Abstract
AIM: Transforming growth factor (TGF)-β1, metalloproteinase (MMP)-1 and its tissue inhibitor (TIMP)-1 are considered predictive biomarkers of chronic hepatitis activity and fibrosis. The aim of this study was to evaluate the effect of lamivudine treatment on the plasma levels of these peptides in patients with chronic hepatitis B.
METHODS: TGF-β1, MMP-1 and TIMP-1 plasma concentrations were measured with an enzyme immunoassay in 40 patients treated with lamivudine for 48 wk. Elimination of HBV-DNA and HBV antigens was evaluated 24 wk after treatment completion.
RESULTS: Baseline TGF-β1 (29.6 ± 2.2 ng/mL) and TIMP-1 (1578 ± 93 ng/mL) significantly exceeded normal values (18.3 ± 1.6 ng/mL and 1102 ± 67 ng/mL respectively). Lamivudine treatment resulted in a significant decrease of TGF-β1 and TIMP-1 during treatment with an increase after 24 wk of treatment. Pretreatment MMP-1 levels (6.7 ± 0.7 ng/mL) were significantly lower than normal values (11.9 ± 0.9 ng/mL) and increased during treatment and follow-up. A significant correlation was noted between TGF-β1 or TIMP-1 and aminotransferases as well as fibrosis scored in liver biopsy specimens. There were no statistically significant differences of TGF-β1, TIMP-1 and MMP-1 between four groups at baseline, 24 and 48 wk of treatment. TGF-β1 and TIMP-1 levels increased significantly in non-responders and normalized in responders at wk 72. MMP-1 also normalized in responders and decreased to values significantly lower than normal in non-responders.
CONCLUSION: These findings support the role of TGF-β1, TIMP-1 and MMP-1 in the pathogenesis of chronic hepatitis B. Because of their association with hepatic injury and antiviral treatment efficacy, determination of these peptides may be useful in disease management.
INTRODUCTION
Transforming growth factor-β1 (TGF-β1) is considered a pivotal inducer of liver fibrosis, acting mostly through activation of hepatic stellate cells (HSCs), which are the main source of extracellular matrix (ECM) proteins[1-3]. The effect of TGF-β1 on liver fibrosis is at least in part related to stimulation of the tissue inhibitor of metalloproteinases-1 (TIMP-1), which affects metalloproteinases (MMP) activity and is responsible for inhibition of ECM proteins breakdown[4]. Apart from a profibrogenic role, TGF-β1 inhibits DNA synthesis serving as a terminator of regenerative cell proliferation and induces apoptosis of hepatocytes in normal liver and during regression of liver hyperplasia[5]. On the other hand, TGF-β may inhibit stellate cells apoptosis and promote their survival, which are at least in part a result of anti-apoptotic effect of TIMP-1[6]. Additionally TGF-β1 exerts regulatory, mostly immunosuppressive effects on the immune system. Since hepatitis B virus (HBV) infection is related to an immune response, cell proliferation and fibrosis, modulation of TGF-β1 might affect the course of chronic viral hepatitis B[7,8]. The possible role of TGF-β1, TIMP-1 and MMP-1 as predictive biomarkers of chronic hepatitis activity and progression was supported by recent clinical studies[9-16], which demonstrated their association with hepatic function impairment or fibrosis, but did not evaluate their effect of antiviral treatment. We undertook this study to evaluate the effect of lamivudine, the most widespread antiviral medication for chronic HBV infection, on plasma TGF-β1, TIMP-1 and MMP-1 levels in patients with chronic hepatitis B.
MATERIALS AND METHODS
Patients
Ethical approval for the study was obtained from the Bioethical Committee of the Medical Academy of Bialystok. Informed consent was obtained from 40 patients (13 females and 27 males, mean age: 45 ± 3 years) with chronic hepatitis B, who were included into the protocol of lamivudine (Zeffix™, Glaxo-Smith-Kline) treatment. Normal values of TGF-β1, TIMP-1 and MMP-1 were collected from 13 healthy volunteers (5 females and 7 males, mean age: 47 ± 2 years). The diagnosis of chronic hepatitis B was confirmed by the presence of HBs and HBe antigens with stable elevated alanine aminotransferase (ALT) activity for at least 6 mo. Additionally the disease activity was confirmed by the presence of viral replication and evaluation of liver biopsy specimens performed by means of the Hepafix System (Braun, Melsungen, Germany). Paraffin-embedded biopsy specimens were stained and evaluated using the Scheuer scoring system[17]. Patients received 100 mg of lamivudine daily for 48 wk. Plasma levels of TGF-β1, TIMP-1 and MMP-1 were measured before treatment and at wk 24, 48 (end of the treatment) and 72. These results were compared to standard laboratory indices of liver injury. To evaluate treatment efficacy patients were divided into four groups with respect to elimination of HBV antigens and HBV-DNA at week 72 (24 wk after completion of treatment). Criteria for inclusion into particular groups were as follows: group I (complete response): HBsAg (-), HBeAg (-), HBV-DNA (-); group II: HBsAg (+), HBeAg (-), HBV-DNA (-); group III: HBsAg (+), HBeAg (-), HBV-DNA (+); group IV (no response): HBsAg (+), HBeAg (+), HBV-DNA (+).
Methods
Venous blood for plasma TGF-β1, TIMP-1 and MMP-1 was collected on ice using tubes with EDTA. Samples for TGF-β1 were immediately activated with acetic acid and urea and assayed with ELISA using recombinant human TGF-β soluble receptor type II (Tβ RII) as a solid phase precoated onto a microplate (Quantikine®, R&D Systems Inc., Minneapolis, USA) as we described previously[18]. TIMP-1 and MMP-1 were assayed by the two-site ELISA sandwich technique (Amersham Pharmacia Biotech, Little Chalfont, Buckinghampshire, England) using specific antibodies as a solid phase. MMP-1 assay recognises total human MMP-1, ie. free and complexed with TIMP-1. TIMP-1 assay recognises total human TIMP-1, including free and complexed with any metalloproteinases bound to the solid phase. TIMP-1 or MMP-1 bound to the solid phase, were detected by peroxidase labelled antibodies. There is no cross-reactivity between TIMP-1 and MMP-1 in these assays.
Liver function tests: ALT, aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyltranspeptidase (GGT) activity and bilirubin concentration were measured using a Cobas Mira instrument (Roche) and the prothrombin index (PI) was determined using Kselmed K-3002 (Poland).
Statistics
Values were expressed as the mean ± SE. The significance of the difference was calculated by two-tailed Student’s t test. For correlation analysis the Pearson product moment correlation was performed. Values of P < 0.05 were considered as statistically significant.
RESULTS
Plasma concentrations of TGF-β1 and TIMP-1 were determined before lamivudine treatment (mean: 29.6 ± 2.2 ng/mL and 1578 ± 93 ng/mL respectively) and significantly exceeded mean normal values (18.3 ± 1.6 ng/mL and 1102 ± 67 ng/mL respectively). Treatment resulted in a significant decrease by wk 24 and a further decline at the end of the treatment. However 24 wk after treatment completion mean values of both TGF-β1 and TIMP-1 increased (Table 1). In contrast, mean MMP-1 baseline level (6.7 ± 0.7 ng/mL) was almost half the normal value (11.9 ± 0.9 ng/mL) and increased during treatment. After completion of treatment the levels still remained lower than normal (Table 1). There was a significant positive correlation between TGF-β1, TIMP-1 and ALT or AST (Table 2). A significant correlation was also demonstrated between TIMP-1 and ALP or GGT, but there was no association between MMP-1 and the biochemical indices of liver injury (Table 2). As demonstrated in Figure 1 a significant positive correlation (r = 0.435; P < 0.01) was observed between TGF-β1 and TIMP-1. There was no correlation between TGF-β1 and MMP-1 (r = 0.069), or between TIMP-1 and MMP-1 (r = -0.143).
Table 1.
Plasma concentrations of TGF-β1, TIMP-1 and MMP-1 during treatment with lamivudine (mean ± SE)
| Normal values |
Time since treatment beginning (wk) |
||||
| 0 | 24 | 48 | 72 | ||
| TGF-β1 (ng/mL) | 18.3 ± 1.6 | 29.6 ± 2.21 | 22.6 ± 1.22 | 21.9 ± 1.52 | 24.1 ± 2.4 |
| TIMP-1 (ng/mL) | 1102.0 ± 67 | 1578.0 ± 931 | 1235.0 ± 672 | 927.0 ± 502 | 1215.0 ± 982 |
| MMP-1 (ng/mL) | 11.9 ± 0.9 | 6.7 ± 0.71 | 8.3 ± 0.51 | 9.6 ± 0.51 | 9.7 ± 0.72 |
Statistical significance in comparison with
normal and
baseline values.
Table 2.
Correlation expressed by r-value and its significance between analyzed biochemical indices and TGF-β1, TIMP-1 or MMP-1 in chronic hepatitis B patients treated with lamivudine
| mean ± SE | TGF-β1 | TIMP-1 | MMP-1 | |
| Bil (mg%) | 1.02 ± 0.06 | 0.049 | 0.119 | -0.059 |
| ALT (U/L) | 74.50 ± 5.9 | 0.201a | 0.247a | 0.005 |
| AST (U/L) | 60.10 ± 5.2 | 0.244a | 0.269a | -0.029 |
| ALP (U/L) | 96.10 ± 3.8 | 0.114 | 0.295a | -0.071 |
| GGT (U/L) | 47.70 ± 4.9 | 0.062 | 0.247a | -0.102 |
| PI (%) | 87.30 ± 1.5 | -0.109 | 0.006 | -0.065 |
Bil: bilirubin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; GGT: gamma glutamyltranspeptidase; PI: prothrombin index.
P < 0.05 vs other groups.
Figure 1.
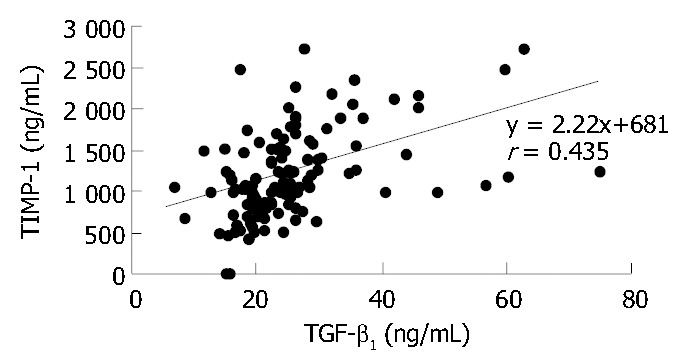
Correlation between plasma TGF-β1 and TIMP-1 in patients with chronic hepatitis B treated with lamivudine.
As demonstrated in Table 3 scored degree of hepatic inflammation and fibrosis was similar before and after the treatment. Histologic observations in biopsies performed before the treatment showed a significant correlation between the degree of fibrosis and plasma TGF-β1 or TIMP-1 levels (Table 4). There was no association between scored inflammation or fibrosis and classical biochemical parameters of liver injury.
Table 3.
Values of scored degree of hepatic inflammation and fibrosis in liver biopsy specimens obtained from patients before and after lamivudine treatment (mean ± SE)
| Before the treatment | After the treatment | |
| Portal inflammation | 2.6 ± 0.1 | 2.5 ± 0.2 |
| Lobular inflammation | 1.9 ± 0.1 | 1.8 ± 0.1 |
| Fibrosis | 2.0 ± 0.2 | 1.8 ± 0.2 |
Table 4.
Correlation expressed by r-value and significance be-tween scored degree of hepatic inflammation or fibrosis and plasma levels of TGF-β1, TIMP-1 and MMP-1 in patients with chronic hepatitis B before lamivudine treatment
|
Inflammation |
Fibrosis | ||
| Portal | Lobular | ||
| TGF-β1 | 0.207 | 0.179 | 0.391a |
| TIMP-1 | 0.224 | 0.319 | 0.620a |
| MMP-1 | 0.009 | -0.094 | -0.135 |
P < 0.05 vs others.
The majority of patients (23%-57.5%) did not respond to treatment and were classified into group IV. A complete response (group I), with elimination of HBs and HBe antigens as well as HBV-DNA, was observed in 5 patients (12.5%). Six patients (15%) demonstrated a partial response (group II), and another six eliminated HBeAg with detectable viral replication (group III). Evaluation of baseline liver function tests and efficacy of treatment showed no statistically significant differences between the four groups. As demonstrated in Table 5, at the end of the treatment, patients in groups III and IV had significantly higher ALT activities than patients in group I. This difference was statistically significant in respect to both ALT and AST activities, 24 weeks after the treatment completion (Table 5). Histologic pictures did not demonstrate significant differences between groups in liver biopsies preformed before and after the treatment. During treatment TGF-β1, TIMP-1 and MMP-1 plasma levels were similar among all patients before divided into the response-related groups (Figure 2, Figure 3, Figure 4). And there were no statistically significant differences in their concentrations between groups at baseline, 24 and 48 weeks of treatment, either Although at week 48 the mean TGF-β1 in the non-responder groups (III and IV) tended to increase, whereas in groups I and II normalized, the difference was not significant (Figure 2). However 24 weeks after treatment completion (wk 72), the difference between TGF-β1 levels in groups I and IV (17.5 ± 0.6 and 26 ± 1.8 ng/mL respectively) became significant (P = 0.04). Moreover the concentration in group IV was significantly (P = 0.009) higher than normal values. TIMP-1 mean concentrations were similar among all groups during the treatment period and normalized at week 48 (Figure 3). However 24 wk later, values in group IV (1350 ± 70 ng/mL) were significantly higher than normal, as well as that in group I (900 ± 131 ng/mL). MMP-1 levels increased in all groups during the treatment. At wk 72, MMP-1 almost normalized in responders and slightly decreased in non-responders (Figure 4). The difference between MMP-1 concentrations in group IV after treatment completion (9.1 ± 0.8 ng/mL) and normal values was statistically significant (P = 0.037).
Table 5.
Activities of alanine and aspartate aminotransferases during the treatment (0, 24 and 48 wk) and 24 wk after its completion (week 72) in four groups (mean ± SE)
|
ALT (U/l) |
AST (U/L) |
|||||||
| 0 | 24 | 48 | 72 | 0 | 24 | 48 | 72 | |
| Group I | 87.0 ± 24.5 | 25.2 ± 5.0 | 23.0 ± 4.8 | 23.2 ± 4.9 | 79.8 ± 15.9 | 30.2 ± 6.7 | 28.0 ± 6.4 | 27.0 ± 6.1 |
| Group II | 108.5 ± 53 | 32.5 ± 9.5 | 31.9 ± 7.5 | 31.7 ± 5.7 | 66.7 ± 24.8 | 44.2 ± 12.7 | 32.1 ± 4.8 | 29.8 ± 3.8 |
| Group III | 127.0 ± 43.4 | 38.7 ± 8.2 | 78.0 ± 30.1a | 88.2 ± 34b | 117 ± 47.2 | 39.2 ± 8.8 | 60.5 ± 15.9 | 63.5 ± 22a |
| Group IV | 101.0 ± 11.8 | 78.6 ± 8.3 | 69.2 ± 6.3b | 68.5 ± 4.2b | 65.9 ± 6.4 | 65.2 ± 21.2 | 59.7 ± 8.4 | 58.7 ± 3.9a |
Statistical significance in comparison with group I (complete response to treatment):
P < 0.05,
P < 0.001 vs other groups.
Figure 2.

Mean TGF-β1 plasma concentrations before and dur-ing lamivudine therapy as well as 24 wk after its completion (wk 72) in respect to the final effect of the treatment. Statistical significance in comparison to normal values are indicated with asterisks and between groups with arrows.
Figure 3.
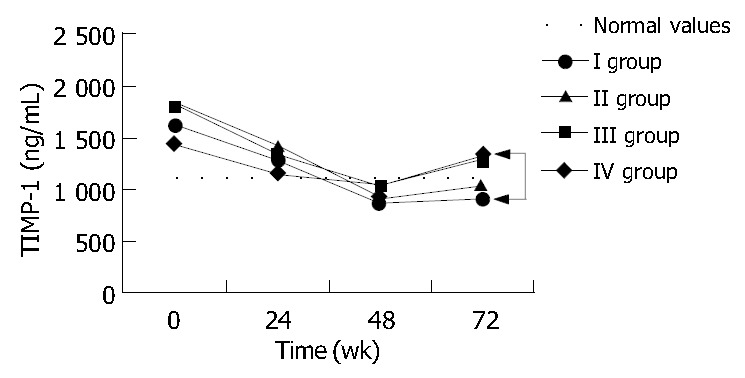
Mean TIMP-1 plasma concentrations before and during lamivudine therapy as well as 24 wk after its completion (wk 72) in respect to the final effect of the treatment. Statistical significance in comparison to normal values are indicated with asterisks and between groups with arrows.
Figure 4.
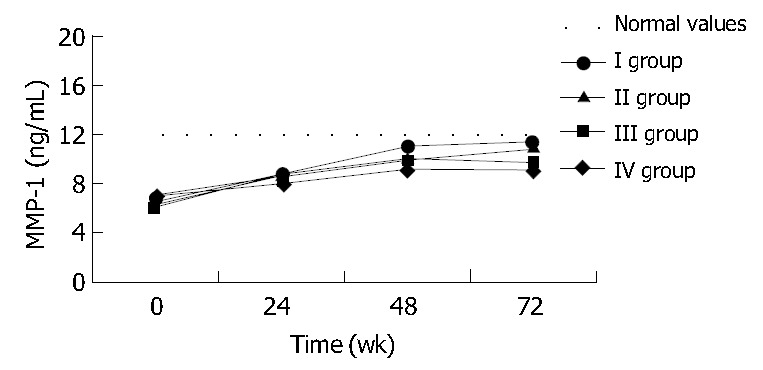
Mean MMP-1 plasma concentrations before and during lamivudine therapy as well as 24 wk after its completion (week 72) in respect to the final effect of the treatment. Statistical significance in comparison to normal values are indicated with asterisks and between groups with arrows.
DISCUSSION
The pivotal role of TGF-β1 in fibrogenesis was finally proved in transgenic mice with an overexpression of TGF-β1, which caused an increase of TGF-β1 plasma levels up to 700 ng/mL and a marked upregulation of TIMP-1 gene expression[19-21]. Chronic liver injury leading to fibrosis displayed diminished ECM degradation mainly through TIMP induction and consequent MMP inhibition[4]. As demonstrated recently by Nie et al in the process of hepatic fibrosis, fibroblasts and myofibroblasts are the major cells that express TIMP-1 and TIMP-2, and their gene expressions correlate with degree of hepatic fibrosis.
The most important factor that affects the measurement of TGF-β1 in human is the preparation of samples with minimal contamination from platelets which are an important source of this cytokine[22]. The Quantikine ELISA System is recommended because of the relatively quick and simple activation with acid and urea, which disrupt the majority of TGF-β1 complexes.
As we have demonstrated previously, TGF-β1 correlates significantly with the degree of liver insufficiency in humans with liver cirrhosis[18]. Moreover both TGF-β1 and TIMP-1 measured in plasma of patients with chronic hepatitis B demonstrate a significant correlation with the degree of hepatocyte injury and fibrosis in liver biopsy specimens[10]. We confirmed these observations in the present study. As demonstrated by Yoo et al[7] HBV antigens induce expression of TGF-β1 in the early stages of infection. According to Lee et al[8] HBV encoded pX protein enhances transcriptional activity and response to TGF-β by amplification of Smadmediated signaling, which can also contribute to HBV-associated liver fibrosis. Therefore the stimulatory effect of HBV antigens on TGF-β1 seems to be an important mechanism of liver fibrosis in addition to TGF-β release caused by hepatocyte injury.
Association between circulating or tissue TGF-β and liver fibrosis has also been confirmed in HCV infection[10,11]. Recent studies of Neuman et al[23], Chen et al and Lu et al[16] demonstrated a similar relationship between different chronic liver diseases including primary biliary cirrhosis and alcoholic liver disease and TGF-β1. Our previous study showed that the positive predictive value of TGF-β1 plasma levels exceeding the upper normal range reached 96% for liver cirrhosis[18]. As demonstrated recently by Zhang et al serum level of TIMP-1 and TIMP-1/MMP-1 could be used as the indices for the diagnosis of hepatic fibrosis in chronic hepatitis B. According to Boeker et al[9], measurement of plasma TIMP-1 detected cirrhosis with a 100% sensitivity but lower specificity. These observations were also confirmed by the association between hepatic fibroproliferation and expression of hepatic TIMP and MMP mRNA[12]. Walsh et al[15], who studied liver histology in patients with chronic hepatitis C, underlined the high sensitivity of TIMP-1 and TIMP-2 in detecting advanced liver disease. According to Nie et al, there was a significant correlation between circulating and liver levels of TIMP-1 in cirrhotics, so its measurement in plasma might be useful in fibrosis management. These observations indicate the usefulness of both TGF-β1 and TIMP-1 as possible early non-invasive biomarkers for liver fibrosis.
The rate of HBeAg seroconversion in our study (27.5%) was in range (18%-32%) demonstrated by numerous authors in previous research with lamivudine treatment[24-26]. In this study we confirmed the association between the degree of hepatocyte injury or liver fibrosis and plasma TGF-β1 or TIMP-1 levels in patients with chronic hepatitis B. Aminotransferases, which are classical surrogate markers of hepatic injury, demonstrated similar association with the outcomes of the treatment. However, in contrast to TGF-β1 and TIMP-1 their activities were not related to liver fibrosis. As the levels of TGF-β1, TIMP-1 and MMP-1 showed a similar change in all groups during therapy, it was unclear whether this was a direct effect of lamivudine on their expressions or an effect caused by HBV inhibition. However, their levels at 24 wk after therapy completion demonstrated their association with infection activity. This relationship was noted in respect to TGF-β1, which increased in group IV (non-responders) at the end of treatment. The effect on TIMP-1 was probably secondary to TGF-β1. The inhibitory activity of excessive TIMP-1 accumulation might be the reason for the decreased MMP-1 plasma concentration. These results are in accordance with our previous findings, demonstrating the strong association between TGF-β1 or TIMP-1 plasma levels and scored hepatic fibrosis evaluated in biopsy specimens of patients with chronic hepatitis B and C[10]. The findings of increased TGF-β1 and TIMP-1 accompanied by an elevation in plasma carboxyterminal cross-linked telopeptide of type 1 procollagen (ICTP), indicative of type I collagen degradation. We suggested that collagenolytic mechanisms preceded TGF-β1/TIMP-1 dependent stimulation of liver fibrosis[10]. Low MMP-1 plasma levels before treatment in the present study is consistent with this observation. It is also in accordance with the report by Murawaki et al[13], who demonstrated a decrease in MMP-1 concentration during histological progression of chronic hepatitis. Moreover, a significantly decreased baseline plasma MMP-1, followed by an increase during treatment, supports the role of TGF-β1/TIMP-1 dependent mechanism of liver fibrosis in patients with active chronic hepatitis B. Similar effects on MMP-1 and TIMP-1 were observed by Ninomiya et al[14] in patients with chronic hepatitis C who showed improvement of liver histology after treatment with interferon-alfa. Reduced progression of liver fibrosis during treatment of chronic hepatitis B with lamivudine has been demonstrated in numerous trials[24-28]. Downregulation of TGF-β1/TIMP-1 causing an increase of MMP-1 activity should be considered as the probable mechanism underlying this effect of lamivudine. However, to confirm this hypothesis further research should be focused on the MMP-1 activity measurement.
These findings support the role of TIMP-1 and MMP-1 balance in the TGF-β1 dependent mechanism of liver fibrosis related to HBV infection. The association of TIMP-1, MMP-1 and TGF-β1 with hepatic injury and antiviral treatment efficacy suggest their possible usefulness in chronic hepatitis B management. Elevated TGF-β1 plasma concentrations during antiviral therapy may indicate medication failure.
Footnotes
Edited by Zhu LH and Xu FM
References
- 1.Knittel T, Janneck T, Müller L, Fellmer P, Ramadori G. Transforming growth factor beta 1-regulated gene expression of Ito cells. Hepatology. 1996;24:352–360. doi: 10.1053/jhep.1996.v24.pm0008690404. [DOI] [PubMed] [Google Scholar]
- 2.Ramadori G, Saile B. The fibrogenic mediators, TGF-β and TNF-α, as surviving factors for activated hepatic stellate cells. In: Gressner AM, Heinrich PC, Matern S, editors. Cytokines in liver injury and repair. Dordrecht/Boston/London: Kluwer Academic Publishers. 2002:184–188. [Google Scholar]
- 3.Williams EJ, Gaça MD, Brigstock DR, Arthur MJ, Benyon RC. Increased expression of connective tissue growth factor in fibrotic human liver and in activated hepatic stellate cells. J Hepatol. 2000;32:754–761. doi: 10.1016/s0168-8278(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 4.Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
- 5.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 6.Saile B, Matthes N, Knittel T, Ramadori G. Transforming growth factor beta and tumor necrosis factor alpha inhibit both apoptosis and proliferation of activated rat hepatic stellate cells. Hepatology. 1999;30:196–202. doi: 10.1002/hep.510300144. [DOI] [PubMed] [Google Scholar]
- 7.Yoo YD, Ueda H, Park K, Flanders KC, Lee YI, Jay G, Kim SJ. Regulation of transforming growth factor-beta 1 expression by the hepatitis B virus (HBV) X transactivator. Role in HBV pathogenesis. J Clin Invest. 1996;97:388–395. doi: 10.1172/JCI118427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DK, Park SH, Yi Y, Choi SG, Lee C, Parks WT, Cho H, de Caestecker MP, Shaul Y, Roberts AB, et al. The hepatitis B virus encoded oncoprotein pX amplifies TGF-beta family signaling through direct interaction with Smad4: potential mechanism of hepatitis B virus-induced liver fibrosis. Genes Dev. 2001;15:455–466. doi: 10.1101/gad.856201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boeker KH, Haberkorn CI, Michels D, Flemming P, Manns MP, Lichtinghagen R. Diagnostic potential of circulating TIMP-1 and MMP-2 as markers of liver fibrosis in patients with chronic hepatitis C. Clin Chim Acta. 2002;316:71–81. doi: 10.1016/s0009-8981(01)00730-6. [DOI] [PubMed] [Google Scholar]
- 10.Flisiak R, Maxwell P, Prokopowicz D, Timms PM, Panasiuk A. Plasma tissue inhibitor of metalloproteinases-1 and transforming growth factor beta 1--possible non-invasive biomarkers of hepatic fibrosis in patients with chronic B and C hepatitis. Hepatogastroenterology. 2002;49:1369–1372. [PubMed] [Google Scholar]
- 11.Kanzler S, Baumann M, Schirmacher P, Dries V, Bayer E, Gerken G, Dienes HP, Lohse AW. Prediction of progressive liver fibrosis in hepatitis C infection by serum and tissue levels of transforming growth factor-beta. J Viral Hepat. 2001;8:430–437. doi: 10.1046/j.1365-2893.2001.00314.x. [DOI] [PubMed] [Google Scholar]
- 12.Lichtinghagen R, Michels D, Haberkorn CI, Arndt B, Bahr M, Flemming P, Manns MP, Boeker KH. Matrix metalloproteinase (MMP)-2, MMP-7, and tissue inhibitor of metalloproteinase-1 are closely related to the fibroproliferative process in the liver during chronic hepatitis C. J Hepatol. 2001;34:239–247. doi: 10.1016/s0168-8278(00)00037-4. [DOI] [PubMed] [Google Scholar]
- 13.Murawaki Y, Ikuta Y, Idobe Y, Kawasaki H. Serum matrix metalloproteinase-1 in patients with chronic viral hepatitis. J Gastroenterol Hepatol. 1999;14:138–145. doi: 10.1046/j.1440-1746.1999.01821.x. [DOI] [PubMed] [Google Scholar]
- 14.Ninomiya T, Yoon S, Nagano H, Kumon Y, Seo Y, Kasuga M, Yano Y, Nakaji M, Hayashi Y. Significance of serum matrix metalloproteinases and their inhibitors on the antifibrogenetic effect of interferon-alfa in chronic hepatitis C patients. Intervirology. 2001;44:227–231. doi: 10.1159/000050052. [DOI] [PubMed] [Google Scholar]
- 15.Walsh KM, Timms P, Campbell S, MacSween RN, Morris AJ. Plasma levels of matrix metalloproteinase-2 (MMP-2) and tissue inhibitors of metalloproteinases -1 and -2 (TIMP-1 and TIMP-2) as noninvasive markers of liver disease in chronic hepatitis C: comparison using ROC analysis. Dig Dis Sci. 1999;44:624–630. doi: 10.1023/a:1026630129025. [DOI] [PubMed] [Google Scholar]
- 16.Lu LG, Zeng MD, Wan MB, Li CZ, Mao YM, Li JQ, Qiu DK, Cao AP, Ye J, Cai X, et al. Grading and staging of hepatic fibrosis, and its relationship with noninvasive diagnostic parameters. World J Gastroenterol. 2003;9:2574–2578. doi: 10.3748/wjg.v9.i11.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 18.Flisiak R, Pytel-Krolczuk B, Prokopowicz D. Circulating transforming growth factor beta(1) as an indicator of hepatic function impairment in liver cirrhosis. Cytokine. 2000;12:677–681. doi: 10.1006/cyto.1999.0660. [DOI] [PubMed] [Google Scholar]
- 19.Kanzler S, Lohse AW, Keil A, Henninger J, Dienes HP, Schirmacher P, Rose-John S, zum Büschenfelde KH, Blessing M. TGF-beta1 in liver fibrosis: an inducible transgenic mouse model to study liver fibrogenesis. Am J Physiol. 1999;276:G1059–G1068. doi: 10.1152/ajpgi.1999.276.4.G1059. [DOI] [PubMed] [Google Scholar]
- 20.Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts AB, Sporn MB, Thorgeirsson SS. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci USA. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clouthier DE, Comerford SA, Hammer RE. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-beta1 transgenic mice. J Clin Invest. 1997;100:2697–2713. doi: 10.1172/JCI119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grainger DJ, Mosedale DE, Metcalfe JC. TGF-beta in blood: a complex problem. Cytokine Growth Factor Rev. 2000;11:133–145. doi: 10.1016/s1359-6101(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 23.Neuman M, Angulo P, Malkiewicz I, Jorgensen R, Shear N, Dickson ER, Haber J, Katz G, Lindor K. Tumor necrosis factor-alpha and transforming growth factor-beta reflect severity of liver damage in primary biliary cirrhosis. J Gastroenterol Hepatol. 2002;17:196–202. doi: 10.1046/j.1440-1746.2002.02672.x. [DOI] [PubMed] [Google Scholar]
- 24.Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 25.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 26.Schalm SW, Heathcote J, Cianciara J, Farrell G, Sherman M, Willems B, Dhillon A, Moorat A, Barber J, Gray DF. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000;46:562–568. doi: 10.1136/gut.46.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tassopoulos NC, Volpes R, Pastore G, Heathcote J, Buti M, Goldin RD, Hawley S, Barber J, Condreay L, Gray DF. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B. Lamivudine Precore Mutant Study Group. Hepatology. 1999;29:889–896. doi: 10.1002/hep.510290321. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki Y, Kumada H, Ikeda K, Chayama K, Arase Y, Saitoh S, Tsubota A, Kobayashi M, Koike M, Ogawa N, et al. Histological changes in liver biopsies after one year of lamivudine treatment in patients with chronic hepatitis B infection. J Hepatol. 1999;30:743–748. doi: 10.1016/s0168-8278(99)80123-8. [DOI] [PubMed] [Google Scholar]


