodium. Eur J Med Res. 1995;1:168–170. [PubMed] [Google Scholar]
- 18.Oh MS, Choi KC, Uribarri J, Sher J, Rao C, Carroll HJ. Prevention of myelinolysis in rats by dexamethasone or colchicine. Am J Nephrol. 1990;10:158–161. doi: 10.1159/000168071. [DOI] [PubMed] [Google Scholar]
- 19.Wszolek ZK, McComb RD, Pfeiffer RF, Steg RE, Wood RP, Shaw BW, Markin RS. Pontine and extrapontine myelinolysis following liver transplantation. Relationship to serum sodium. Transplantation. 1989;48:1006–1012. doi: 10.1097/00007890-198912000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Soni BM, Vaidyanthan S, Watt JW, Krishnan KR. A retrospective study of hyponatremia in tetraplegic/paraplegic patients with a review of the literature. Paraplegia. 1994;32:597–607. doi: 10.1038/sc.1994.95. [DOI] [PubMed] [Google Scholar]
- 21.Palmer BF, Gates JR, Lader M. Causes and management of hyponatremia. Ann Pharmacother. 2003;37:1694–1702. doi: 10.1345/aph.1D105. [DOI] [PubMed] [Google Scholar]
- 22.Sterns RH, Cappuccio JD, Silver SM, Cohen EP. Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective. J Am Soc Nephrol. 1994;4:1522–1530. doi: 10.1681/ASN.V481522. [DOI] [PubMed] [Google Scholar]
- 23.Mueller AR, Platz KP, Bechstein WO, Schattenfroh N, Stoltenburg-Didinger G, Blumhardt G, Christe W, Neuhaus P. Neurotoxicity after orthotopic liver transplantation. A comparison between cyclosporine and FK506. Transplantation. 1994;58:155–170. [PubMed] [Google Scholar]
- 24.Kanwal F, Chen D, Ting L, Gornbein J, Saab S, Durazo F, Yersiz H, Farmer D, Ghobrial RM, Busuttil RW, et al. A model to predict the development of mental status changes of unclear cause after liver transplantation. Liver Transpl. 2003;9:1312–1319. doi: 10.1016/j.lts.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Lien YH. Role of organic osmolytes in myelinolysis. A topographic study in rats after rapid correction of hyponatremia. J Clin Invest. 1995;95:1579–1586. doi: 10.1172/JCI117831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn CJ, Wagstaff AJ, Perry CM, Plosker GL, Goa KL. Cyclosporin: an updated review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (neoral) 1 in organ transplantation. Drugs. 2001;61:1957–2016. doi: 10.2165/00003495-200161130-00006. [DOI] [PubMed] [Google Scholar]
- 27.Bird GL, Meadows J, Goka J, Polson R, Williams R. Cyclosporin-associated akinetic mutism and extrapyramidal syndrome after liver transplantation. J Neurol Neurosurg Psychiatry. 1990;53:1068–1071. doi: 10.1136/jnnp.53.12.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbasoglu O, Goldstein RM, Vodapally MS, Jennings LW, Levy MF, Husberg BS, Klintmalm GB. Liver transplantation in hyponatremic patients with emphasis on central pontine myelinolysis. Clin Transplant. 1998;12:263–269. [PubMed] [Google Scholar]
- 29.Harris CP, Townsend JJ, Baringer JR. Symptomatic hyponatraemia: can myelinolysis be prevented by treatment. J Neurol Neurosurg Psychiatry. 1993;56:626–632. doi: 10.1136/jnnp.56.6.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez J, Benito-León J, Molina JA, Ramos A, Bermejo F. [Central pontine myelinolysis associated with cyclosporin in liver transplantation] Neurologia. 1998;13:437–440. [PubMed] [Google Scholar]
- 31.Bernsen HJ, Prick MJ. Improvement of central pontine myelinolysis as demonstrated by repeated magnetic resonance imaging in a patient without evidence of hyponatremia. Acta Neurol Belg. 1999;99:189–193. [PubMed] [Google Scholar]
- 32.Bekiesińska-Figatowska M, Bulski T, Rózyczka I, Furmanek M, Walecki J. MR imaging of seven presumed cases of central pontine and extrapontine myelinolysis. Acta Neurobiol Exp (Wars) 2001;61:141–144. doi: 10.55782/ane-2001-1395. [DOI] [PubMed] [Google Scholar]
- 33.Kato T, Hattori H, Nagato M, Kiuchi T, Uemoto S, Nakahata T, Tanaka K. Subclinical central pontine myelinolysis following liver transplantation. Brain Dev. 2002;24:179–182. doi: 10.1016/s0387-7604(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 34.Soupart A, Ngassa M, Decaux G. Therapeutic relowering of the serum sodium in a patient after excessive correction of hyponatremia. Clin Nephrol. 1999;51:383–386. [PubMed] [Google Scholar]
- 35.Bronster DJ, Emre S, Boccagni P, Sheiner PA, Schwartz ME, Miller CM. Central nervous system complications in liver transplant recipients--incidence, timing, and long-term follow-up. Clin Transplant. 2000;14:1–7. doi: 10.1034/j.1399-0012.2000.140101.x. [DOI] [PubMed] [Google Scholar]
- 36.Zheng SS, Liang TB, Xu X, Wang WL, Shen Y, Zhang M, Huang DS. Ten year's experience on liver transplantation in a single organ transplantation center. Zhonghua Putong Waike Zazhi. 2003;18:71–73. [Google Scholar]
- 37.Kokudo N, Sugawara Y, Imamura H, Sano K, Makuuchi M. Sling suspension of the liver in donor operation: a gradual tape-repositioning technique. Transplantation. 2003;76:803–807. doi: 10.1097/01.TP.0000080982.03297.A7. [DOI] [PubMed] [Google Scholar]
- 38.Yan LN, Wang W, Li B, Lu SC, Wen TF, Zeng Y, Cheng NS, Zhao JC, Lin QY, Chen XL, et al. Venovenous bypass ahead of mobilization of the liver in orthotopic liver transplantation. Hepatobiliary Pancreat Dis Int. 2003;2:44–47. [PubMed] [Google Scholar]


