Abstract
AIM: To evaluate the safety of Curcuma aromatica oil gelatin microspheres (CAO-GMS) infused via hepatic artery against primary liver cancer.
METHODS: The safety of CAO-GMS was evaluated in view of its acute toxicity in rats, long-term toxicity in Beagle dogs and general pharmacology in rats and mongrel dogs.
RESULTS: The 50% lethal dose (LD50) of CAO-GMS infused via the hepatic artery was 17.19 mg/kg, and the serum biochemical indices of dying rats after the administration changed markedly while those of survived rats did not. Subsequent pathological examination of the tissues from the dead rats indicated improper embolism. Similar edema and small necrotic foci in the hepatic lobule were found in the hepatic tissue of rats receiving 10 and 5 mg/kg CAO-GMS and GMS 60 d after the last administration, while not in the rats of the blank control group, indicating that microspheres infused via the hepatic artery may induce irreversible liver damage dose-dependently. General pharmacological study showed that the activities (posture and gait), respiration frequency, blood pressure or heart rate of the dogs were not affected by CAO-GMS, nor were salivation, tremor or pupil changes of the rats observed or their balancing ability compromised, suggesting CAO-GMS infused via the hepatic artery did not significantly affect the nervous, respiratory and cardiovascular systems.
CONCLUSION: CAO-GMS embolization administered via the hepatic artery is safe but undesired embolization induced by vascular variation should be given due attention in its clinical application. Individualized embolization dosage and super-selective catheterization technique are recommended to avoid undesired embolism and reduce complications.
INTRODUCTION
Curcuma aromatica oil (CAO) is the oil mixture extracted from Curcuma aromatica consisting of multiple effective ingredients, such as β-elemene and curcumol[1-7]. Researchers have demonstrated that CAO, with relatively low toxic effects, is the main anti-neoplasm ingredient of Curcuma aromatica[8-12], which can be used for therapy against neoplasmic diseases, especially liver neoplasm[13-19]. Clinically, CAO has been applied in interventional therapy administered via the hepatic artery, and produced favorable effects in patients with primary liver cancer[20-24] and experimentally, in rats with transplanted liver tumor as well[25]. As an oil preparation, CAO is often difficult to achieve total embolization in interventional therapy with far too short retention time in the neoplastic lesions. CAO microsphere is suggested to be ideal for increasing the retention time, enhancing the inhibitory effects on cancer cells, and also for blocking the trophic vessels for the hepatoma, so to produce dual effects of chemotherapy and embolization. Therefore, we employed the microsphere degradation technique to entrap the CAO into the gelatin medium and prepared CAO-GMS[26], which has shown potent therapeutic efficacy against transplanted liver cancer in rats[27-29] and from which the released CAO exhibited marked dose-dependent inhibition of the growth of human hepatoma cell line SMMC-7721[30].
CAO-GMS is a preparation of traditional Chinese herbal drugs possible for quality control[26]. Considering its special pharmaceutical form and administration route, we adopted the interventional procedures described by Lindell et al[31] similar to clinical situations, so as to study CAO-GMS in view of its acute toxicity, long-term toxicity and general pharmacology in rats and dogs. The results may help evaluate the safety of the interventional therapy with CAO-GMS embolization administered via the hepatic artery in the treatment of primary liver cancer and provide experimental evidence for its potential clinical application.
MATERIALS AND METHODS
Drugs and reagents
CAO-GMS (50-100 µm in diameter, Batch No.: 971006), 8% CAO and blank gelatin microspheres (50-100 µm in diameter, Batch No.: 97050) were provided by the Department of Pharmaceutics, Shenyang Pharmaceutical University. Sodium carboxylmethyl cellulose (CMC-Na) was purchased from Guangzhou Chemical Reagents and Glassware Co. (Batch No.: 950808). The microspheres were suspended in 3 g/L CMC-Na solution for use.
Animals
A total of 104 SD rats (clean animals, including 52 males and 52 females weighing 220-300 g, Certification No.: 152) used in this study were purchased from Shanghai Sino-British SIPPR/BK Lab Animal Co. Ltd. Twenty-one mongrel dogs (conventional animals, 11 males and 10 females, weighing 13.5-21.0 kg, Certification No.: 19980610) were purchased from Guangzhou Shima Experimental Animal Company and quaranteed by the Experimental Animal Center of Guangzhou University of Traditional Chinese Medicine. Eighteen Beagle dogs (specific pathogen-free animals, 9 males and 9 females, weighing 12.5-13.1 kg, Certification No.: 97A025) were purchased from the Experimental Animal Center of Guangzhou Medicine Industry Institute.
Six-channel physiological recorder (Plugsys, Hugo Sachs Elektonik, Germany), blood pressure sensor (Isotec, Hugo Sachs Elektonik, Germany), thermosensitive recorder (WR500, Isotec, Hugo Sachs Elektonik, Germany), digital subtraction angiography-interventional therapy apparatus (DSA, Siemens, Germany), automated blood analyzer (Counter-5, Japan), automated blood coagulation analyzer (ACT-3000 plus, Germany), clinical biochemical analyzer (CL-7200, Japan) and electrocardiograph (ECG-6511, Japan) were used in this study.
Acute toxicity in rats
Seventy-four SD rats were randomly divided into 7 groups including CAO-GMS-1 group (n = 10) receiving CAO-GMS 5 mg/kg, CAO-GMS-2 group (n = 12) receiving CAO-GMS 10 mg/kg, CAO-GMS-3 group (n = 14) receiving CAO-GMS 20 mg/kg, CAO-GMS-4 group (n = 12) receiving CAO-GMS 40 mg/kg, GMS control group (n = 10) receiving gelatin microspheres 40 mg/kg, blank control group (n = 8) receiving 3.5 mL/kg CMC-Na (3 g/L) in a volume equivalent to that of injected microspheres and ZT group (n = 8) receiving 3.2 mg/kg CAO. All rats were anesthetized with 45 mg/kg intraperitoneal pentobarbital injection prior to drug administration following the method described by Lidell et al[31] (Figure 1). In brief, a catheter was inserted through the gastroduodenal artery to the proper hepatic artery, the distal end of the gastroduodenal artery was ligated, and the common hepatic artery as well as the right branch of the proper hepatic artery was temporarily blocked to receive the agents through the left branch of the proper hepatic artery. The general activities, mortality and survival time of the rats were consecutively observed and recorded for 14 d after drug administration. Blood samples of the dying rats were collected and determined for ALT, AST, total bilirubin (T-BIL), direct bilirubin (D-BIL), blood creatinine (BCr) and blood urea nitrogen (BUN). Blood samples were also taken from the survived rats on d 14 for determination of the above indices. Autopsy was performed on the dead rats and the vital organs such as the liver, spleen, lung and kidney etc. were pathologically examined.
Figure 1.
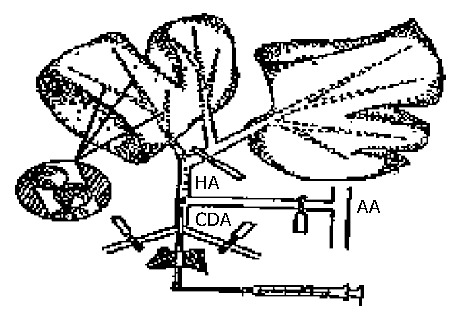
Administration method (from Lindell[31]).
Long-term toxicity in Beagle dogs
Eighteen Beagle dogs were randomly divided into 4 groups including CAO-GMS high-dose group (CAO-GMS-H, 5 dogs) receiving 15 mg/kg CAO-GMS every 4 wk, CAO-GMS low-dose group (CAO-GMS-L, 5 dogs) receiving 7.5 mg/kg CAO-GMS every 4 wk, GMS control group (4 dogs) receiving 15 mg/kg gelatin microspheres every 4 wk, blank control group (4 dogs) receiving 10 mL/kg saline in the same volume as that of the microspheres every 4 wk. Each dog was anaesthetized with 30 mg/kg pentobarbital, and a catheter was super-selectively inserted into the hepatic artery through the femoral artery puncture under perspective monitor. The hepatic artery was examined by intra-arterial digital subtraction angiography with the injection of 8 mL meglumine diatrizoate (760 g/L) at the rate of 2 mL/s, 150 Psi by a high-pressure syringe. The microspheres of specified doses were mixed with the contrast media and slowly injected. To prevent improper embolism due to reflux of the liquid, the injection rate was strictly controlled. The catheter was washed with saline after the injection, and angiography of the hepatic artery was performed for confirmation of the range and degree of embolization. The catheter was withdrawn after the surgery, and the puncture pressed for 15 min and bandaged with pressure. The procedures were repeated twice every 4 wk. Half of the dogs in each group were sacrificed on d 30 after the last treatment and the other half on d 60. The tissue samples were fixed in 40 g/L neutral formaldehyde, processed by the standard histological techniques, and stained with hematoxylin and eosin (HE) for light microscopic examination. The liver, kidney, lung, adrenal, pancreas, stomach, duodenum, ileum, colon, prostate, brain, spinal cord, heart, spleen, sternum (bone and marrow), bladder, uterus, ovary, thyroid, thymus and testis of the dogs were all examined pathologically to assess the long-term toxicity, reversible toxicity and delayed toxicity of CAO-GMS.
General effect on nervous system of rats
Thirty rats were randomly divided into 3 groups including high-dose CAO-GMS group (CAO-GMS-H, 12 rats) receiving 10 mg/kg CAO-GMS, low-dose CAO-GMS group (CAO-GMS-L, 10 rats) receiving 5 mg/kg CAO-GMS, and blank control group (8 rats) receiving 3.5 mL/kg CMC-Na (3 g/L, in equivalent volume). The administration of the agents followed the procedures described above. The general activities (posture and gait), salivation, tremor or pupil changes of rats were recorded. Balance test of rotarod model was performed on d 3, 5, 7 and 14, respectively, in which the rats were put on a 90-cm-long rod 2.5 cm in diameter to observe the dropping frequency. The rats dropping more than 3 times were defined to have incoordination with nervous system impairment.
General effect on respiratory and cardiovascular systems of dogs
Twenty-one mongrel dogs were randomly divided into 4 groups, namely high-dose CAO-GMS group (CAO-GMS-H, 6 dogs receiving 0.10 g CAO-GMS), low-dose CAO-GMS group (CAO-GMS-L, 6 dogs receiving 0.03 CAO-GMS), CAO group (4 dogs receiving 6.4 μL/kg CAO) and blank control group (5 dogs) receiving 10 mL/kg CMC-Na (3 g/L, in equivalent volume). Under anesthesia with pentobarbital, the blood pressure of the dogs was measured with catheterization through the common carotid artery (connected by Isotec blood pressure sensor). Two-lead electrocardiogram and respiratory frequency were also recorded. Thirty minutes later, the dogs were given specified agents with the method described above. The respiratory frequency, blood pressure and electrocardiogram of the dogs before and 30, 60, 120, 180 min after administration were measured using six-channel physiology recorder.
Statistical analysis
Data were expressed as mean ± SD. The 50% lethal dose (LD50) was calculated with Bliss method. The biochemical indices were assessed by multiple ANOVA. Changes in blood pressure, respiratory frequency and heart rate before and after administration of the agents within the same group were assessed by paired t test using SPSS 10.0 statistical software. The difference was considered significant when P value was less than 0.05.
RESULTS
Acute toxicity in rats
All rats in CAO-GMS-4 group fell in a state of drowsiness with hair erection and died within 24 h after administration of the agent. Death of the rats in CAO-GMS-3 group occurred 24-48 h after the administration, with the survived rats exhibiting signs of fatigue, hair erection, and emaciation accompanied by reduced activity and anorexia; 2 rats developed ascites and survived until d 14. The rats in CAO-GMS-2 group also showed fatigue within the first 2 d after administration of the agent but resumed normal condition on d 3. The rats in CAO-GMS-1, blank control and CAO group did not show signs of discomfort. The rats of GMS group exhibited changes similar to those of CAO-GMS-4 group. LD50 of CAO-GMS embolization via the hepatic artery was 17.19 mg/kg, with the 95% confidential interval of 13.08-23.67 mg/kg (Table 1).
Table 1.
Deaths of the rats after administration of CAO-GMS
| Group | Number of rats | Dosage (mg/kg) | Number of dead rats | Mortality (%) | Peak time of death occurrence (h) |
| CAO-GMS-1 | 10 | 5 | 0 | 0 | - |
| CAO-GMS-2 | 12 | 10 | 4 | 33.33 | 24-48 |
| CAO-GMS-3 | 14 | 20 | 8 | 57.14 | 24-48 |
| CAO-GMS-4 | 12 | 40 | 12 | 100 | < 24 |
| GMS | 10 | 40 | 7 | 71.42 | 24-48 |
| Blank control | 8 | 3.51 | 0 | 0 | - |
| CAO | 8 | 3.2 | 0 | 0 | - |
Measured in mL/kg.
Compared with those of the blank control group, all the dying rats of the CAO-GMS groups showed marked increase in ALT, AST, T-BL, D-BL, BCr and BUN (Table 2), but none of the survived rats showed marked changes in these indices (Table 3).
Table 2.
Changes in biochemical indices of the dying rats receiving CAO-GMS (mean ± SD)
| Group | Nurmber of rats | ALT (U/L) | AST (U/L) | T-BiL (mmol/L) | D-BiL (μmol/L) | BCr (μmol/L) | BUN (mmol/L) |
| CAO-GMS-1 | 0 | - | - | - | - | - | - |
| CAO-GMS-2 | 4 | 200 ± 54b | 805 ± 533b | 12.0 ± 5b | 46.0 ± 90b | 50.0 ± 34b | 20.0 ± 12a |
| CAO-GMS-3 | 8 | 186 ± 89b | 700 ± 522b | 12.4 ± 8.6b | 59.0 ± 88b | 49.0 ± 43b | 20.0 ± 14a |
| CAO-GMS-4 | 12 | 241 ± 289b | 715 ± 614b | 15.0 ± 19b | 78.0 ± 162b | 58.0 ± 43b | 22.0 ± 13a |
| GMS | 7 | 232 ± 168b | 685 ± 503b | 13.8 ± 9.6b | 54.0 ± 126b | 52.0 ± 36b | 20.0 ± 13a |
| Blank control | 0 | 38 ± 11 | 278 ± 114 | 4.5 ± 1.3 | 3.2 ± 1.6 | 38.7 ± 3.5 | 8.6 ± 11.8 |
| CAO | 0 | - | - | - | - | - | - |
P < 0.05;
P < 0.01 vs blank control.
Table 3.
Changes in biochemical indices of survived rats receiving CAO-GMS (mean ± SD)
| Group | Nurmber of rats | ALT (U/L) | AST (U/L) | T-BiL (mmol/L) | D-BiL (μmmol/L) | BCr (μmmol/L) | BUN (mmol/L) |
| CAO-GMS-1 | 10 | 37.7 ± 5.5 | 310 ± 96 | 5.4 ± 1.4 | 3.8 ± 1.4 | 40.3 ± 5.2 | 8.42 ± 0.69 |
| CAO-GMS-2 | 8 | 39.2 ± 7.7 | 301 ± 74 | 5.2 ± 1.7 | 3.6 ± 1.8 | 41.8 ± 9.3 | 10.00 ± 2.2 |
| CAO-GMS-3 | 6 | 39.0 ± 10 | 314 ± 59 | 7.4 ± 4.7 | 4.5 ± 2.9 | 39.0 ± 13 | 9.20 ± 2.9 |
| CAO-GMS-4 | 0 | - | - | - | - | - | - |
| GMS | 3 | 46.0 ± 6.5 | 265 ± 114 | 4.0 ± 1.5 | 2.9 ± 1.6 | 35.0 ± 1.4 | 7.60 ± 1.4 |
| Blank control | 8 | 38.0 ± 11 | 278 ± 114 | 4.5 ± 1.3 | 3.2 ± 1.6 | 38.7 ± 3.5 | 8.60 ± 1.8 |
| CAO | 8 | 36.0 ± 7 | 307 ± 84 | 4.8 ± 1.9 | 3.2 ± 1.7 | 39.4 ± 2.4 | 8.00 ± 1.6 |
The tissue samples of the rats in CAO-GMS and GMS groups showed damage of hepatic tissues, as were mostly manifested in CAO-GMS-3, 4 groups and GSM group. The rats in CAO-GMS-3 and -4 groups were found to have hemorrhagic damage in lung tissues and necrosis of renal tubules, indicating dose-dependent damage of the kidney and lung by CAO-GMS. We identified traces of the microspheres in a tissue sample of CAO-GMS-2 group, suggesting that the toxic effects on kidney tissues in CAO-GMS-1, -2, -3, -4 groups arose from improper embolization of the renal artery. The tissue samples from the 4 groups showed dose-independent damage of the spleen tissues. Abnormal changes were not observed in vital organs in blank control and CAO groups.
We found that CAO-GMS induced untoward responses and death of the rats dose-dependently. Since the rats in blank control and CAO groups showed no untoward response as those in GMS group did, we presumed that GMS, instead of CAO, caused renal and hepatic injuries. The most probable cause of the renal damage might be undesired embolization of the renal artery by GMS.
Long-term toxicity in Beagle dogs
The dogs of CAO-GMS-H, CAO-GMS-L and GMS groups showed necrotic foci in the hepatic tissue with color attenuation 1-2 mm in diameter. The blank control group showed no marked change in the color and texture of the hepatic tissue.
Figure 2 displays the pathological changes in hepatic tissues of the dogs. Two dogs in CAO-GMS-H group exhibited whole lobule coagulation necrosis in the hepatic tissue with total destruction of the normal structures. The remaining hepatic tissue exhibited extensive moderate cellular edema with occasional cellular inflammatory edema in the necrotic region. In a dog died during the experiment, whole lobule coagulation necrosis was observed in the hepatic tissue, which was also seen in two dogs sacrificed on d 30 with mild cellular edema in the remaining hepatic tissue and destruction of normal structures.
Figure 2.
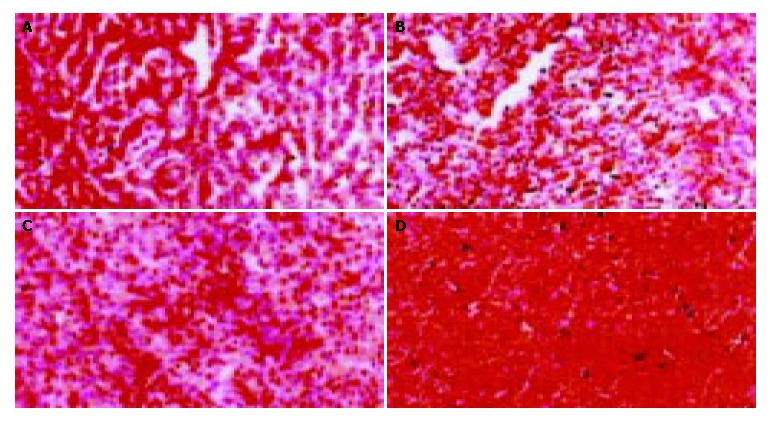
Long-term toxicity of CAO-GMS on the liver of dogs. A: CAO-GMS-H group receiving 15 mg/kg CAO-GMS every 4 wk; B: CAO-GMS-L group receiving 7.5 mg/kg CAO-GMS every 4 wk; C: GMS group receiving 15 mg/kg GMS every 4 wk; D: Blank control group receiving 10 mL/kg saline every 4 wk.
Two dogs of CAO-GMS-L group developed extensive moderate to severe hepatic cellular necrosis. The central region of the liver lobules showed moderate cellular edema with fatty degeneration and foci of coagulation necrosis, where neutrophilic leukocyte infiltration could be seen. Two dogs sacrificed on d 30 had extensive moderate cellular necrosis in the hepatic tissue and small necrotic foci, with ruptured normal structure.
In GMS group, two dogs were found to have whole lobular coagulation necrosis in the hepatic tissue where normal structure was disrupted. The other regions of the liver lobules showed moderate cellular edema with lymphocyte infiltration in the necrotic region. Two dogs sacrificed on d 30 exhibited extensive mild cellular necrosis in the hepatic tissue and spotty necrosis of the hepatic cells.
In the blank control group, mild cellular edema in the hepatic tissue was found in 2 dogs, with disrupted normal structure. Two dogs sacrificed on d 30 exhibited mild cellular necrosis in the hepatic tissue.
Pathological examination of the other vital organs showed no abnormal changes in the morphology of the kidney, lung, adrenal, pancreas, stomach, duodenum, ileum, colon, prostate, brain, spinal cord, heart, spleen, sternum (bone and marrow), bladder, uterus, ovary, thyroid, thymus and testis.
The above results demonstrated that CAO-GMS infused via the hepatic artery may cause irreversible ischemic necrosis of the liver without visible damage on other organs. Delayed toxic response was not evident. The toxic effects on liver were identical with those observed in study of the acute toxicity. Partly due to the super-selective catheterization technique employed in this study, no improper embolization was found, indicating the importance of the technique in the clinical application for reducing complications.
Effects on nervous system of rats
The rats receiving 5 or 10 mg/kg CAO-GMS or 3 g/L CMC-Na showed emaciation, reduced activity and anorexia in the first 2 postoperative days, but resumed normal conditions on d 3, probably due to operative injury. No pupil abnormality or tremor was observed. All rats dropped less than 3 times in the balance test of rotarod model, indicating that CAO-GMS did not affect the balancing ability of the rats.
Effects on respiratory and cardiovascular systems of dogs
The effects of CAO-GMS on respiratory frequency, blood pressure and heart rate are shown in Table 4. CAO infused via the hepatic artery produced no marked effects on blood pressure and heart rate of the dogs (P > 0.05). The respiratory frequency of the dogs within 3 h after administration of the agent was lowered, but not statistically (P > 0.05 vs indices before embolization).
Table 4.
Changes in respiration, rates blood pressure and ECG in dogs at different time points after CAO (6.4 μL/kg) interventional therapy (mean ± SD)
| Time point | Nurmber of rats | Respiration frequency (breath/min) | Heart rate (beat/min) |
Blood pressure (mmHg) |
|
| Systolic | Diastolic | ||||
| Preoperation | 4 | 17.0 ± 10.2 | 198.5 ± 38.4 | 147.8 ± 8.2 | 116.0 ± 11.5 |
| 30 min postoperative | 4 | 15.0 ± 7.0 | 193.5 ± 36.1 | 141.5 ± 8.2 | 114.8 ± 9.7 |
| 60 min postoperative | 4 | 16.0 ± 8.5 | 189.3 ± 27.1 | 140.5 ± 8.9 | 115.8 ± 9.3 |
| 120 min postoperative | 4 | 17.8 ± 9.5 | 190.5 ± 23.6 | 141.5 ± 3.3 | 117.0 ± 5.0 |
| 180 min postoperative | 4 | 13.8 ± 6.0 | 189.0 ± 28.4 | 143.5 ± 5.8 | 119.0 ± 6.3 |
CAO-GMS or blank GMS produced no marked effects on blood pressure and heart rate of the dogs (P > 0.05, Table 5, Table 6).
Table 5.
Changes in respiration, blood pressure and ECG in dogs at different time points after CAO-GMS interventional therapy (mean ± SD)
| Time point | Dosage | Number of rats | Respiration frequency (/min) | Heart rate (/min) |
Blood pressure (mmHg) |
|
| Systolic | Diastolic | |||||
| Preoperation | 0.10 | 6 | 19.0 ± 5.2 | 219.0 ± 22.7 | 142.5 ± 9.0 | 112.7 ± 9.1 |
| 0.03 | 6 | 13.5 ± 4.9 | 196.0 ± 28.5 | 144.7 ± 12.2 | 116.2 ± 9.8 | |
| 30 min postoperative | 0.10 | 6 | 20.5 ± 6.7 | 214.7 ± 19.1 | 142.7 ± 7.3 | 113.0 ± 8.6 |
| 0.03 | 6 | 14.0 ± 4.9 | 198.3 ± 17.7 | 146.3 ± 11.3 | 118.8 ± 12.6 | |
| 60 min postoperative | 0.10 | 6 | 19.7 ± 7.7 | 211.5 ± 18.7 | 142.3 ± 6.4 | 113.5 ± 8.1 |
| 0.03 | 6 | 12.3 ± 3.4 | 194.0 ± 16.6 | 144.0 ± 10.7 | 119.2 ± 13.6 | |
| 120 min postoperative | 0.10 | 6 | 19.5 ± 7.8 | 211.7 ± 25.0 | 142.3 ± 10.0 | 114.0 ± 10.7 |
| 0.03 | 6 | 15.3 ± 5.5 | 193.7 ± 20.2 | 145.0 ± 9.1 | 118.8 ± 9.2 | |
| 180 min postoperative | 0.10 | 6 | 20.3 ± 8.9 | 209.3 ± 30.2 | 141.5 ± 12.3 | 114.5 ± 13.1 |
| 0.03 | 6 | 16.8 ± 6.8 | 193.7 ± 21.6 | 143.8 ± 9.5 | 119.2 ± 9.5 | |
Table 6.
Changes in respiration, blood pressure and ECG in dogs at different time points after GMS (0.1 g) interventional therapy (mean ± SD)
| Time point | Number of rats | Respiration frequency (/min) | Heart rate (/min) |
Blood pressure (mmHg) |
|
| Systolic | Diastolic | ||||
| Preoperation | 5 | 20.4 ± 6.2 | 186.6 ± 28.3 | 133.4 ± 16.0 | 109.4 ± 16.9 |
| 30 min postoperative | 5 | 18.0 ± 5.8 | 178.2 ± 28.6 | 139.8 ± 20.0 | 116.0 ± 14.9 |
| 60 min postoperative | 5 | 18.2 ± 5.8 | 179.0 ± 29.2 | 143.8 ± 20.7 | 117.2 ± 17.4 |
| 120 min postoperative | 5 | 17.4 ± 3.8 | 183.8 ± 34.1 | 146.0 ± 17.7 | 121.0 ± 18.9 |
| 180 min postoperative | 5 | 18.0 ± 4.1 | 180.0 ± 37.5 | 142.6 ± 20.0 | 116.8 ± 19.7 |
The respiratory frequency of the dogs within 3 h after the administration was lowered, but not significantly (P > 0.05 vs indices before embolization).
Effects on respiratory blood pressure and heart rate graphs
The recorded graphical data also indicated the same results (Figure 3) as above.
Figure 3.
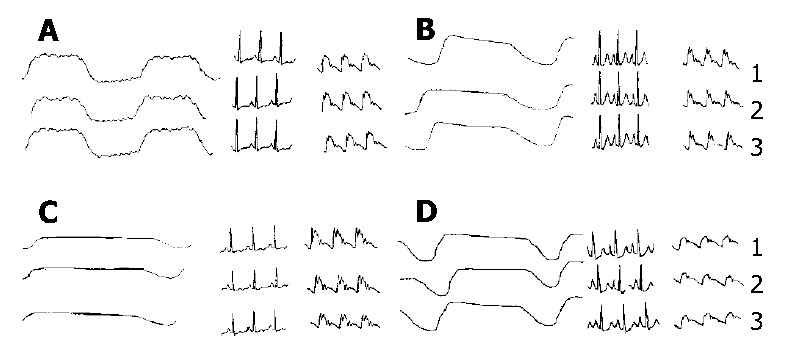
Effects of CAO-GMS infused via the hepatic artery on respiration, blood pressure and heart rate of dogs. A: Blank control group (with 3 g/L CMC-Na, 10 mL/kg); B: CAO-GMS group (with CAO-GMS, 0.1 g); C: CAO-GMS group (with CAO-GMS, 0.03 g); D: CAO group (with CAO, 6.4 μL/kg); 1: Before administration; 2: 60 min after administration; 3: 180 min after administration.
DISCUSSION
Transarterial chemoembolization (TACE) is most commonly adopted for palliative treatments in patients with hepatocellular carcinoma (HCC)[32,33], but the shrinkage of neoplasm comes at the price of sacrificing the hepatic function and in the long-term effects, it does not significantly differ from conservative therapy[34]. Improvement in the operative technique and in the agents used for interventional therapy is therefore much desired to produce better anti-tumor effect with low toxicity and liver protection. By producing CAO-GMS, we take full advantage of CAO of its anti-tumor, immune-enhancing effects with low-toxicity[12-26]. To enhance the effect of embolizing the trophic vessels for hepatoma, CAO was coupled with biodegradable microsphere (50-100 μm in diameter).
Previous studies demonstrated that CAO-GMS exhibited marked inhibitory effects on transplanted liver cancer in rats, which were attributed to the blocking of trophic vessels of the hepatoma by the microspheres and the therapeutic effects of CAO released slowly from the degraded microspheres[27-30].
In this study, CAO-GMS was not found to affect respiration, blood pressure and heart rate, etc., nor did it elicit acute or long-term toxic response, but at high doses, CAO-GMS embolization via the hepatic artery might induce significant increase in AST and ALT as well as irreversible ischemic necrosis of the liver. This was a sign of hyperreactive acute liver injury, as was similar to the clinical effect of other chemotherapeutic microspheres at high doses[35,36], and predominantly responsible for its untoward, or even lethal effects.
CAO-GMS or blank GMS administered via the hepatic artery in normal animals caused embolization in the kidney and untargeted liver lobes, leading to the death of animals, which, however, did not happen with CAO or saline. This result indicates that CAO alone normally does not induce the adverse effects, which arise from undesired embolization of CAO-GMS as with other chemotherapeutic microspheres[36,37]. Based on these facts, we concluded that the most probable reason for the acute hyperreactive response was the sudden accumulative embolization in the liver or undesired embolization as in the renal artery.
The dose adopted for embolization and the operation technique are crucial for clinical application of CAO-GMS. Most researchers preferred the use of computed tomography (CT) to determine individualized dosage rather than a conventionally fixed dosage, along with super-selective catheterization techniques to reduce reflux embolization of the microspheres. These techniques were believed to enhance the safety of the CAO-GMS interventional therapy.
In general, the results of this study demonstrated that CAO-GMS is fairly safe for interventional therapy but individual difference should be fully considered in its clinical application. Clinicians are advised to determine the embolization dosage and interventional method individually to avoid the undesired embolization caused by the microspheres. We propose the use of superselective catheterization technique to precisely block the trophic artery of the hepatoma, and the exact dosage of CAO-GMS should be determined according to the size, location and trophic vessels of the tumors to prevent undesired embolization or complications after the interventional therapy.
Footnotes
Supported by the National Medical Science and Technology Foundation during the 9th Five-Year Plan Period, No. 96-906-07-04
Edited by Chen WW Proofread by Zhu LH and Xu FM
References
- 1.Wang Y, Wang MZ. Study on the quality of Rhizoma Curcuma. Yaoxue Xuebao. 2001;36:849–853. [Google Scholar]
- 2.Li AQ, Hu XJ, Deng YH, Yao CS, Wang SJ, Chen JM. The chemical constituents of the volatile oil of Curcuma Wenyujin Y. H. Chen et C. Ling. Zhongcaoyao. 2001;33:782–783. [Google Scholar]
- 3.Huang KX, Tao ZM, Zhang AJ, Peng SL, Ding LS. [Studies on chemical constituents of Curcuma aromatica salisb] Zhongguo Zhongyao Zazhi. 2000;25:163–165. [PubMed] [Google Scholar]
- 4.Guo YT, Chu XK, Chen YR, Wu XY, Chen G, Lu XR. [Studies on constituents of wenezhu (Curcuma aromatica Salise) (author's transl)] Yaoxue Xuebao. 1980;15:251–252. [PubMed] [Google Scholar]
- 5.Yang SD, Chen YH. [The determination of curcumol in the volatile oil of Curcuma aromatica by phloroglucinol spectrophotometry (author's transl)] Yaocue Xuebao. 1980;15:228–233. [PubMed] [Google Scholar]
- 6.Yang S, Chen J, Chen Y. [Determination of curcumol in the volatile oil of Curcuma aromatica (author's transl)] Yaoxue Xuebao. 1979;14:356–361. [PubMed] [Google Scholar]
- 7.Li CZ. [Anti-inflammatory effect of the volatile oil from Curcuma aromatica] Zhongyao Tongbao. 1985;10:38–40. [PubMed] [Google Scholar]
- 8.Fu NW. [Antitumor effect and pharmacological actions of beta-elemene isolated from the rhizome of Curcuma aromatica] Zhongyao Tongbao. 1984;9:83–87. [PubMed] [Google Scholar]
- 9.Zeng LL, Wang GY, Wang DX, Chen CH, Xu SY. [Studies on the active ingredient of Curcuma aromatica Salisb and its effects] Yaoxue Xuebao. 1982;17:946–950. [PubMed] [Google Scholar]
- 10.Dai LM, Chen MZ, Xu SY. [The therapeutic effect of the Chinese drug Yu-Jin (Curcuma aromatica salisb) on experimental allergic encephalomyelitis of the guinea pig] Yaoxue Xuebao. 1982;17:692–695. [PubMed] [Google Scholar]
- 11.Qian ZC, Liu JY, Zhao SR, Yu LH, Zhang DY, Wei WH. [Prophylactic effect of active immunization with Curcuma aromatica treated tumor cells--exploration of mechanism of action of Curcuma aromatica (author's transl)] Yaoxue Xuebao. 1981;16:892–896. [PubMed] [Google Scholar]
- 12.Li GD, Xu F, Shen AJ. Research progress of Curcuma aromatica oil. Zhongguo Yaoxue Zazhi. 2002;37:806–809. [Google Scholar]
- 13.Tan M, Bin XN, Wu WY, Wang B, Zhang WB, Xiao CM. Effect of curcuma aromatica oil (CAO) on cellular apoptosis of hepatoma carried by mice. Zhongxiyi Jiehe Ganbing Zazhi. 2002;12:290–291. [Google Scholar]
- 14.Wang J, Wang SQ, Ni H, Chen L, Song WQ. Experimental studies in the inhibitory effects of Curcuma aromatica oil on human hepatic cancer cell line SMMC-7721 growth. Tianjin Zhongyiyao. 2003;20:48–50. [Google Scholar]
- 15.Shi LC, Wang B, Wu WY, Xiao CM, He QL. Study on molecule mechanism of inhibiting hepatoma of Curcuma aramatica oil in mice. Zhongyao Yaoli Yu Linchuang. 2002;18:6–7. [Google Scholar]
- 16.Shi LC, Wu WY, Zhang WB, Ou YQ, Tan M, Xiao CM. Effect of curcuma aromatica oil on proliferating cell nuclear antigen of hepatoma in mice. Shijie Huaren Xiaohua Zazhi. 1999;7:156–157. [Google Scholar]
- 17.Wu WY, Luo YJ, Cheng JH, Chang G, Xu Q, Liu WS, Li RX. Image cytometric DNA analysis of hepatic carcinomas carried by mice treaed by Curcuma aromatica oil (CAO) Zhongxiyi Jiehe Ganbing Zazhi. 1999;9:18–20. [Google Scholar]
- 18.Wu WY, Xu Q, Shi LC, Zhang WB. Inhibitory effects of Curcuma aromatica oil on proliferation of hepatoma in mice. World J Gastroenterol. 2000;6:216–219. doi: 10.3748/wjg.v6.i2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu W, Liu K, Tang X. [Preliminary study on the antitumor immuno-protective mechanism of beta-elemene] Zhonghua Zhongliu Zazhi. 1999;21:405–408. [PubMed] [Google Scholar]
- 20.Li B, Liang N. [Advances in the clinical application and experimental study of Curcurma zedoaria oil preparations] Zhongyaocai. 2003;26:68–71. [PubMed] [Google Scholar]
- 21.Han MJ, Ren K, Zhao ZC, Zhang XT. Oleum curcuma com-pound used in hepatic arterial embolization to treat hepatocel-lular carcinoma: follow up report of 84 cases. Linchuang Fangshexue Zazhi. 1998;17:112–114. [Google Scholar]
- 22.Cheng JH, Chang G, Wu WY. [A controlled clinical study between hepatic arterial infusion with embolized curcuma aromatic oil and chemical drugs in treating primary liver cancer] Zhongguo Zhongxiyi Jiehe Zazhi. 2001;21:165–167. [PubMed] [Google Scholar]
- 23.Chen CY, Xu K, Zhu DY, Wu WY, Deng H. Treatment of secondary hepatocarcinoma by hepatic artery perfusion embo-lism of oleum Curcumae: a clinical observation of 28 cases. Xinzhongyi. 2003;35:23–24. [Google Scholar]
- 24.Cheng JH, Wu WY, Liu WS, Chang G, Liu YL, Yang ZG. Thera-peutic effect of Curcuma aromatica oil infused via hepatic artery against primary liver neoplasms: 17 cases. Shijie Huaren Xiaohua Zazhi. 1999;7:92. [Google Scholar]
- 25.Wu WY, Luo YJ, Cheng JH, Chang G, Liu WS, Li RX. Therapeu-tic effect of Curcuma aramatica oil infused via hepatic artery against transplanted hepatoma in rats. Huaren Xiaohua Zazhi. 1998;6:859–861. [Google Scholar]
- 26.Deng R, Chen JM, Gao SC, Ding Y, Xu HD. Determination of Zedoary turmeric oil gelatin microspheres. Zhongguo Yiyuan Yaoxue Zazhi. 2001;21:79–81. [Google Scholar]
- 27.Deng R, Chen JM, Wu WY. The anti-tumor activity of Zedoary turmeric oil gelatin microspheres for heparical arterial embolization. Shenyang Yaoke Daxue Xuebao. 2000;17:197–199. [Google Scholar]
- 28.Wu W, Deng R, Ou Y. [Therapeutic efficacy of microsphere-entrapped curcuma aromatica oil infused via hepatic artery against transplanted hepatoma in rats] Zhonghua Ganzangbing Zazhi. 2000;8:24–26. [PubMed] [Google Scholar]
- 29.Deng R, Chen JM, Yao CS, Wu WY. Zedoary turmeric oil gelatin microspheres for hepatic arterial embolization. Yaoxue Xuebao. 2000;35:539–543. [Google Scholar]
- 30.Wu WY, Guo WJ, Chang G. Effect of CAO released from MS-CAO on human hepatoma cell line SMMC-7721. Shijie Huaren Xiaohua Zazhi. 2003;11:260–263. [Google Scholar]
- 31.Lindell B, Aronsen KF, Rothman U. Repeated arterial embo-lization of rat livers by degradable microspheres. Eur Surg Res. 1977;9:347–356. doi: 10.1159/000127954. [DOI] [PubMed] [Google Scholar]
- 32.Qian J, Feng GS, Vogl T. Combined interventional therapies of hepatocellular carcinoma. World J Gastroenterol. 2003;9:1885–1891. doi: 10.3748/wjg.v9.i9.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng JH, Liu XH. [Survey and progress in the research of Chinese medicinal herbs intervention therapy in treating primary liver carcinoma] Zhongguo Zhongxiyi Jiehe Zazhi. 1997;17:187–190. [PubMed] [Google Scholar]
- 34.A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med. 1995;332:1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 35.Li ZH, Li L, Yan LN, Lu WS, Xie XD, Luo YL. The side effect and its prevention of intra radioembolization using phosphorus-32 labelled glass microspheres for patients with nonresectable liver cancer. Huaxi Yixue. 2002;17:29–32. [Google Scholar]
- 36.Luo YK, Liang P, Dong BW, Yu XL, Su L. Selective puncture to embolize the portal vein with NBCA injection in rats: study on its efficacy and safety. Zhongguo Chaosheng Yixue Zazhi. 2002;18:168–172. [Google Scholar]
- 37.Li L, Yan L, Chen X. [The side effect and complication of intraarterial phosphorus-32 glass microspheres for patients with advanced hepatoma] Shengwu Yixue Gongchengxue Zazhi. 1997;14:376–379. [PubMed] [Google Scholar]


