Abstract
AIM: To determine the effects of total parenteral nutrition and somatostatin on patients with chylous ascites.
METHODS: Five patients were diagnosed with chylous ascites on the basis of laboratory findings of ascites sample from Nov 1999 to May 2003. Total parenteral nutrition and somatostatin or its analogue was administered to 4 patients, while the other one only received total parenteral nutrition. All the patients had persistent peritoneal drainage, with the quantity and quality of drainage fluid observed daily. Necessary supportive treatments were given to the patients individually during the therapy.
RESULTS: Two of 4 patients who received somatostatin therapy obtained complete recovery within 10 d without any recurrence while on a normal diet. In these 2 patients, the peritoneal drainage reduced to zero in one and the other's decreased from 2000 mL to 80 mL with a clear appearance and negative qualitative analysis of chyle. Recurrent chylous ascites, though relieved effectively by the same method every time, developed in one patient with advanced pancreatic cancer. The other patient's lymphatic fistula was blocked with the fibrin glue after conservative treatment. The patient who only received total parenteral nutrition was cured 24 d after therapy.
CONCLUSION: Total parenteral nutrition along with somatostatin can relieve the symptoms and close the fistula in patients with chylous ascites rapidly. It appears to be an effective therapy available for the treatment of chylous ascites caused by various disorders.
INTRODUCTION
Chylous ascites, an uncommon disease usually caused by obstruction or rupture of the peritoneal or retroperitoneal lymphatic glands, is defined as the accumulation of chyle in the peritoneal cavity[1]. It is a difficult disorder due to the serious mechanical, nutritional and immunological consequences of the constant loss of protein and lymphocytes[2]. Morton's dramatic and detailed account in 1694 of a 2-year-old boy with tuberculosis who died of chylous ascites was the first clear report of chyloperitoneum.
Many pathological conditions can result in this disease, including congenital defects of the lymphatic system, nonspecific bacterial, parasitic and tuberculous peritoneal infection, liver cirrhosis, malignant neoplasm, blunt abdominal trauma and surgical injury[1]. Over all, the most common cause in adults is believed to be abdominal malignancy, while congenital lymphatic abnormalities in pediatric population. Press et alreported an incidence of 1 per 20464 admissions at the Massachusetts General Hospital during a 20-year period[3]. They found, however, a 1 per 11589 incidence in the last years of their study. Most investigators believe that the incidence of chylous ascites is increasing because of more aggressive thoracic and retroperitoneal surgery and with the prolonged survival of patients with cancer[4-8]. Some new techniques, such as laparoscopic surgery and transplantation, also lead to postoperative chylous ascites[9-11]. Kaas et al[12] found that 12 (7.4%) of 163 patients with complex surgical procedures developed chylous ascites.
Though the incidence of chylous ascites has increased in recent years, the treatment remains unsatisfactory in some cases because of prolonged duration of disease. Conservative treatment of chylous ascites, recommended in most patients, involves paracentesis, a medium chain triglyceride (MCT) based diet, total parenteral nutrition (TPN), recently used somatostatin and so on. Surgery is only recommended when conservative treatment fails[2]. In a review of 156 patients with chylous ascites resolved after intervention, 51 patients were successfully treated surgically; 105 patients were treated conservatively[13]. Usually the MCT based diet is the first choice, TPN is recommended after dietary manipulation has failed, and somatostatin therapy is attempted only if chylous ascites has been refractory to all conservative measures. It will take several weeks to 2 mo to close the lymphatic fistula adequately with routine conservative regimens[13].
Here we report on our successful use of persistent peritoneal drainage, TPN as well as somatostatin in treatment of 5 cases of chylous ascites.
MATERIALS AND METHODS
Five adult patients with chylous ascites were admitted to our hospital from November 1999 to May 2003. On admission, computerized tomography (CT) examination was performed to reveal the etiology, and the diagnosis was confirmed by analyzing the cloudy ascites fluid obtained through paracentesis or peritoneal drainage. Table 1 shows the clinical features, CT findings and laboratory findings of ascites samples from 5 patients.
Table 1.
Clinical features, CT and laboratory findings for 5 patients with chylous ascites
| Patient | Age in yr/Sex | Medical/Surgical condition | CT findings |
Laboratory findings of ascites |
|||
| Qualitative analysis of chyle | Leukocyte count (/mm3)/Lymphocyte (%) | Total protein/Albumin (g/L) | Cholesterol/Triglyceride (mmol/L) | ||||
| 1 | 48/M | Two wk after radical distal subtotal gastrectomy for gastric cancer | Large volume of ascites | + | 1600/95 | 49.4/30.9 | 1.92/7.14 |
| 2 | 50/M | Five mo after pancreaticoduodenectomy for pancreatic cancer | Large volume of ascites, multiple metastases involving liver and lymph nodes | + | 120/95 | 15.0/10.1 | 1.59/2.41 |
| 3 | 65/F | Half a year after cure of the tuberculous peritoneal infection | Large volume of ascites, liver cirrhosis | + | 560/50 | 23.2/14.2 | 2.11/5.68 |
| 4 | 50/F | One wk after finding of 400 mL milky fluid in pelvic cavity during laparoscopy cholecystectomy | Small volume of ascites in pelvic cavity | + | 190/88 | 52.8/32.0 | 0.67/3.01 |
| 5 | 44/F | 10 d after radical pelvic lymphadenectomy for ovarian cancer | Small volume of fluid in pelvic cavity | + | 890/92 | 47.7/31.9 | 1.47/7.11 |
As soon as the diagnosis was confirmed, every patient was put in fasting state and received fluid replenishment until disturbance of water, electrolytes and acid-base was corrected. A single lumen central venous catheter was inserted into the peritoneal cavity for continuous drainage in 3 patients, while peritoneal cavity drainage tubes inserted during the operation were reserved in patient 4 and 5. The quality and quantity of drainage fluid were monitored daily. Then TPN (non-protein calorie 25 kcal/(kg·d), nitrogen 0.2-0.25 g/(kg·d), glucose: fat ratio 6:4) via central vein was administered to patients at gradually increasing dose. Somatostatin (Stilamin, Laboratoires Serono S. A.) was administered to patient 2 and 4 through continuous intravenous infusion at a dose of 3 mg per 12 h. Patient 3 and 5 received subcutaneous administration of the somatostatin analogue, octreotide (Sandostatin, Novartis Pharma AG), at a dose from 100 μg to 200 μg 3 times daily. Necessary supportive treatments, such as albumin, diuretics and antibiotics, were given to the patients individually during the therapy. In addition, patient 5 received abdominal cavity and peripheral venous chemotherapy at the same time.
RESULTS
Figure 1 shows the change of drainage volume, the duration of TPN and somatostatin therapy of 5 patients. Once the peritoneal drainage was zero (in patient 1 and 4) or was proved non-chylous ascites (in patient 2 and 3), TPN and somatostatin dose would diminish gradually along with the recovery of oral low fat diet. Patient 3 and 4, who received somatostatin therapy, obtained complete recovery within 10 d, while patient 1 who only received TPN was cured 24 d after therapy. The drainage of patient 3 decreased from 2000 mL to 80 mL within 10 d with a clear appearance and negative qualitative analysis of chyle, and the volume remained unchanged when she received normal diet. Then patient 3 was referred to department of gastroenterology for further treatment of the established liver cirrhosis, which caused the remaining ascites. All these 3 patients' drainage catheters were removed after they had normal diet for 3 d. The drainage volume of patient 5, though dropped from 600 mL to 200 mL within 10 d, remained unchanged for 11 d with positive qualitative analysis of chyle. Then we successfully used fibrin glue to block up the lymphatic fistula that was proved mature with X-ray fistulography. CT follow-up examinations did not reveal the presence of ascitic fluid. The nutritional status of them was well maintained during therapy. Follow-up study found no recurrence in these 4 patients while on normal diet after 6 mo. Though the drainage decreased from 2000 mL to 100 mL within 11 d and was proved non-chylous ascites, repeated recurrences developed in patient 2 who died of advanced pancreatic cancer 3 mo later.
Figure 1.
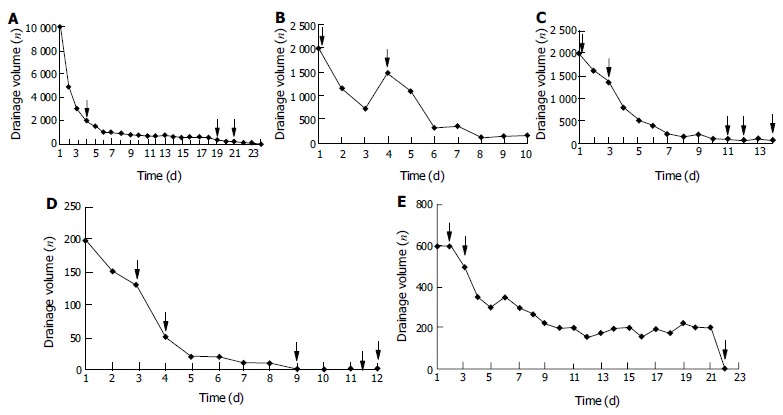
Change of drainage volume and the duration of TPN and somatostatin therapy in 5 patients. ↓The beginning and the end of TPN, ↓the beginning and the end of somatostatin therapy, ↓the beginning of food intake, ↓blockage of the fistula. A: Patient 1 who only received TPN recovered fully after 24 d of therapy; B: Patient 2 suffered from repeated recurrence though the drainage volume decreased from 2000 mL to 100 mL within 11 d; C: Chylous ascites never recurred in patient 3 after the drainage volume decreased to 80 mL within 10 d with negative qualitative analysis of chyle; D: Patient 4 recovered fully within 9 d; E: Chylous fistula of patient 5 showed refractory to therapy and was sealed with fibrin glue.
DISCUSSION
Our results showed that TPN along with somatostatin appears to be an effective therapy available for the treatment of chylous ascites caused by various disorders.
There are multiple causes of chylous ascites. The most common ones in Western countries are abdominal malignancy and cirrhosis, which account for over two thirds of all cases. In contrast, infectious etiologies, such as tuberculosis and filariasis, account for the majority of cases of chylous ascites in Eastern and developing countries[14]. Previous studies showed the effect of some regimen in the treatment of chylous ascites caused by one kind of pathological condition. In the present series, various etiological factors including surgical injury (patient 1 and 5), pancreatic cancer (patient 2), liver cirrhosis (patient 3) and idiopathic cause (patient 4) demonstrate the wide indications of our treatment. It also indicates the change of etiology spectrum in China, operation and cancer have become the common causes now.
Treatment of the underlying cause of chylous ascites is of pivotal importance in managing patients with chylous ascites, especially those having an infectious, inflammatory, or hemodynamic cause. But conservative treatment is usually vital for most patients to relieve the symptoms and restore nutritional deficits. Paracentesis is not only a diagnostic but also therapeutic method in the management of chylous ascites. Despite several definite drawbacks and complications, repeated paracentesis is commonly included in the nonoperative treatment regimens to relieve abdominal distention[13]. In three patients of this series, repeated paracentesis was replaced by persistent peritoneal drainage with a single lumen central venous catheter, which permitted us to monitor the quality and quantity of drainage daily. In patient 4 and 5, peritoneal drainage tube inserted during the operation was reserved until recovery. No tube blockage, catheter-related sepsis or any other complications developed in all patients. So persistent peritoneal drainage may be a much better and accepted choice than repeated paracentesis.
Fasting, together with TPN, can decrease the lymph flow in thoracic duct dramatically from 220 mL/(kg·h) to 1 mL/(kg·h)[13]. Furthermore, TPN restores nutritional deficits and balances metabolic impairments imposed by long-standing chylous ascites and repeat sessions of paracentesis. So fasting and TPN are essential in nonoperative management of chylous ascites. In the past, however, TPN was usually recommended as the second line treatment when enteral dietary manipulation failed[13]. Routine conservative treatment, using TPN only or combined with an MCT diet, needed 2 to 6 wk to cure 60% to 100% of cases[14,15]. In our study, patient 1 who only received fasting and TPN recovered completely after 24 d.
Initial experience with continuous intravenous high dose somatostatin for the closure of postoperative lymphorrhagia was reported in 1990 by Ulibarri et al[16]. The exact mechanisms of somatostatin on drying lymphatic fistulas are not completely understood. It has been previously shown to decrease the intestinal absorption of fats, lower triglyceride concentration in the thoracic duct and attenuate lymph flow in the major lymphatic channels. In addition, it also decreases gastric, pancreatic and intestinal secretions, inhibits motor activity of the intestine, slows the process of intestinal absorption and decreases splanchnic blood flow, which may further contribute to decreased lymph production. It has also been speculated that somatostatin improves chylous ascites by inhibition of lymph fluid excretion through specific receptors found in the normal lymphatic vessels of intestinal wall[17,18]. Shapiro et al[17] reported rapid resolution of chylous ascites after liver transplantation within 2 d after administration of the octreotide combined with total parenteral nutrition. Satisfactory results were also achieved by others[16,19-23]. To our knowledge, however, most of these articles included just one case. Somatostatin therapy also remains an indefinite or second-line method in treatment of chylous ascites. The recommended algorithm for the management of chylous ascites in a review in 2000, only regarded somatostatin in combination with TPN as an unproved alternative method[13]. Another algorithm in 2002 recommended somatostatin therapy only after a period of combined dietary intervention and TPN had failed[14]. Our results showed that 2 of 4 patients treated with somatostatin or its analogue recovered completely within 10 d with well maintained nutritional status and no significant side effect, while the symptoms of patient 2 relieved despite of relapse. As to patient 5, the conservative treatment decreased the fistula drainage greatly and paved the way for the subsequent management. In this case we are inclined to surmise that chemotherapy may affect the closure of lymphatic fistula. These results suggest that somatostatin along with TPN can close the lymphatic leakage or relieve the symptom effectively and rapidly, in comparison with conventional regimens.
The outcome of chylous ascites mostly depends on the underlying pathological condition causing lymphatic leakage. The mortality of chylous ascites, especially those caused by surgery, has decreased much than before[15], but that caused by malignancy remains high. In our study, TPN and somatostatin could not control the chylous ascites completely in patient 2, so necessary adjustment of this regimen or more aggressive therapy should be applied to stop the lymphatic leakage in these patients. Peritoneovenous shunt has been proved to be a valuable method especially for those cancer patients who are refractory to conservative treatment[24-26].
We conclude that TPN with somatostatin should be the first-line therapy for chylous ascites caused by various disorders, and started as soon as possible. Some other methods should also be attempted to close the fistula that is refractory to conservative treatment. Further studies of multicenter clinical trials involving more patients to compare the efficacy and cost between this regimen and the others are suggested.
Footnotes
Edited by Zhu LH Proofread by Chen WW and Xu FM
References
- 1.Browse NL, Wilson NM, Russo F, al-Hassan H, Allen DR. Aetiology and treatment of chylous ascites. Br J Surg. 1992;79:1145–1150. doi: 10.1002/bjs.1800791110. [DOI] [PubMed] [Google Scholar]
- 2.Leibovitch I. Postoperative chylous ascites--the urologist's view. Drugs Today (Barc) 2002;38:687–697. doi: 10.1358/dot.2002.38.10.820141. [DOI] [PubMed] [Google Scholar]
- 3.Press OW, Press NO, Kaufman SD. Evaluation and management of chylous ascites. Ann Intern Med. 1982;96:358–364. doi: 10.7326/0003-4819-96-3-358. [DOI] [PubMed] [Google Scholar]
- 4.Halkic N, Abdelmoumene A, Suardet L, Mosimann F. Postoperative chylous ascites after radical gastrectomy. A case report. Minerva Chir. 2003;58:389–391. [PubMed] [Google Scholar]
- 5.Warner RR, Croen EC, Zaveri K, Ratner L. A carcinoid tumor associated with chylous ascites and elevated tumor markers. Int J Colorectal Dis. 2002;17:156–160. doi: 10.1007/s00384-001-0367-2. [DOI] [PubMed] [Google Scholar]
- 6.Amin R. Chylous ascites from prostatic adenocarcinoma. Urology. 2002;59:773. doi: 10.1016/s0090-4295(02)01536-4. [DOI] [PubMed] [Google Scholar]
- 7.Tokiwa K, Fumino S, Ono S, Iwai N. Results of retroperitoneal lymphadenectomy in the treatment of abdominal neuroblastoma. Arch Surg. 2003;138:711–715. doi: 10.1001/archsurg.138.7.711. [DOI] [PubMed] [Google Scholar]
- 8.Sexton WJ, Wood CG, Kim R, Pisters LL. Repeat retroperitoneal lymph node dissection for metastatic testis cancer. J Urol. 2003;169:1353–1356. doi: 10.1097/01.ju.0000052372.06901.de. [DOI] [PubMed] [Google Scholar]
- 9.Senyüz OF, Sentürk H, Taşçi H, Kaya G, Ozbay G, Sariyar M. Chylous ascites after liver transplantation with mesentero-portal jump graft. J Hepatobiliary Pancreat Surg. 2001;8:571–572. doi: 10.1007/s005340100027. [DOI] [PubMed] [Google Scholar]
- 10.Shafizadeh SF, Daily PP, Baliga P, Rogers J, Baillie GM, Rajagopolan PR, Chavin KD. Chylous ascites secondary to laparoscopic donor nephrectomy. Urology. 2002;60:345. doi: 10.1016/s0090-4295(02)01743-0. [DOI] [PubMed] [Google Scholar]
- 11.Bacelar TS, de Albuquerque AC, de Arruda PC, Ferraz AA, Ferraz EM. Postoperative chylous ascites: a rare complication of laparoscopic Nissen fundoplication. JSLS. 2003;7:269–271. [PMC free article] [PubMed] [Google Scholar]
- 12.Kaas R, Rustman LD, Zoetmulder FA. Chylous ascites after oncological abdominal surgery: incidence and treatment. Eur J Surg Oncol. 2001;27:187–189. doi: 10.1053/ejso.2000.1088. [DOI] [PubMed] [Google Scholar]
- 13.Aalami OO, Allen DB, Organ CH. Chylous ascites: a collective review. Surgery. 2000;128:761–778. doi: 10.1067/msy.2000.109502. [DOI] [PubMed] [Google Scholar]
- 14.Leibovitch I, Mor Y, Golomb J, Ramon J. The diagnosis and management of postoperative chylous ascites. J Urol. 2002;167:449–457. doi: 10.1016/S0022-5347(01)69064-5. [DOI] [PubMed] [Google Scholar]
- 15.Lee YY, Soong WJ, Lee YS, Hwang B. Total parenteral nutrition as a primary therapeutic modality for congenital chylous ascites: report of one case. Acta Paediatr Taiwan. 2002;43:214–216. [PubMed] [Google Scholar]
- 16.Collard JM, Laterre PF, Boemer F, Reynaert M, Ponlot R. Conservative treatment of postsurgical lymphatic leaks with somatostatin-14. Chest. 2000;117:902–905. doi: 10.1378/chest.117.3.902. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro AM, Bain VG, Sigalet DL, Kneteman NM. Rapid resolution of chylous ascites after liver transplantation using somatostatin analog and total parenteral nutrition. Transplantation. 1996;61:1410–1411. doi: 10.1097/00007890-199605150-00023. [DOI] [PubMed] [Google Scholar]
- 18.Reubi JC, Horisberger U, Waser B, Gebbers JO, Laissue J. Preferential location of somatostatin receptors in germinal centers of human gut lymphoid tissue. Gastroenterology. 1992;103:1207–1214. doi: 10.1016/0016-5085(92)91505-x. [DOI] [PubMed] [Google Scholar]
- 19.Rimensberger PC, Müller-Schenker B, Kalangos A, Beghetti M. Treatment of a persistent postoperative chylothorax with somatostatin. Ann Thorac Surg. 1998;66:253–254. doi: 10.1016/s0003-4975(98)00361-0. [DOI] [PubMed] [Google Scholar]
- 20.Laterre PF, Dugernier T, Reynaert MS. Chylous ascites: diagnosis, causes and treatment. Acta Gastroenterol Belg. 2000;63:260–263. [PubMed] [Google Scholar]
- 21.Al-Sebeih K, Sadeghi N, Al-Dhahri S. Bilateral chylothorax following neck dissection: a new method of treatment. Ann Otol Rhinol Laryngol. 2001;110:381–384. doi: 10.1177/000348940111000416. [DOI] [PubMed] [Google Scholar]
- 22.Leibovitch I, Mor Y, Golomb J, Ramon J. Chylous ascites after radical nephrectomy and inferior vena cava thrombectomy. Successful conservative management with somatostatin analogue. Eur Urol. 2002;41:220–222. doi: 10.1016/s0302-2838(01)00034-3. [DOI] [PubMed] [Google Scholar]
- 23.Leong RW, House AK, Jeffrey GP. Chylous ascites caused by portal vein thrombosis treated with octreotide. J Gastroenterol Hepatol. 2003;18:1211–1213. doi: 10.1046/j.1440-1746.2003.02850.x. [DOI] [PubMed] [Google Scholar]
- 24.Wagayama H, Tanaka T, Shimomura M, Ogura K, Shiraki K. Pancreatic cancer with chylous ascites demonstrated by lymphoscintigraphy: successful treatment with peritoneovenous shunting. Dig Dis Sci. 2002;47:1836–1838. doi: 10.1023/a:1016461015632. [DOI] [PubMed] [Google Scholar]
- 25.Manolitsas TP, Abdessalam S, Fowler JM. Chylous ascites following treatment for gynecologic malignancies. Gynecol Oncol. 2002;86:370–374. doi: 10.1006/gyno.2002.6754. [DOI] [PubMed] [Google Scholar]
- 26.Camoglio FS, Dipaola G, Cervellione RM, Chironi C, Giacomello L, Zanatta C, Ottolenghi A. Treatment of neonatal chylous ascites using a modified Denver peritoneovenous shunt: a case report. Pediatr Med Chir. 2003;25:145–147. [PubMed] [Google Scholar]


