Abstract
AIM: To investigate the effect of transcatheter arterial embolization (TAE) on angiogenesis of hepatic tumor.
METHODS: Twenty New Zealand White rabbits were randomly divided into two groups of 10 each and VX2 carcinoma was implanted in the left medial lobes of the livers. Fourteen days later, a silicon catheter was inserted into the left hepatic artery of rabbit with VX2 hepatic tumor and infusion was performed via the hepatic artery using Lipiodol (the TAE group) or saline (the control group). Rabbits were sacrificed 7 d after treatment and tumor tissues were excised. Expression of vascular endothelial growth factor (VEGF) protein and microvessel density (MVD) of tumors were examined using immunohistochemistry. The staining intensity of VEGF was evaluated with a computer-assisted image-analyzer. Reverse transcription-polymerase chain reaction (RT-PCR) was used to detect the VEGF mRNA expression of tumors.
RESULTS: MVD was higher in the TAE group compared with the control group (28.6 ± 10.6 vs 16.3 ± 6.9, P < 0.01). Expression of VEGF protein was enhanced after TAE. The staining intensity of VEGF in the TAE group was 0.162 ± 0.018, significantly higher than in the control group (0.142 ± 0.01, P < 0.01). At mRNA level, VEGF165 mRNA was significantly higher in the TAE group compared with the control group (2.58 ± 0.42 vs 1.99 ± 0.21, P < 0.001). MVD was well correlated to VEGF expression in both the TAE group (r = 0.69, P < 0.05) and the control group (r = 0.72, P < 0.05).
CONCLUSION: TAE promotes the development of neovascularization of residual tumors through up-regulation of VEGF expression, possibly due to hypoxic insult.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in Asia, including China. Surgical resection is still the treatment of first choice for patients with HCC nowadays[1]. However, because of tumor extension, poor hepatic functional reserve due to underlying liver cirrhosis, or both, only a small portion of patients are suitable candidates for surgery[2-4]. Transcatheter arterial chemoembolization (TACE) is considered to be an effective treatment for patients with HCC who are not candidates for surgical resection[5,6]. TACE could reduce tumor size[7-8]. However, intrahepatic or extrahepatic metastasis after TACE is the major factor limiting its overall therapeutic effect[9].
Angiogenesis, the process leading to the formation of new blood vessels from preexisting vessels, plays a central role in the survival of tumor cells, in local tumor growth, and in the development of distant metastasis[10]. Microvessel density (MVD) has been the gold standard for the quantification of tumor angiogenesis, showing a good correlation with the prognosis in a variety of cancers including HCC[11-15]. Among the known angiogenic factors produced by tumor cells, vascular endothelial growth factor (VEGF) is one of the most potent angiogenic factors involved in tumor development. VEGF is a specific mitogenic factor for vascular endothelial cells, which stimulates endothelial cells proliferation, promotes neovascularization and increases vascular permeability. Evidence from preclinical and clinical studies indicates VEGF is associated with formation of metastases and poor prognosis in various cancers[16-18].
VEGF protein is produced by alternative splicing of the primary transcript leading to the production of at least four major isoforms of VEGF: 121, 165, 189, and 206[19]. VEGF189 and VEGF206 contain heparin-binding domains and are highly concentrated in the extracellular matrix. VEGF165 and VEGF121 are diffusible and predominate in most tissues, lacking this particular domain. The presence or absence of these domains affects the properties, actions, and distribution of the isoform produced[19].
Little is known of the changes of angiogenesis and VEGF expression in HCC after TACE. Thus, we established a rabbit model of transcatheter arterial embolization (TAE) with hepatic VX2 carcinoma, and examined MVD and VEGF expression in the hepatic tumors after TAE.
MATERIALS AND METHODS
Implantation procedure
The rabbit VX2 carcinoma was selected for implantation in the liver because of the similarities of its blood supply to that of human hepatomas[20,21]. The VX2 strain (obtained from Dr. Li Xin of Department of Radiology, the Union Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China) was maintained by serial passage in rabbit hindlimbs. Twenty male New Zealand White rabbits (3.0 to 3.5 kg) were used for all experiments. Studies with these animals were approved by the Animal Care and Use Committee of Tongji Medical College of Huazhong University of Science and Technology and carried out according to their guidelines. Tumor implantation was performed as described previously[22] with a slight modification. Briefly, rabbits were anesthetized by i.v. injection of sodium pentobarbital (30 mg/kg) via a marginal ear vein. The abdominal area was shaved and sterilized, and a midline subxyphoid incision was made. The left medial lobe of rabbit liver was gently exposed, and one piece of the VX2 tumor tissue excised from the carrier rabbit and broken into fine pieces (1 mm × 1 mm × 1 mm in size per piece) in Hanks’ solution was implanted into the subcapsule of the left medial hepatic lobe. The implanted site was closed with gelatin sponge to prevent the leakage of tumor cells into the peritoneal cavity. The hepatic lobe was then returned to the abdominal cavity. This method allows the growth of a single solitary, well-demarcated tumor in the liver of each recipient rabbit. The incision was closed in two layers. The tumors were allowed to grow for 2 wk, and studies were performed when the tumors reached 1 to 2 cm in diameter.
TAE procedure
Twenty rabbits were divided into two groups of 10 rabbits each randomly. The animals were relaparotomized through a midline abdominal incision under the general anesthesia as described above, and a silicone catheter (outer diameter 0.8 mm) with a tip in the shape of a hockey-stick was inserted retrogradely into the left hepatic artery via the gastroduodenal artery under the operating microscope (Zeiss OPMI 6-S, Aalen, Germany). The catheter was tightly secured and hepatic arterial infusion was made, with 0.5 mL/kg of lipiodol in TAE group or with the same volume of saline in control group. After the injection, the catheter was removed and the gastroduodenal artery was ligated. The incision was closed in two layers. CT scan was performed 2 d after TAE, and confirmed that the lipiodol had been retained in the tumors of all cases (Figure 1).
Figure 1.
CT scan 2 d following lipiodol infusion.
Tissue preparation
The rabbits were sacrificed 7 d after treatment, and the tumor was removed. Part of the specimen was flash-frozen at –70 °C for RT-PCR, and the rest was fixed in 40 g/L formaldehyde and embedded in paraffin for immunohistochemical study.
Immunohistochemistry
Consecutive 4-μm sections were cut and mounted on glass slides. Sections were stained with H&E. VEGF expression was examined immunohistochemically with an anti-VEGF mouse monoclonal antibody (JH121, Neomarkers, Inc., Fremont, CA) at a dilution of 1:80, and by using streptavidin-peroxidase technique with SPtm kit (Zymed Laboratories, Inc., USA). The JC/70 monoclonal antibody (Dako, Glostrup, Denmark) against CD31 was used for microvessel staining using the LSAB method (Dako LSAB kit; Glostrup, Denmark). Negative controls were prepared by PBS substitution for the primary antibodies staining, and known positive controls were included in each staining run.
Morphometric analyses
The positive expression cells of VEGF were ones with brown-yellow granular patterns in the cytoplasm. In intensively positive staining area, three fields of vision were selected at × 400 magnification. Staining intensity of VEGF was assessed morphometrically with a computer-assisted image-analyzer (HPIAS-1000 high precision color-image measure system, Tongji Qiangping Image Engineer Company). The image analyzer is an integrated system of Windows-based software specially designed for immunohistochemical analysis.
Determination of MVD
MVD was evaluated according to the method described by Gasparini et al.[23] The stained sections were screened at low power field (× 40), and five areas with the most intense neovascularization (hot spots) were selected. Microvessel counts of these areas were performed at high power field (× 200). Any brown-stained endothelial cell or endothelial cell cluster clearly separated from adjacent microvessels, tumor cells, and other connective-tissue elements was counted as one microvessel, irrespective of the presence of a vessel lumen. The mean microvessel count of the five richest vascular areas was taken as the MVD, which was expressed as the absolute number of microvessels per 0.74 mm2 (× 200).
RNA preparation and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TriZol reagent (Life Technologies, Grand Island, NY) according to the manufacturer’s recommendations. Briefly, the tissue was homogenized with a Polytron homogenizer in 1 mL TriZol per 100 mg tissue. The suspension was left at room temperature for 10 min before being centrifuged at 12000 g at 4 °C for 10 min. The aqueous supernatant containing the RNA was carefully removed and transferred to a fresh tube. Total RNA was precipitated by addition of isopropanol and centrifuged at 12000 g at 4 °C for 10 min. The RNA pellet was washed with 750 mL/L ethanol and re-dissolved in DEPC-treated water. Before being used in RT-PCR these samples were phenol/chloroform extracted and phenol precipitated. The RNA concentration and quality were determined by UV spectrophotometer at absorbances of 260 and 280 nm.
cDNA was synthesized from total RNA with Avian Myeloblastosis Virus and oligo dT-Adaptor Primer (TaKaRa RNA PCR Kit, TaKaRa Bioteth., Dalian, CHN) according to the manufacturer’s instructions. Amplification of cDNA was performed using published sense and antisense primers for rabbit β-actin and VEGF[19,24,25]. The primer sequence for β-actin sense was 5’-CCTTCCTGCGCATGGAGTCCTGG-3’, and the primer sequence for β-actin antisense was 5’-GGAGCAATGATCTTGATCTTC-3’. The sense primer sequence for VEGF was 5’-CAGTGAATTCGAGATGAGCTTCCTACAGCAC-3’, and the antisense primer sequence was 5’-CCTGGAATTCTCACCGCCTCGGCTTGTCAC-3’. The PCR cycle profile was at 94 °C for 30 s, 59 °C for 60 s and 72 °C for 60 s, with 30 cycles.
After visualization of the PCR products by 20 g/L agarose gel electrophoresis with ethidium bromide staining gel, images were obtained and the densities of the products were quantified using a digital gel image analysis system (GDS8000, UVP Co., UK). The PCR fragments were identified according to their molecular mass using a DNA marker (DL2000 marker, TaKaRa Bioteth., Dalian, China). The relative expression levels were calculated as the density of the product of the respective target genes after normalization with the β-actin internal control.
Statistical analysis
All data are expressed as mean ± SD. Differences in staining intensity of VEGF, quantity of variant VEGF isoforms and MVD between the TAE group and the control group were compared with Student’s t test. The correlation of VEGF and MVD was performed with linear regression analysis. P < 0.05 was considered statistically significant. Statistical analysis was done using SPSS 10.0 for Windows.
RESULTS
MVD and its pathologic features
Figure 2A shows microvessels were stained in brown color. Microvessels were heterogeneously distributed inside the tumor, and, in general, maximal density was observed near the margins of the invasive tumor or in the central regions of tumor where necrosis could be seen. The MVD was 28.6 ± 10.6 in the TAE group, and 16.3 ± 6.9 in the control group. The MVD was significantly higher in the TAE group than that of the control group (P < 0.01).
Figure 2.
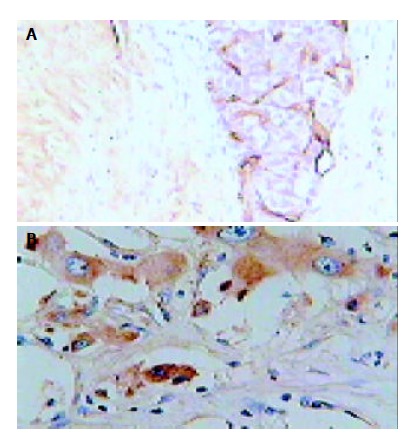
Immunohistochemical staining in hepatic tumor tis-sues obtained from a rabbit underwent TAE. A: CD31, origi-nal magnification × 200; B: VEGF, original magnification × 400.
Expression of VEGF protein
Immunoreactive products of anti-VEGF antibody were positively detected in the cytoplasm of hepatoma cells and scarcely detected in endothelial cells. In general, hepatoma cells showed stronger VEGF immunoreactivity than endothelial cells. The intensity of immunoreactive products was heterogeneous (Figure 2B). The staining intensity of VEGF in the TAE group was 0.162 ± 0.018, significantly higher compared to the control group (0.142 ± 0.01, P < 0.01).
There was a significant correlation between MVD and VEGF protein expression in both the TAE group (r = 0.69, P < 0.05) and the control group (r = 0.72, P < 0.05).
Expression of VEGF mRNA
PCR amplification of cDNA prepared from frozen sections showed the expression of three bands of 110, 242 and 314 bp corresponding to VEGF121, VEGF165 and VEGF189, respectively (Figure 3A). The band corresponding to VEGF206 was not detected. In both the TAE group and the control group, VEGF165 was strongly detected (Figure 3B). The intensity of VEGF165 mRNA was significantly higher in the TAE group (2.58 ± 0.42), as compared with the control group (1.99 ± 0.21, P < 0.001). Although the signal of VEGF121 mRNA was slightly higher in the TAE group (1.14 ± 0.19) than that of the control group (0.99 ± 0.11), there was no statistical significance. No significant difference between VEGF189 mRNA in the TAE group (1.03 ± 0.14) and the control group (1.01 ± 0.11) was demonstrated (Figure 3B).
Figure 3.
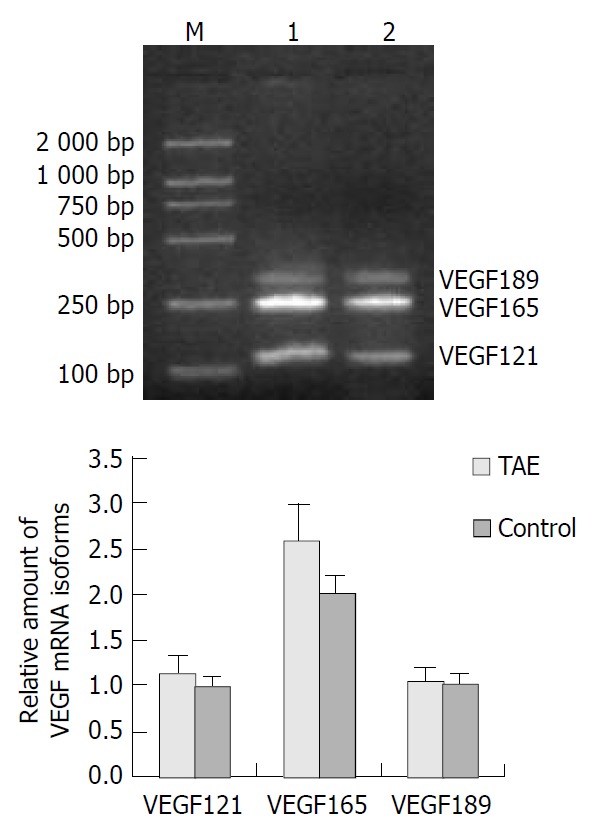
Expression of VEGF mRNA in hepatic tumor tissues. A: Results of RT-PCR of VEGF mRNA in TAE (lane 1), control (lane 2) and M (molecular mass marker); B: quantification of RT-PCR data.
DISCUSSION
TACE has been accepted as one of the most effective palliative treatments for patients with unresectable HCC on the basis of the characterization of its blood supply. Since the blood supply of HCC is derived almost exclusively from hepatic arteries, hepatoma cells undergo ischemic necrosis in TAE. The addition of chemotherapy aimed to enhance the antitumor effect of the ischemia. However, it was only a small proportion of HCC that occurred complete necrosis after TACE[26]. We have occasionally noticed rapid growth and extensive metastasis after multiple TACE treatments. TACE prior to surgical resection may facilitate recurrence of HCC[27]. The recurrence rate after 3 years of treatment is 30%-60%[28,29]. It is possible that in addition to the limited ability of chemoembolization to eliminate cancer cells, it may play a role in enhancing the malignant potential of the tumor and the ability of some cells to escape necrosis after treatment[30]. Huang et al[26] and Kim et al[31] reported that the proliferative activity of tumor cells was increased after TACE.
In the present study, we investigated the change of angiogenesis in VX2 hepatic tumor that underwent TAE using immunohistochemical techniques. The results showed that MVD of the tumors markedly increased after TAE. MVD is currently considered the gold standard for histological assessment of the degree of angiogenesis within a tumor. It is an independent predictor for the growth, metastasis and prognosis of tumor. Increasing evidence suggests that there is a clear relationship between the MVD and HCC prognosis[1,14,32,33]. These findings thus provide evidence that the development of neovascularization in hepatic residual tumors after TAE may be one of the reasons why the majority of tumor nodes do not show complete necrosis and why tumors have an increased tendency to metastasize after TAE.
Until recently, VEGF was the only growth factor proven to be specific and critical for blood vessel formation[34]. Many studies have indicated that VEGF is not only correlated with HCC angiogenesis, but also closely correlated with the invasion and metastasis of HCC. The levels of VEGF in tumor and peripheral blood of patients with HCC might be a useful indicator for both HCC metastasis and poor prognosis[18,35-41]. In our study, the expression of VEGF protein was well correlated to MVD, and the level of VEGF expression in TAE group was increased significantly compared with the control. These findings show that VEGF plays a vital role in the process of angiogenesis in hepatic tumors after TAE.
It has been known that four alternatively spliced products can be generated from the single VEGF gene, yielding different protein products composed of 121, 165, 189 and 206 amino acids, designated as VEGF121, VEGF165, VEGF189 and VEGF206, respectively. Among them, only VEGF121 and VEGF165 are secreted and can induce mitogenesis of endothelial cells; VEGF189 and VEGF206 are membrane anchored and act as vascular permeability factors. To identify the changes of VEGF mRNA isoform expression in hepatic tumors after TAE, we performed RT-PCR. In our study three bands of 110, 242 and 314 bp were shown, corresponding to VEGF121, VEGF165 and VEGF189, respectively. The major VEGF isoform expressed in VX2 hepatic tumor was VEGF165. The significantly enhanced expression of VEGF165 mRNA in the TAE group was observed compared with the control group. Although VEGF121 mRNA was slightly higher in the TAE group than in the control group, there was no statistical significance. We detected no VEGF206 mRNA, consistent with the very restricted expression of this splice variant.
The possible reason for up-regulation of VEGF expression after embolization was anoxia and ischemia of tumor tissues. Hypoxia is known to be a very important stimulus for the new vessel formation seen in tumor angiogenesis by stimulating the expression of VEGF[42,43]. Kim et al[44] reported that hypoxia might induce HCC cells to secrete more VEGF and IGF-II. Moreover, the combination of hypoxia and IGF-II brought an additional induction of VEGF. In the current study, it was found that more microvessel was observed in the tumor margins or areas around the necrotic tissues, which might be resulted from more severe local anoxia in these areas.
Song et al[45] reported that plasma IGF-II level was increased after TACE, which implied that expression of other angiogenic factors besides VEGF might also be changed after TACE.
In conclusion, these results indicate that TAE is involved in the development of neovascularization of hepatic residual tumors after TAE through up-regulation of VEGF expression, possibly due to hypoxic insult. Therefore, the combination of TAE and antiangiogenic therapy may be superior to TAE alone in the treatment of HCC.
Footnotes
Edited by Zhu LH and Chen WW Proofread by Xu FM
References
- 1.Tang Z, Zhou X, Lin Z, Yang B, Ma Z, Ye S, Wu Z, Fan J, Liu Y, Liu K, et al. Surgical treatment of hepatocellular carcinoma and related basic research with special reference to recurrence and metastasis. Chin Med J (Engl) 1999;112:887–891. [PubMed] [Google Scholar]
- 2.Yan FH, Zhou KR, Cheng JM, Wang JH, Yan ZP, Da RR, Fan J, Ji Y. Role and limitation of FMPSPGR dynamic contrast scanning in the follow-up of patients with hepatocellular carcinoma treated by TACE. World J Gastroenterol. 2002;8:658–662. doi: 10.3748/wjg.v8.i4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faivre J, Forman D, Estève J, Obradovic M, Sant M. Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe. EUROCARE Working Group. Eur J Cancer. 1998;34:2184–2190. doi: 10.1016/s0959-8049(98)00330-x. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology. 2001;33:62–65. doi: 10.1053/jhep.2001.21041. [DOI] [PubMed] [Google Scholar]
- 5.Acunaş B, Rozanes I. Hepatocellular carcinoma: treatment with transcatheter arterial chemoembolization. Eur J Radiol. 1999;32:86–89. doi: 10.1016/s0720-048x(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 6.Tang ZY. Hepatocellular carcinoma. J Gastroenterol Hepatol. 2000;15 Suppl:G1–G7. doi: 10.1046/j.1440-1746.2000.02257.x. [DOI] [PubMed] [Google Scholar]
- 7.Poon RT, Ngan H, Lo CM, Liu CL, Fan ST, Wong J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol. 2000;73:109–114. doi: 10.1002/(sici)1096-9098(200002)73:2<109::aid-jso10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 9.Liou TC, Shih SC, Kao CR, Chou SY, Lin SC, Wang HY. Pulmonary metastasis of hepatocellular carcinoma associated with transarterial chemoembolization. J Hepatol. 1995;23:563–568. doi: 10.1016/0168-8278(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 11.Zheng HC, Sun JM, Li XH, Yang XF, Zhang YC, Xin Y. Role of PTEN and MMP-7 expression in growth, invasion, metastasis and angiogenesis of gastric carcinoma. Pathol Int. 2003;53:659–666. doi: 10.1046/j.1440-1827.2003.01542.x. [DOI] [PubMed] [Google Scholar]
- 12.Abramson LP, Grundy PE, Rademaker AW, Helenowski I, Cornwell M, Katzenstein HM, Reynolds M, Arensman RM, Crawford SE. Increased microvascular density predicts relapse in Wilms' tumor. J Pediatr Surg. 2003;38:325–30; discussion 325-330. doi: 10.1053/jpsu.2003.50102. [DOI] [PubMed] [Google Scholar]
- 13.Pruneri G, Ponzoni M, Ferreri AJ, Decarli N, Tresoldi M, Raggi F, Baldessari C, Freschi M, Baldini L, Goldaniga M, et al. Microvessel density, a surrogate marker of angiogenesis, is significantly related to survival in multiple myeloma patients. Br J Haematol. 2002;118:817–820. doi: 10.1046/j.1365-2141.2002.03654.x. [DOI] [PubMed] [Google Scholar]
- 14.Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, Wong J. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775–1785. doi: 10.1200/JCO.2002.07.089. [DOI] [PubMed] [Google Scholar]
- 15.Ng IO, Poon RT, Lee JM, Fan ST, Ng M, Tso WK. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116:838–845. doi: 10.1309/FXNL-QTN1-94FH-AB3A. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi R, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K. Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tu-mor of the stomach. Oncology. 2003;64:266–274. doi: 10.1159/000069316. [DOI] [PubMed] [Google Scholar]
- 17.White JD, Hewett PW, Kosuge D, McCulloch T, Enholm BC, Carmichael J, Murray JC. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 2002;62:1669–1675. [PubMed] [Google Scholar]
- 18.Torimura T, Sata M, Ueno T, Kin M, Tsuji R, Suzaku K, Hashimoto O, Sugawara H, Tanikawa K. Increased expression of vascular endothelial growth factor is associated with tumor progression in hepatocellular carcinoma. Hum Pathol. 1998;29:986–991. doi: 10.1016/s0046-8177(98)90205-2. [DOI] [PubMed] [Google Scholar]
- 19.Watkins RH, D'Angio CT, Ryan RM, Patel A, Maniscalco WM. Differential expression of VEGF mRNA splice variants in newborn and adult hyperoxic lung injury. Am J Physiol. 1999;276:L858–L867. doi: 10.1152/ajplung.1999.276.5.L858. [DOI] [PubMed] [Google Scholar]
- 20.Geschwind JF, Artemov D, Abraham S, Omdal D, Huncharek MS, McGee C, Arepally A, Lambert D, Venbrux AC, Lund GB. Chemoembolization of liver tumor in a rabbit model: assessment of tumor cell death with diffusion-weighted MR imaging and histologic analysis. J Vasc Interv Radiol. 2000;11:1245–1255. doi: 10.1016/s1051-0443(07)61299-8. [DOI] [PubMed] [Google Scholar]
- 21.Ko YH, Pedersen PL, Geschwind JF. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett. 2001;173:83–91. doi: 10.1016/s0304-3835(01)00667-x. [DOI] [PubMed] [Google Scholar]
- 22.Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002;62:3909–3913. [PubMed] [Google Scholar]
- 23.Gasparini G, Harris AL. Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J Clin Oncol. 1995;13:765–782. doi: 10.1200/JCO.1995.13.3.765. [DOI] [PubMed] [Google Scholar]
- 24.Ozaki NK, Beharry KD, Nishihara KC, Akmal Y, Ang JG, Sheikh R, Modanlou HD. Regulation of retinal vascular endothelial growth factor and receptors in rabbits exposed to hyperoxia. Invest Ophthalmol Vis Sci. 2002;43:1546–1557. [PubMed] [Google Scholar]
- 25.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 26.Huang J, He X, Lin X, Zhang C, Li J. Effect of preoperative transcatheter arterial chemoembolization on tumor cell activity in hepatocellular carcinoma. Chin Med J (Engl) 2000;113:446–448. [PubMed] [Google Scholar]
- 27.Adachi E, Matsumata T, Nishizaki T, Hashimoto H, Tsuneyoshi M, Sugimachi K. Effects of preoperative transcatheter hepatic arterial chemoembolization for hepatocellular carcinoma. The relationship between postoperative course and tumor necrosis. Cancer. 1993;72:3593–3598. doi: 10.1002/1097-0142(19931215)72:12<3593::aid-cncr2820721208>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, Miyayama S, Takashima T, Unoura M, Kogayashi K. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79–83. doi: 10.1148/radiology.188.1.8390073. [DOI] [PubMed] [Google Scholar]
- 29.Ohnishi K, Yoshioka H, Kosaka K, Toshima K, Nishiyama J, Kameda C, Ito S, Fujiwara K. Treatment of hypervascular small hepatocellular carcinoma with ultrasound-guided percutaneous acetic acid injection: comparison with segmental transcatheter arterial embolization. Am J Gastroenterol. 1996;91:2574–2579. [PubMed] [Google Scholar]
- 30.Seki T, Tamai T, Ikeda K, Imamura M, Nishimura A, Yamashiki N, Nakagawa T, Inoue K. Rapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percutaneous radiofrequency ablation in the primary tumour region. Eur J Gastroenterol Hepatol. 2001;13:291–294. doi: 10.1097/00042737-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Kim YB, Park YN, Park C. Increased proliferation activities of vascular endothelial cells and tumour cells in residual hepato-cellular carcinoma following transcatheter arterial embolization. Histopathology. 2001;38:160–166. doi: 10.1046/j.1365-2559.2001.01064.x. [DOI] [PubMed] [Google Scholar]
- 32.Sun HC, Tang ZY, Li XM, Zhou YN, Sun BR, Ma ZC. Microvessel density of hepatocellular carcinoma: its relationship with prognosis. J Cancer Res Clin Oncol. 1999;125:419–426. doi: 10.1007/s004320050296. [DOI] [PubMed] [Google Scholar]
- 33.Tanigawa N, Lu C, Mitsui T, Miura S. Quantitation of sinusoid-like vessels in hepatocellular carcinoma: its clinical and prognostic significance. Hepatology. 1997;26:1216–1223. doi: 10.1053/jhep.1997.v26.pm0009362365. [DOI] [PubMed] [Google Scholar]
- 34.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 35.Jinno K, Tanimizu M, Hyodo I, Nishikawa Y, Hosokawa Y, Doi T, Endo H, Yamashita T, Okada Y. Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroenterol. 1998;33:376–382. doi: 10.1007/s005350050099. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Tang ZY, Fan J, Wu ZQ, Li XM, Liu YK, Liu F, Sun HC, Ye SL. Expression of platelet-derived endothelial cell growth factor and vascular endothelial growth factor in hepatocellular carcinoma and portal vein tumor thrombus. J Cancer Res Clin Oncol. 2000;126:57–61. doi: 10.1007/s004320050009. [DOI] [PubMed] [Google Scholar]
- 37.Iguchi H, Yokota M, Fukutomi M, Uchimura K, Yonemasu H, Hachitanda Y, Nakao Y, Tanaka Y, Sumii T, Funakoshi A. A possible role of VEGF in osteolytic bone metastasis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2002;21:309–313. [PubMed] [Google Scholar]
- 38.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Huber J, Nakatani T, Tsujinoue H, Yanase K, et al. Synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in murine hepatocellular carcinoma. Hepatology. 2002;35:834–842. doi: 10.1053/jhep.2002.32541. [DOI] [PubMed] [Google Scholar]
- 39.Chao Y, Li CP, Chau GY, Chen CP, King KL, Lui WY, Yen SH, Chang FY, Chan WK, Lee SD. Prognostic significance of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin in patients with resectable hepatocellular carcinoma after surgery. Ann Surg Oncol. 2003;10:355–362. doi: 10.1245/aso.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Poon RT, Ng IO, Lau C, Zhu LX, Yu WC, Lo CM, Fan ST, Wong J. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann Surg. 2001;233:227–235. doi: 10.1097/00000658-200102000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorrin-Rivas MJ, Arii S, Mori A, Takeda Y, Mizumoto M, Furutani M, Imamura M. Implications of human macrophage metalloelastase and vascular endothelial growth factor gene expression in angiogenesis of hepatocellular carcinoma. Ann Surg. 2000;231:67–73. doi: 10.1097/00000658-200001000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Marschall Z, Cramer T, Höcker M, Finkenzeller G, Wiedenmann B, Rosewicz S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut. 2001;48:87–96. doi: 10.1136/gut.48.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 44.Kim KW, Bae SK, Lee OH, Bae MH, Lee MJ, Park BC. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res. 1998;58:348–351. [PubMed] [Google Scholar]
- 45.Song BC, Chung YH, Kim JA, Lee HC, Yoon HK, Sung KB, Yang SH, Yoo K, Lee YS, Suh DJ. Association between insulin-like growth factor-2 and metastases after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma: a prospective study. Cancer. 2001;91:2386–2393. [PubMed] [Google Scholar]


