Abstract
AIM: To evaluate the role of survivin and caspase-3 in apoptosis of gastric carcinoma, as well as in prognosis of patients with gastric carcinoma.
METHODS: Expressions of survivin and caspase-3 were investigated immunohistochemically in 80 gastric carcinoma patients without a history of chemo-radiation therapy. Tumor cell apoptosis was examined by TUNEL method.
RESULTS: Immunohistochemical analysis showed that survivin expression was positive in 61 of 80 patients (76%) with gastric carcinoma. In contrast, no expression of survivin in adjacent normal tissues was detected. Expression level of caspase-3 was higher in normal tissues than in carcinoma. Patients with higher expression of survivin had worse histological grades and pathological stages. Expression of caspase-3 was significantly associated with histological stages, but not with the pathological stages. Although survivin expression in carcinoma was not inversely related to caspase-3, patients with survivin (-) and caspase-3 (+) had the maximum apoptosis index.
CONCLUSION: Expression level of survivin was associated with histological grades and pathological stages of the tumor, indicating that survivin may be a poor prognosis factor for gastric carcinoma. Unlike caspase-3, survivin (an apoptosis inhibitor) can markedly inhibit the apoptosis of tumor cells.
INTRODUCTION
Abnormalities in cell death control are implicated as a cause or contributing factor in a range of diseases, including cancer, autoimmunity, and degenerative disorders[1]. This control involves several proteins that promote or inhibit apoptosis and an evolutionarily conserved multistep cascade[2]. A number of proteins, such as Bcl-2, Fas and Bax affect upstream of the cascade[3,4]. Survivin, a recently discovered inhibitor of apoptosis, may prolong cell survival by targeting the terminal effector caspase-3[5,6]. Located at the end of cascade, caspase-3 acts as both initiators and executors in the apoptotic process. So survivin and caspase-3 have been the focus of debate regarding apoptosis.
In the last decade, molecular abnormalities of tumor cells have emerged as important prognostic indicators of gastric carcinoma. As a candidate molecule to influence the apoptosis balance, survivin has unique properties such as undetectable in normal adults tissues and overexpression in a variety of human cancers in vivo[7]. Although studies indicated that survivin was a prognostic tumor marker[8-13], little is known about its potential role in gastric carcinoma. In this study we sought to investigate the expression of survivin and caspase-3 in gastric carcinoma and to dissect their potential prognostic value, and discuss the relationship between survivin, caspase-3 and tumor cell apoptosis.
MATERIALS AND METHODS
Patients and samples
A total of 80 patients with gastric adenocarcinoma did not receive any treatment prior to surgery. Of them 56 were males and 24 were females, with a mean age of 60 years. Surgically resected specimens were fixed in 10% neutral formalin, embedded in paraffin, and stained by haematoxylin-eosin. Histological grades and pathological stages were conformed to the criteria of UICC (Figure 1). The subjects consisted of 17 cases in stage I, 34 cases in stage II, and 29 cases in stage III. Tumor tissues and normal tissues from every patient were detected.
Figure 1.
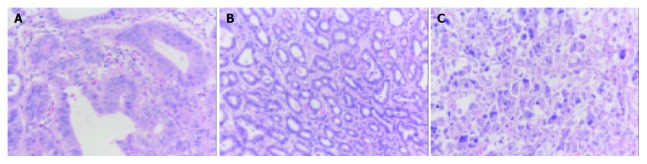
HE staining of gastric carcinoma. A: well differentiated gastric carcinoma; B: Moderately differentiated gastric carcinoma; C: Poorly differentiated gastric carcinoma (Original magnification: × 200).
Immunohistochemical staining for survivin and caspase-3
A pilot study using the anti-survivin antibody and anti-caspase-3 antibody was conducted on various neoplasms, including gastric carcinoma, lung cancer, breast cancer and non-Hodgkin’s lymphoma to determine an appropriate dilution. The immunostaining was performed, and negative control slides processed without primary antibody were incubated for each staining. Paraffin-embedded slides were deparaffinized and put in 400 mL EDTA solution (0.001 mol/L, pH6.0). Then the solution was heated in a pressure cooker and boiled for 2 min while maintaining the pressure. After cooling the slides were incubated with the primary antibody (mouse anti-human survivin or caspase-3 monoclonal antibody purchased from NEO MAEKERS) overnight at 4 °C and rinsed by PBS (0.01 mol/L, pH7.4) three times. Then the slides were incubated with an anti-mouse conjugate containing horseradish peroxidase at 37 °C for 30 min and rinsed by PBS three times. Finally 3,3-diaminobenzidine was used for color development and hematoxylin was used for counterstaining. The mean percentage of positive tumor cells was determined in at least five areas at 400-fold magnification and assigned to one of the following five categories[14]: -, < 5%; +, 5%-25%; ++, 26%-50%; +++, 51%-75%; ++++, > 75%.
Histochemical detection of apoptosis
All cases received detection of apoptosis except those with both survivin and caspase-3 negativities. Apoptotic cells and apoptotic bodies were detected by in situ labeling using a TUNEL kit purchased from Borrinman Company. In brief, deparaffinized and rehydrated sections were digested with proteinase K for 20 min at room temperature and washed. After quenching in 30 mL/L hydrogen peroxide for 10 min and washing with PBS, terminal deoxynucleotidyl transferase enzyme was pipetted onto the sections, which were then incubated at 37 °C for 1 h. After stopping the reaction by putting sections in PBS and washing, anti-digoxin-peroxidase was added to the slides. Finally slides were washed with PBS, stained with 3,3-diaminobenzidine, and counterstained with hematoxylin. Substitution for terminal deoxynucleotidyl transferase with distilled water was used as negative control. Positive cells were determined according to the method described previously[15]. In brief, positive cells had dark or dark brown nuclei and some morphological characteristics, including chromatin condensation, nuclear disintegration, and formation of crescent caps of condensed chromatin at the nuclear periphery. Counting method was the same as described previously.
Statistical analysis
Differences of positivity rates between different groups were assessed by t-test. Kruskal-Wallis’ rank sum test was used to assess the differences between ranked data. Linear correlation hypothesis test was used to evaluate the extent of correlation between two groups. All of the statistical analyses were performed with SAS statistical package.
RESULTS
Immunohistochemical staining revealed that anti-survivin mAb 8E2 specifically reacted with gastric carcinoma cells, with positive staining in cytoplasm and near the Golgi apparatus, whereas no expression of survivin was observed in adjacent normal tissues. A total of 61 cases of gastric carcinoma in this series were defined as positive staining (76%, Figure 2), with the mean percentage of 29.83%.
Figure 2.
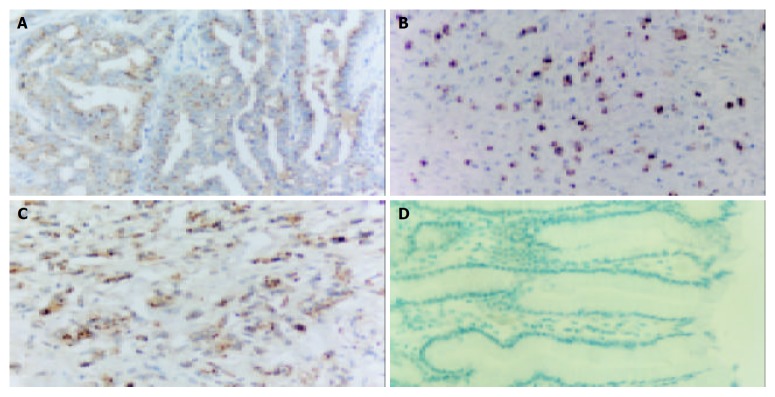
Survivin expression in gastric carcinoma. A: well differentiated gastric carcinoma; B: Moderately differentiated gastric carcinoma; C: Poorly differentiated gastric carcinoma; D: Substitution for antibody with PBS as negative control (Original magnification: × 200).
Of the 80 cases of gastric carcinomas, 75 cases (94%) of the adjacent normal tissues were positive for caspase-3, while 68 cases (85%) of the tumors were caspase-3 positive (Figure 3). Student’s t-test showed that caspase-3 expressed higher in normal tissue than in carcinoma. Survivin and caspase-3 were not positive at the same position in cancer cells. Expression of survivin in carcinomas showed a negative but not linear correlation with that of caspase-3 (r = -0.18, P > 0.05) (Table 1, Table 2, Table 3, Table 4).
Figure 3.
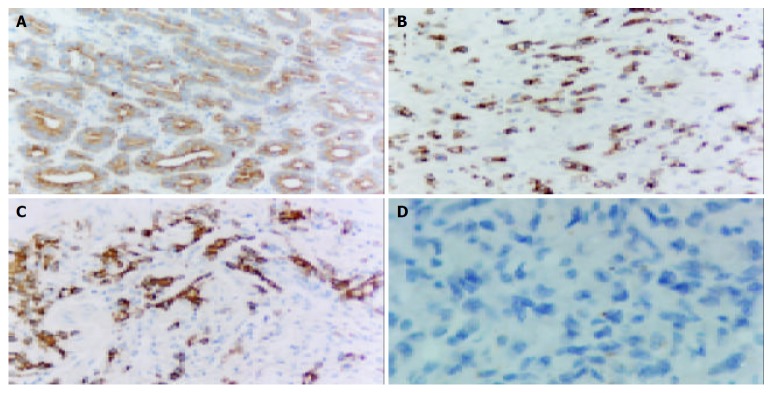
Expression of caspase-3 in gastric carcinoma. A: well differentiated gastric carcinoma; B: Moderately differentiated gastric carcinoma; C: Poorly differentiated gastric carcinoma; D: Substitution for antibody with PBS as negative control (Original magnification: × 200).
Table 1.
Correlation between histological grades and survivin expression
| Positive | Poorly | Moderately | Well | Sum | Mean |
| degree | differentiated | differentiated | differentiated | ranks | |
| + | 1 | 13 | 4 | 18 | 9.5 |
| ++ | 8 | 11 | 1 | 20 | 28.5 |
| +++ | 10 | 5 | 1 | 16 | 46.5 |
| ++++ | 4 | 3 | 0 | 7 | 58 |
| n | 23 | 32 | 6 | 61 |
Hc = 12.8, P < 0.005 between poorly, moderately and well differ-entiated gastric carcinomas.
Table 2.
Correlation between pathological stages and expres-sion of survivin
| Positive degree | Stage I | Stage II | Stage III | Sum | Mean ranks |
| + | 8 | 6 | 4 | 18 | 9.5 |
| ++ | 3 | 15 | 2 | 20 | 28.5 |
| +++ | 1 | 3 | 12 | 16 | 46.5 |
| ++++ | 1 | 2 | 4 | 7 | 58 |
| n | 13 | 26 | 22 | 61 |
Hc = 15.1, P < 0.005 between stages I, II and III.
Table 3.
Correlation between histological grades and expres-sion of caspase-3
| Positive | Poorly | Moderately | Well | Sum | Mean |
| degree | differentiated | differentiated | differentiated | ranks | |
| + | 6 | 2 | 0 | 8 | 3.5 |
| ++ | 10 | 14 | 1 | 25 | 17.5 |
| +++ | 8 | 19 | 2 | 29 | 42.5 |
| ++++ | 1 | 1 | 4 | 6 | 63 |
| n | 25 | 36 | 7 | 68 |
Hc = 11.7, P < 0.005 between poorly, moderately and well differ-entiated gastric carcinomas.
Table 4.
Correlation between pathological stages and expres-sion of caspase-3
| Positive degree | Stage I | Stage II | Stage III | Sum | Mean ranks |
| + | 1 | 5 | 2 | 8 | 4.5 |
| ++ | 7 | 7 | 11 | 25 | 21 |
| +++ | 5 | 14 | 10 | 29 | 48 |
| ++++ | 3 | 2 | 1 | 6 | 65.5 |
| n | 16 | 28 | 24 | 68 |
Hc = 0.54, P > 0.75 between stages I, II and III.
Through Kruskal-Wallis’ rank sum test, we found that the expression of both survivin and caspase-3 had significant differences between tissues with different histological grades (Table 1, Table 3). The expression of survivin was significantly associated with pathological stages, but caspase-3 was not (Table 2, Table 4).
Apoptotic cells and apoptotic bodies were observed in gastric carcinoma by using in situ labeling (Figure 4). The mean apoptotic index (AI) of the 80 cases was 0.84%. The mean AI in survivin-positive tumors was 0.59%, which was significantly lower than the mean AI of 1.26% observed in survivin-negative tumors (P < 0.005). The mean AI in caspase-3-positive tumors (0.97%) was significantly higher than that in caspase-3-negative tumors (0.56%, P < 0.05). Tumors with survivin(-) and caspase-3 (+) had the highest AI of 1.58%.
Figure 4.
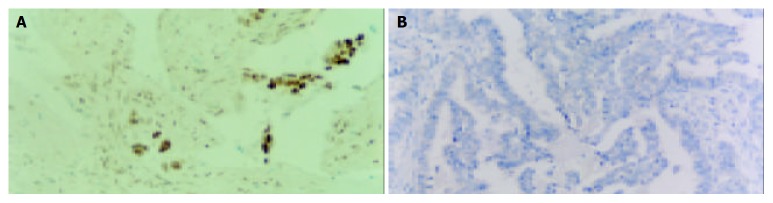
Apoptosis in gastric carcinoma. A: Positive; B: Substitution for terminal deoxynucleotidyl transferase with distilled water as negative control (Original magnification: × 200).
DISCUSSION
Recently, several inhibitors of apoptosis (IAP) related to the baculovirus IAP gene have been identified in humans, mice, and Drosophila[4]. Recombinant expressions of IAP proteins counteract various forms of apoptosis in vivo[16] and in vitro[17]. These molecules are thought to block an evolutionarily conserved step in apoptosis. At least in the case of X-linked IAP, this may involve direct inhibition of the terminal effectors caspase-3 and caspase-7 through a BIR-dependent recognition[18]. Among the recently described IAP family, survivin is characterized by a unique structure with a single BIR and no-zinc-binding domain known as the RING finger[19,20], and by the selective distribution in common human cancers[4,7]. In this study, specific staining for survivin was detected in 61 cases (76%), with a variable proportion of positive tumor cells (10%-85%). In contrast, the adjacent normal tissues or the infiltrating lymphocytes did not express survivin, consistent with similar studies[13]. As the histological differentiation decreased and pathological stage increased, positivity rate and expression level of survivin were elevated. So survivin expression has prognostic value in human gastric carcinoma.
Alessandra[21] reported that survivin was expressed in G2-M phase of the cell cycle in a cell cycle-regulated manner and associated with mitotic spindle microtubules. In this study, survivin-positive patients had lower AI as compared with survivin-negative patients, suggesting that the overexpression of survivin in cancer might obliterate the checkpoint of the cell cycle and allow aberrant progression through the G2-M phase checkpoint in gastric carcinoma.
In normal gastric tissues, caspase-3 was mainly expressed in gastric surface mucous cells, being in accord with the gastric cell turnover. Caspase-3 expression was increased in well differentiated tumors and apoptotic cells were increased in tumors with caspase-3 positivity, indicating that the expression of caspase-3 promoted apoptosis of tumor cells. It is well known that a number of genetic alterations are required for malignant transformation. Therefore we can speculate that abnormal differentiation leads to decreased expression of caspase-3 in tumor cells.
As a key effector molecule of apoptosis, caspase-3 can inactivate number proteins, which are associated with the structure and cycle of normal cells. Survivin showed an inversed function compared with caspase-3, which can be illustrated by the results that cases with survivin (-) and caspase-3 (+) had higher AI than cases with survivin (+) and caspase-3 (-). Then what we wanted to know was, if survivin inhibited directly the expression or function of caspase-3. Although our results indicated that expression of survivin was inversely associated with that of caspase-3, statistic analysis showed no linear correlation. So we think survivin did not inhibit the function of caspase-3 by directly inhibiting its expression. One of the relevant points to this issue was that survivin induced caspase-9 deactivation first[22,23], and then caspase-3 could not be activated at the end of the cascade. Some other reports suggested that survivin inhibited caspase pathway of apoptosis, and controled apoptosis and mitotic spindle checkpoint[19,24]. We speculate that survivin mainly oppresses caspase-3 activation or possesses effect on the upstream promoter activity, but not its expression. Further studies are needed to confirm the process of how survivin inhibits caspase-3.
As long as survivin affects terminal effector of apoptosis and exists in tumor cells, it would be an ideal target of apoptosis-based therapy. One of the roles of chemotherapy is inducing apoptosis, and caspase-3 has been proved to participate in this process[25], so acting on this target might also enhance sensitivity to chemotherapy or reduce the effect of drug-resistance. A recent in vitro study demonstrated that anti-survivin RNA down-regulated the expression of endogenous survivin in transformed cells and induced apoptotic cell death[26]. Targeted antagonists of survivin may offer a new therapeutic method for gastric carcinoma. A homeland study[27] revealed that antisense oligonucleotide targeting survivin induced decrease of survivin expression, increase of cell apoptosis, inhibition of cell proliferation in hepG2 cells. It also has been reported[28] that survivin-based plasmids could induce apoptosis in gastric cancer cells and sensitize gastric cancer cells to chemotherapeutic agents. Gene therapy targeting survivin gene expression may offer a new approach to cancer therapy.
Footnotes
Edited by Chen WW and Zhu LH Proofread by Xu FM
References
- 1.Cross M, Dexter TM. Growth factors in development, transformation, and tumorigenesis. Cell. 1991;64:271–280. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- 2.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 3.Barinaga M. Death by dozens of cuts. Science. 1998;280:32–34. doi: 10.1126/science.280.5360.32. [DOI] [PubMed] [Google Scholar]
- 4.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 5.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 6.Adida C, Haioun C, Gaulard P, Lepage E, Morel P, Briere J, Dombret H, Reyes F, Diebold J, Gisselbrecht C, et al. Prognostic significance of survivin expression in diffuse large B-cell lymphomas. Blood. 2000;96:1921–1925. [PubMed] [Google Scholar]
- 7.Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071–5074. [PubMed] [Google Scholar]
- 8.Islam A, Kageyama H, Hashizume K, Kaneko Y, Nakagawara A. Role of survivin, whose gene is mapped to 17q25, in human neuroblastoma and identification of a novel dominant-negative isoform, survivin-beta/2B. Med Pediatr Oncol. 2000;35:550–553. doi: 10.1002/1096-911x(20001201)35:6<550::aid-mpo12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6:127–134. [PubMed] [Google Scholar]
- 10.Asanuma K, Moriai R, Yajima T, Yagihashi A, Yamada M, Kobayashi D, Watanabe N. Survivin as a radioresistance factor in pancreatic cancer. Jpn J Cancer Res. 2000;91:1204–1209. doi: 10.1111/j.1349-7006.2000.tb00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998;58:1808–1812. [PubMed] [Google Scholar]
- 12.Lu C, Tanigawa N. Spontaneous apoptosis is inversely related to intratumoral microvessel density in gastric carcinoma. Cancer Res. 1997;57:221–224. [PubMed] [Google Scholar]
- 13.Duckett CS, Nava VE, Gedrich RW, Clem RJ, Van Dongen JL, Gilfillan MC, Shiels H, Hardwick JM, Thompson CB. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 14.Hay BA, Wassarman DA, Rubin GM. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell. 1995;83:1253–1262. doi: 10.1016/0092-8674(95)90150-7. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- 16.Giodini A, Kallio MJ, Wall NR, Gorbsky GJ, Tognin S, Marchisio PC, Symons M, Altieri DC. Regulation of microtubule stability and mitotic progression by survivin. Cancer Res. 2002;62:2462–2467. [PubMed] [Google Scholar]
- 17.Reed JC. The Survivin saga goes in vivo. J Clin Invest. 2001;108:965–969. doi: 10.1172/JCI14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y. Survivin structure: crystal unclear. Nat Struct Biol. 2000;7:620–623. doi: 10.1038/77904. [DOI] [PubMed] [Google Scholar]
- 19.Shankar SL, Mani S, O'Guin KN, Kandimalla ER, Agrawal S, Shafit-Zagardo B. Survivin inhibition induces human neural tumor cell death through caspase-independent and -dependent pathways. J Neurochem. 2001;79:426–436. doi: 10.1046/j.1471-4159.2001.00596.x. [DOI] [PubMed] [Google Scholar]
- 20.Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 21.Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki H, Sheng Y, Kotsuji F, Tsang BK. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 2000;60:5659–5666. [PubMed] [Google Scholar]
- 23.Hauser HP, Bardroff M, Pyrowolakis G, Jentsch S. A giant ubiquitin-conjugating enzyme related to IAP apoptosis inhibitors. J Cell Biol. 1998;141:1415–1422. doi: 10.1083/jcb.141.6.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller LK. An exegesis of IAPs: salvation and surprises from BIR motifs. Trends Cell Biol. 1999;9:323–328. doi: 10.1016/s0962-8924(99)01609-8. [DOI] [PubMed] [Google Scholar]
- 25.Sun C, Cai M, Gunasekera AH, Meadows RP, Wang H, Chen J, Zhang H, Wu W, Xu N, Ng SC, et al. NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature. 1999;401:818–822. doi: 10.1038/44617. [DOI] [PubMed] [Google Scholar]
- 26.Kappler M, Kotzsch M, Bartel F, Füssel S, Lautenschläger C, Schmidt U, Würl P, Bache M, Schmidt H, Taubert H, et al. Elevated expression level of survivin protein in soft-tissue sarcomas is a strong independent predictor of survival. Clin Cancer Res. 2003;9:1098–1104. [PubMed] [Google Scholar]
- 27.Wang Y, Wang JL. Antisense oligonucleotide targeting survivin inducing apoptosis of hepatic cancer cells. Zhonghua Xiaohua Zazhi. 2003;23:11–14. [Google Scholar]
- 28.Tan JH, Tu SP, Zou B, Ma TL, Zhong J, Zhang CL, Qiao MM, Jiang SH. Experimental study on apoptosis induced by pcDNA3-survivin mutant in gastric cancer cell lines. Zhonghua Xiaohua Zazhi. 2003;23:199–202. [Google Scholar]


