Abstract
AIM: To evaluate whether cell apoptosis and regeneration were existed in normal liver cells adjacent to carcinoma after transarterial chemoembolization (TACE).
METHODS: Fifty rabbits with hepatic carcinoma were divided into 5 groups at random: group A (control group), groups B and C (TACE treatment groups), groups D and E (partial hepatectomy groups). There were 10 rabbits in each group. Rabbits in groups B-E were treated by transarterial chemoembolization (TACE) and partial hepatectomy (PH) respectively. The changes of S-phase cell fraction (SPF), proliferation index (PI) and cell apoptosis in the normal liver tissue were determined with flow cytometry (FCM) after operations on the first and third days. We determined the mitosis index (MI) with histo-pathological method and the apoptosis index (AI) with TUNEL method at the same time.
RESULTS: Twenty-four hours after operations, compared with control group, the rabbits in TACE group had much higher index of SPF, PI and MI (MI: t = 4.89, P < 0.001; SPF: t = 5.27, P < 0.001; PI: t = 4.87, P < 0.001). Moreover, the proliferation of liver cells in TACE group was much weaker than that of the cells treated by partial hepatectomy, and the differences were significant (MI: t = 7.02, P < 0.001; SPF: t = 4.06, P < 0.001; PI: t = 2.70, P < 0.05). Seventy-two h after operations, FCM showed a small sub-G1 peak in TACE group and PH group, compared with the control group, but there was no difference between them (t = 0.41, P > 0.05). TACE showed that AI in the treated rabbits was higher than that in control group (t = 3.07, P < 0.05), and there were no differences between TACE group and PH group, either (t = 0.93, P > 0.05).
CONCLUSION: Cell apoptosis and regeneration exist in rabbit liver tissues after TACE in some degree, which may be associated with the selective embolization of iodised oil, chemotherapeutic drug and free radical damage.
INTRODUCTION
The efficacy of transarterial chemoembolization (TACE) is greatly determined by the liver function reserve of liver cancer patients. More than 40% patients died of liver failure after TACE, in which most of them were accompanied by poor liver function and damage due to chemotherapy. Therefore, it is important to study the effects of TACE on non-neoplastic liver tissue to decrease the death rate. We have established the animal model of rabbit VX2 hepatic carcinoma. All the rabbits were treated by TACE to detect the effect on cell cycle of non-neoplastic liver tissues after operations[1].
MATERIALS AND METHODS
Establishment of the animal model
Fifty Japanese alpine hares, weighing 2.5-3.3 kg, provided by the Experiment Animal Center of Tongji Medical College were used in this experiment. The rabbits were implanted hepatocellular carcinoma cell VX2, and observed for 3 wk before experiment[2].
Treatment and radiological examination[3,4]
Fifty rabbits with VX2 hepatic carcinoma were randomly divided into 5 groups: Group A (control group), groups B and C (TACE treatment groups), groups D and E (partial hepatectomy groups), 10 rabbits in each group. Three weeks later, at laparotomy of the rabbits, the feeding artery of liver carcinoma was punctured and arteriography was made[5]. A 1 mL of physiological saline was administered to group A. Groups B and C were treated with 0.6-0.8 mL of a mixture of iodine oil (10 mL) and mitomycin (10 mg)[6,7]. Groups D and E were treated by hepatectomy. Groups B and C were performed CT and MRI after TACE[8,9].
Flow cytometry analysis
Rabbits in groups A, B and C were killed on the first day after the procedure, and those in groups D and E were killed on the third day after the procedure. As soon as the rabbits died, a piece of non-neoplastic liver tissue was cut from each rabbit and digested with enzymes. The cells were fixed by 800 mL/L ice ethanol, hatched by 40 µL PC on ice for 30 min, and washed in 1 g/L PBS. At last, they were stained by at 100 µL PI 10 mg/L and 50 µL 1 g/L RNA enzyme for 20 min, then flow cytometry was performed. The cells were detected by FAC Sort FCM machine (Beton Dickson Co, USA). The fluorescence of cells was analyzed with Cellquest software and the cell cycle was studied. The other liver tissues were photographed, fixed with 40 g/L formaldehyde, and stained by HE[10].
Groups B and C were performed CT after TACE, and accumulation of iodized oil was observed. General pathological changes of carcinoma were detected in all the rabbits. Under the microscope, we calculated the number of liver cells in the mitosis phase per 1000 liver cells, which was called mitosis index (MI).
FCM analysis
S phase cell fraction (SPF) was calculated according to the equation: SPF = S/(S + G1/G0 + G2/M) × 100%. Proliferation index (PI) was calculated according to the equation: PI = (S + G2/M)/(S + G1/G0 + G2/M) × 100%. Apoptosis index (AI) was analyzed by TUNEL method[11], the number of apoptotic cells was calculated under microscope[12].
Statistic analysis
All the data were analyzed by t test.
RESULTS
General pathological and radiological findings
Three wk after tumor implantation, the size of VX2 liver carcinoma was 3.5 ± 1.6 cm on average, and appeared as white nodules near the liver surface. There were no definite margins between carcinomas and normal liver tissues. On DSA, there was increased and irregular angiogenesis of the tumor vessels, with tumor stained mainly at the periphery (Figure 1). On MR, the signal of cancer was slightly lower than that of normal liver tissue on T1WI and slightly higher on T2WI (Figure 2). The iodized oil deposited well after TACE on CT[13] (Figure 3).
Figure 1.
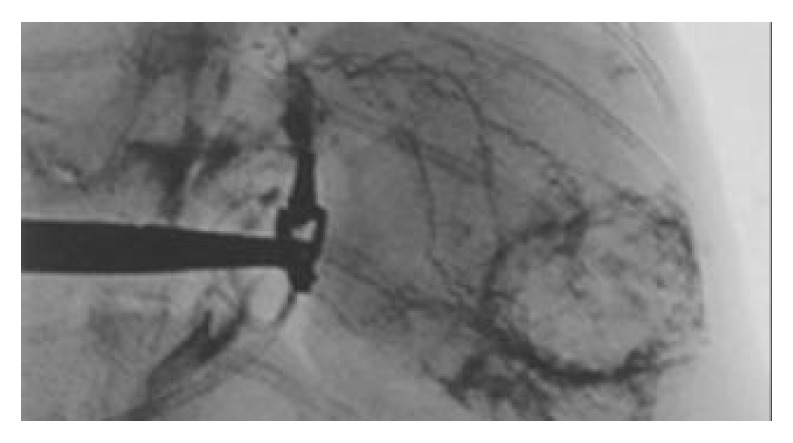
Hepatic arteriography of rabbits. There was increased angiogenesis with thickening and irregularity of the vessels. There was also tumor staining mainly in the periphery of the tumor.
Figure 2.
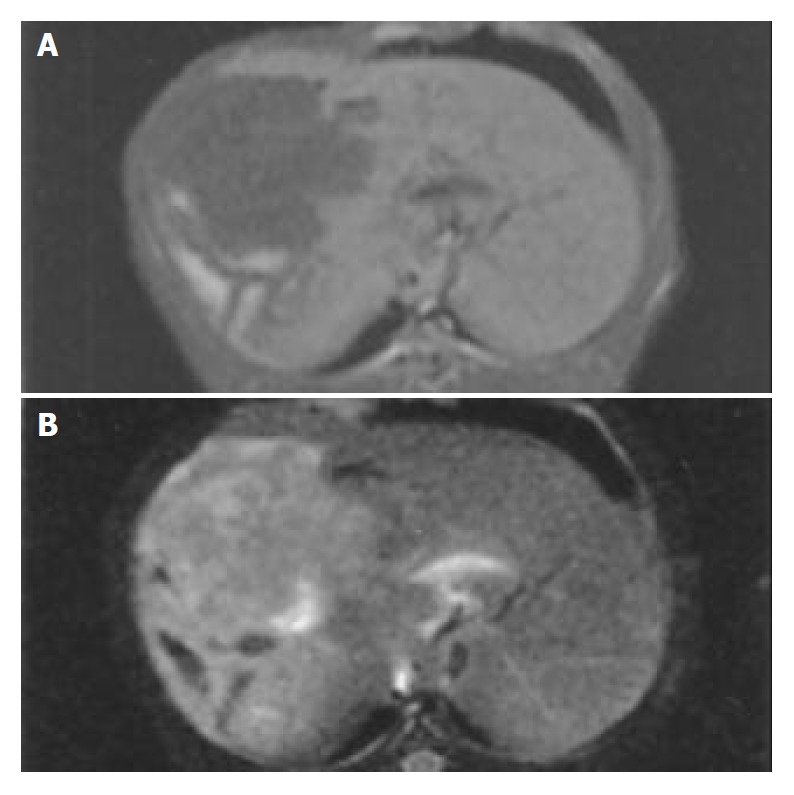
MRI of rabbit liver. The tumor appeared hypointense on T1WI (A) and hyperintense on T2WI (B).
Figure 3.
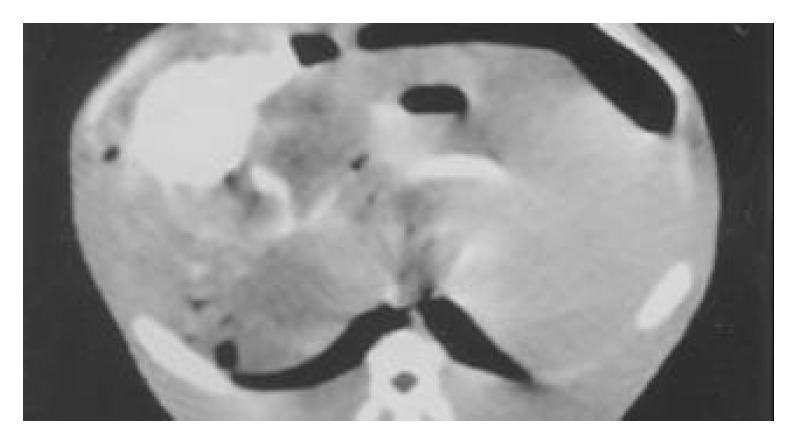
CT scan shows massive retention of iodized oil in tumor after TACE.
Cytological changes
Red fluorescent light could be seen in the cells under laser confocal microscope (Figure 4). Under light microscope (Olympus), we could find cell mitosis clearly, and mitosis index (MI) could be calculated. Flow cytometry showed that S phase and G2/M phase cells in TACE groups and partial hepatectomy groups increased much more on the first day after operations than that in the control group (Figure 5). On the third day after operations, the amount of S-phase and G2/M phase cells in TACE group and partial hepatectomy group was about the same as control group (Figure 6). In the cell apoptosis index (AI) determined by TUNEL method, no significant difference existed between TACE group (Figure 7) and partial hepatectomy group.
Figure 4.
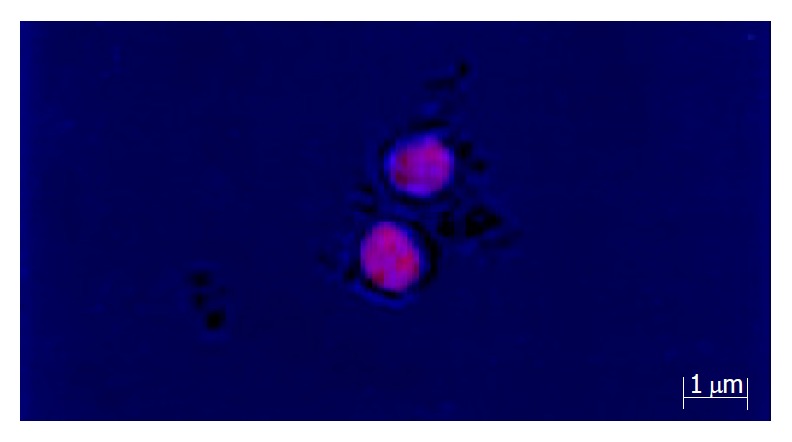
Cells giving out red fluorescent light under laser confocal microscope.
Figure 5.
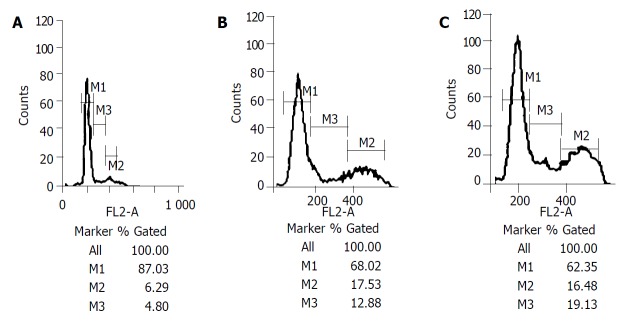
A: Shows the S phase and G2/M phase cells were small in proportion on the first day after operations (4.80%, 6.29%) in control group. B: Shows the S phase and G2/M phase cells grew much more on the first day after operations (17.53%, 12.88%) in TACE group. C: Shows the S phase and G2/M phase cells grew much more on the first day after operations (16.48%, 19.13%) in PH group. *M1 is G0/G1 phase cell ratio/M2 is G2/M phase cell ratio, M3 is S phase cell ratio.
Figure 6.
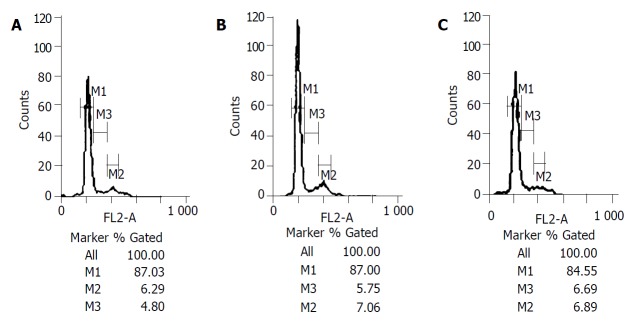
A: Shows the S phase and G2/M phase cells were small in proportion on the third day after operations (4.80%, 6.29%) in control group. B and C: Show the S phase and G2/M phase cells had slight changes on the third day after operations in TACE group and PH group.
Figure 7.
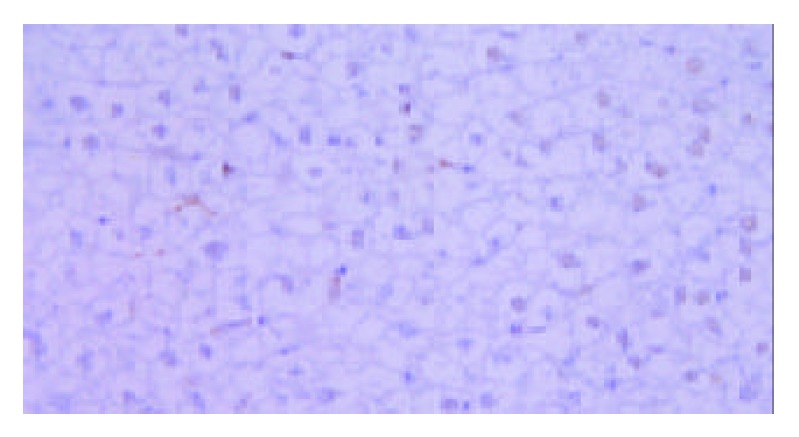
Analysis of apoptosis cells by TUNEL method in TACE group. The nuclei of apoptosis cells appeared brown while those of normal cells appeared blue. It was found that part of the cells were apoptotic cells.
The t test values of MI, SPF and PI of TACE groups and control group are listed in Table 1. The t test values of MI, SPF and PI of TACE groups and partial hepatectomy groups are listed in Table 2.
Table 1.
t test value of MI, SPF and PI of TACE groups and control group on the first day after operations
| Index | TACE group | Control group | t value | P value |
| MI (%) | 1.42 ± 0.55 | 0.51 ± 0.21 | 4.89 | P < 0.001 |
| SPF (%) | 12.46 ± 4.18 | 4.90 ± 1.76 | 5.27 | P < 0.001 |
| PI (%) | 23.38 ± 8.31 | 9.93 ± 2.68 | 4.87 | P < 0.001 |
Table 2.
t test value of MI, SPF and PI of TACE groups and partial hepatectomy group on the first day after operations
| Index | TACE group | Partial hepatectomy group | t value | P value |
| MI (%) | 1.42 ± 0.55 | 3.13 ± 0.54 | 7.02 | P < 0.001 |
| SPF (%) | 12.46 ± 4.18 | 21.76 ± 5.92 | 4.06 | P < 0.001 |
| PI (%) | 23.38 ± 8.31 | 32.51 ± 6.75 | 2.70 | P < 0.05 |
The t test values of MI, SPF of TACE groups and control group are listed in Table 3. There was no significant difference between the two groups (P > 0.05). However, the P value of AI and sub-G1 was less than 0.05. The t test values of MI, SPF, AI and PI of TACE groups and partial hepatectomy groups are listed in Table 4.
Table 3.
t test value of MI, SPF, AI, Sub-G1 and PI of TACE groups and control group on the third day after operations
| Index | TACE group | Control group | t value | P value |
| MI (%) | 0.62 ± 0.33 | 0.51 ± 0.21 | 0.88 | P > 0.05 |
| SPF (%) | 5.70 ± 1.93 | 4.90 ± 1.76 | 0.95 | P > 0.05 |
| PI (%) | 11.69 ± 1.74 | 9.93 ± 2.68 | 1.68 | P > 0.05 |
| Sub-G1 (%) | 2.31 ± 1.57 | 1.18 ± 0.45 | 2.18 | P < 0.05 |
| AI (%) | 20.33 ± 2.36 | 13.68 ± 1.97 | 3.07 | P < 0.05 |
Table 4.
t test value of MI, SPF, sub-G1 and PI of TACE groups and partial hepatectomy group on the third day after operations
| Index | TACE group | Partial hepatectomy group | t value | P value |
| MI(%) | 0.62 ± 0.33 | 0.81 ± 0.30 | 1.28 | P > 0.05 |
| SPF(%) | 5.70 ± 1.93 | 6.62 ± 1.56 | 1.34 | P > 0.05 |
| PI(%) | 11.69 ± 1.74 | 11.85 ± 2.00 | 0.17 | P > 0.05 |
| Sub-G1 (%) | 2.31 ± 1.57 | 2.05 ± 1.07 | 0.41 | P > 0.05 |
| AI(%) | 20.33 ± 2.36 | 22.69 ± 3.79 | 0.93 | P > 0.05 |
DISCUSSION
Transarterial chemoembolization (TACE) has been one of the most important and commonly used methods for treatment of hepatocarcinoma[14]. Because most patients died of liver failure after TACE[15,16], it is important to study the condition of postoperative normal liver tissues[17]. The most precise method to evaluate hepatocyte reproduction and apoptosis has been the study of cell cycle[18]. In our research, normal hepatocytes were isolated, fixed and stained, flow cytometry (FCM) was performed and DNA was analyzed. Results showed SPF, PI and MI value of TACE group was much higher than that of control group (P < 0.001, Table 1). We could conclude that there existed liver regeneration after TACE. From Table 2 at the same time, we could find that hepatocyte regeneration in PH group was stronger than that in TACE group on the first day (P < 0.05). On the third day after TACE, the difference between TACE group and control group was not significant in all indexes. Furthermore, the difference between TACE group and PH group was not significant either on the third day. From these data, we could conclude that the regeneration process stopped, most of the cells entered the dormancy phase (G0 phase). However, the value of hypodiploid peak (sub-G1) increased on the third day after TACE (t = 2.18, P < 0.05). The difference of sub-G1 between TACE group and PH group was obvious (P > 0.05, Table 3, Table 4). The apoptosis index (AI) became greater on the third day after TACE.
Cell apoptosis (programmed cell death) means the maintenance of stability of internal environment, i.e. the process of autonomic programmed cell death has been controlled by gene[19]. After partial hepatectomy, the hepatocytes would regenerate soon. At the peak of regeneration, cell apoptosis begins. Accompanying cell reproduction, cell apoptosis would eliminate the overgrown cells, and rebuilding of the tissue constitution is achieved. Some authors[20]pointed out that the process was mitogen→cell reproduction→regeneration→cell apoptosis. It was different from the necrosis caused by liver cytotoxic material, which was in the order of cytotoxic material→necrosis→compensatory hyperplasia→cell regeneration. Liver tissue regeneration after PH has been studied comprehensively. When the liver is resected less than 70%, the regeneration is proportional to the volume of the resected portion. Mitosis reaches the peak in 24 h, and completes in 72 h. Liver tissue regeneration exists also after TACE, this is due to the effect of super-selective embolization with iodized oil. The liver regeneration in TACE group was weaker than that in PH group, the reason was that the extent of “medical hepatectomy” was much smaller[21]. However, from Table 3 and Table 4, we could find the apoptosis levels between TACE and hepatectomy groups. The apoptosis level following liver regeneration was directly proportional to the regeneration level. It is easy to draw the conclusion that there must exist a new mechanism, which promotes cell apoptosis after TACE. The mechanism might be that mitomycin depressed DNA synthesis and promoted cell apoptosis[22]. Some authors[23] pointed out that mitomycin (5 mg/mL) could cause cell apoptosis after 24 h. Almost all chemotherapeutic drugs could induce cell apoptosis by different ways[24]. For example, vincristine (VCR) and colchicine could induce cell apoptosis by disturbance of microtubule function→loss of hepatocyte function→identification of the injury by hepatocytes→decrease of protein synthesis→increase of [Ca2+] activation of endonuclease and protein kinase→DNA split→cell apoptosis[25]. On the other hand, there were ischemia and hypoxia of locally embolized tissue, and release of a large amount of free radicals and cell toxins[26,27]. They could induce cell apoptosis[28,29]. For example, free radicals could injure DNA which would lead to activation of polydioadenosine phosphate pibotransferase[30]and accumulation of p53. A cell calcium ion would cause cell apoptosis. It could activate nucleus transcription factors such as NF-κB which would induce cell apoptosis[31]. Normal hepatocyte apoptosis was mainly resulted from the effects of chemotherapeutic drugs and free radical injury rather than from normal tissue repair[32,33].
The regeneration and apoptosis level of normal hepatocytes usually determine the patients’ prognosis after transarterial chemoembolization. The obvious apoptosis after TACE was due to chemotherapeutic drugs and free radicals, which made the normal process of apoptosis more rapid. Therefore, excessive dosage of chemotherapeutic drugs may promote apoptosis and injury of normal hepatocytes in non-neoplastic area of the liver, and deteriorate the liver function of patients. We can draw the conclusion that it is important to use chemotherapeutic drugs, because they can protect the liver function of patients and improve their survival rate after treatment.
Footnotes
Supported by the Natural Science Foundation of Hubei Province, No. 2002AB130
Edited by Wang XL and Zhu LH Proofread by Xu FM
References
- 1.El Khaddari S, Gaudin JL, Abidi H, Picaud G, Rode A, Souquet JC. [Chemoembolization in hepatocellular carcinoma: multivariate analysis of survival prognostic factors after the first session] Gastroenterol Clin Biol. 2002;26:728–734. [PubMed] [Google Scholar]
- 2.Lin WY, Chen J, Lin Y, Han K. Implantation of VX2 carcinoma into the liver of rabbits: a comparison of three direct-injection methods. J Vet Med Sci. 2002;64:649–652. doi: 10.1292/jvms.64.649. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Zheng CS, Feng GS, Zhuo CK, Zhao JG, Liu X. An implantable rat liver tumor model for experimental transarterial chemoembolization therapy and its imaging features. World J Gastroenterol. 2002;8:1035–1039. doi: 10.3748/wjg.v8.i6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiozawa S, Tsuchiya A, Endo S, Kumazawa K, Ogawa K. [Transradial approach for transcatheter arterial chemoembolization in patients with hepatocellular carcinoma] Nihon Shokakibyo Gakkai Zasshi. 2002;99:1450–1454. [PubMed] [Google Scholar]
- 5.Yi J, Liao X, Yang Z, Li X. Study on the changes in microvessel density in hepatocellular carcinoma following transcatheter arterial chemoembolization. J Tongji Med Univ. 2001;21:321–331. doi: 10.1007/BF02886568. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Li J, Zhang Y. [Transarterial chemoembolization with high dose iodized oil for the treatment of large hepatocellular carcinoma] Zhonghua Zhongliu Zazhi. 2001;23:165–167. [PubMed] [Google Scholar]
- 7.Pacella CM, Bizzarri G, Cecconi P, Caspani B, Magnolfi F, Bianchini A, Anelli V, Pacella S, Rossi Z. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219:669–678. doi: 10.1148/radiology.219.3.r01ma02669. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Li D, Yang S, Wu Y. [Efficacy of TACE in treatment of intraportal tumor thrombi with double helical CT] Hunan Yike Daxue Xuebao. 1999;24:47–49. [PubMed] [Google Scholar]
- 9.Kim HC, Kim AY, Han JK, Chung JW, Lee JY, Park JH, Choi BI. Hepatic arterial and portal venous phase helical CT in patients treated with transcatheter arterial chemoembolization for hepatocellular carcinoma: added value of unenhanced images. Radiology. 2002;225:773–780. doi: 10.1148/radiol.2253011346. [DOI] [PubMed] [Google Scholar]
- 10.Jia HS, Quan XY, Zeng S, Wen ZB. [Dynamic evaluation of rabbit VX2 hepatic carcinoma with CT and MRI] Diyi Junyi Daxue Xuebao. 2002;22:141–144. [PubMed] [Google Scholar]
- 11.Xiao E, Li D, Shen S, Zhou S, Tan L, Wang Y, Luo J, Wu Y, Tan C, Liu H, et al. Effect of preoperative transcatheter arterial chemoembolization on apoptosis of hepatocellular carcinoma cells. Chin Med J (Engl) 2003;116:203–207. [PubMed] [Google Scholar]
- 12.Sano B, Sugiyama Y, Kunieda K, Sano J, Saji S. Antitumor effects induced by the combination of TNP-470 as an angiogenesis inhibitor and lentinan as a biological response modifier in a rabbit spontaneous liver metastasis model. Surg Today. 2002;32:503–509. doi: 10.1007/s005950200085. [DOI] [PubMed] [Google Scholar]
- 13.Vogl TJ, Eichler K, Zangos S, Mack M, Hammerstingl R. [Hepatocellular carcinoma: Role of imaging diagnostics in detection, intervention and follow-up] Rofo. 2002;174:1358–1368. doi: 10.1055/s-2002-35349. [DOI] [PubMed] [Google Scholar]
- 14.Barone M, Ettorre GC, Ladisa R, Schiavariello M, Santoro C, Francioso G, Vinciguerra V, Francavilla A. Transcatheter arterial chemoembolization (TACE) in treatment of hepatocellular carcinoma. Hepatogastroenterology. 2003;50:183–187. [PubMed] [Google Scholar]
- 15.Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S211–S221. doi: 10.1016/s1051-0443(07)61789-8. [DOI] [PubMed] [Google Scholar]
- 16.Huang YS, Chiang JH, Wu JC, Chang FY, Lee SD. Risk of hepatic failure after transcatheter arterial chemoembolization for hepatocellular carcinoma: predictive value of the monoethylglycinexylidide test. Am J Gastroenterol. 2002;97:1223–1227. doi: 10.1111/j.1572-0241.2002.05709.x. [DOI] [PubMed] [Google Scholar]
- 17.Grieco A, Marcoccia S, Miele L, Marmiroli L, Caminiti G, Ragazzoni E, Cotroneo AR, Cefaro GA, Rapaccini GL, Gasbarrini G. Transarterial chemoembolization (TACE) for unresectable hepatocellular carcinoma in cirrhotics: functional hepatic reserve and survival. Hepatogastroenterology. 2003;50:207–212. [PubMed] [Google Scholar]
- 18.Schuchmann M, Galle PR. Apoptosis in liver disease. Eur J Gastroenterol Hepatol. 2001;13:785–790. doi: 10.1097/00042737-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, Arthur MJ, Iredale JP, Mann DA. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685–698. doi: 10.1053/gast.2001.27188. [DOI] [PubMed] [Google Scholar]
- 20.Neuman MG. Apoptosis in diseases of the liver. Crit Rev Clin Lab Sci. 2001;38:109–166. doi: 10.1080/20014091084182. [DOI] [PubMed] [Google Scholar]
- 21.Saccheri S, Lovaria A, Sangiovanni A, Nicolini A, De Fazio C, Ronchi G, Fasani P, Del Ninno E, Colombo M. Segmental transcatheter arterial chemoembolization treatment in patients with cirrhosis and inoperable hepatocellular carcinomas. J Vasc Interv Radiol. 2002;13:995–999. doi: 10.1016/s1051-0443(07)61863-6. [DOI] [PubMed] [Google Scholar]
- 22.Durand RE, LePard NE. Effects of mitomycin C on the oxygenation and radiosensitivity of murine and human tumours in mice. Radiother Oncol. 2000;56:245–252. doi: 10.1016/s0167-8140(00)00180-8. [DOI] [PubMed] [Google Scholar]
- 23.Castañeda F, Kinne RK. Effects of doxorubicin, mitomycin C, and ethanol on Hep-G2 cells in vitro. J Cancer Res Clin Oncol. 1999;125:1–8. doi: 10.1007/s004320050235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato N, Mizumoto K, Maehara N, Kusumoto M, Nishio S, Urashima T, Ogawa T, Tanaka M. Enhancement of drug-induced apoptosis by antisense oligodeoxynucleotides targeted against Mdm2 and p21WAF1/CIP1. Anticancer Res. 2000;20:837–842. [PubMed] [Google Scholar]
- 25.Crenesse D, Gugenheim J, Hornoy J, Tornieri K, Laurens M, Cambien B, Lenegrate G, Cursio R, De Souza G, Auberger P, et al. Protein kinase activation by warm and cold hypoxia- reoxygenation in primary-cultured rat hepatocytes-JNK(1)/SAPK(1) involvement in apoptosis. Hepatology. 2000;32:1029–1036. doi: 10.1053/jhep.2000.19065. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Ari Z, Hochhauser E, Burstein I, Papo O, Kaganovsky E, Krasnov T, Vamichkim A, Vidne BA. Role of anti-tumor necrosis factor-alpha in ischemia/reperfusion injury in isolated rat liver in a blood-free environment. Transplantation. 2002;73:1875–1880. doi: 10.1097/00007890-200206270-00004. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi T, Sugawara Y, Ohkubo T, Imamura H, Makuuchi M. Effects of amrinone on hepatic ischemia-reperfusion injury in rats. J Hepatol. 2002;37:31–38. doi: 10.1016/s0168-8278(02)00084-3. [DOI] [PubMed] [Google Scholar]
- 28.Barros LF, Stutzin A, Calixto A, Catalán M, Castro J, Hetz C, Hermosilla T. Nonselective cation channels as effectors of free radical-induced rat liver cell necrosis. Hepatology. 2001;33:114–122. doi: 10.1053/jhep.2001.20530. [DOI] [PubMed] [Google Scholar]
- 29.Karbowski M, Kurono C, Wozniak M, Ostrowski M, Teranishi M, Nishizawa Y, Usukura J, Soji T, Wakabayashi T. Free radical-induced megamitochondria formation and apoptosis. Free Radic Biol Med. 1999;26:396–409. doi: 10.1016/s0891-5849(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 30.Song BC, Suh DJ, Yang SH, Lee HC, Chung YH, Sung KB, Lee YS. Lens culinaris agglutinin-reactive alpha-fetoprotein as a prognostic marker in patients with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization. J Clin Gastroenterol. 2002;35:398–402. doi: 10.1097/00004836-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Factor V, Oliver AL, Panta GR, Thorgeirsson SS, Sonenshein GE, Arsura M. Roles of Akt/PKB and IKK complex in constitutive induction of NF-kappaB in hepatocellular carcinomas of transforming growth factor alpha/c-myc transgenic mice. Hepatology. 2001;34:32–41. doi: 10.1053/jhep.2001.25270. [DOI] [PubMed] [Google Scholar]
- 32.Won JY, Lee DY, Lee JT, Park SI, Kim MJ, Yoo HS, Suh SH, Park SJ. Supplemental transcatheter arterial chemoembolization through a collateral omental artery: treatment for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2003;26:136–140. doi: 10.1007/s00270-002-2629-y. [DOI] [PubMed] [Google Scholar]
- 33.Zaragoza A, Díez-Fernández C, Alvarez AM, Andrés D, Cascales M. Mitochondrial involvement in cocaine-treated rat hepatocytes: effect of N-acetylcysteine and deferoxamine. Br J Pharmacol. 2001;132:1063–1070. doi: 10.1038/sj.bjp.0703909. [DOI] [PMC free article] [PubMed] [Google Scholar]


