Abstract
AIM: To clone, identify and study new NS5ATP2 gene and its spliced variant transactivated by hepatitis C virus non-structural protein 5A.
METHODS: On the basis of subtractive cDNA library of genes transactivated by NS5A protein of hepatitis C virus, the coding sequence of new gene and its spliced variant were obtained by bioinformatics method. Polymerase chain reaction (PCR) was conducted to amplify NS5ATP2 gene.
RESULTS: The coding sequence of a new gene and its spliced variant were cloned and identified successfully.
CONCLUSION: A new gene has been recognized as the new target transactivated by HCV NS5A protein. These results brought some new clues for studying the biological functions of new genes and pathogenesis of the viral proteins.
INTRODUCTION
Hepatitis C virus (HCV) is the major causative agent of non-A, non-B hepatitis worldwide, which often leads to cirrhosis and an increased risk of hepatocellular carcinoma. The single-stranded RNA genome of HCV is a 9.6 kb-long positive-sense molecule, belonging to the Flaviviridae family. The viral genome encodes a single polyprotein precursor of approximately 3 010 amino acids, which is cleaved by both host and viral proteases to generate putative structural proteins (core, E1, and E2/p7) and the nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B)[1-3]. The nonstructural protein 5A (NS5A) is a phosphoprotein consisting of 447 amino acid residues. NS5A exists in two forms of polypeptide p56 and p58, which are phosphorylated mainly at serine residues both in vitro and in vivo[4,5].
It was previously shown that NS5A could function as a transcriptional trans-activator. Although these reports implicate a functional role of NS5A in transcription, the exact nature of its role or the mechanism (s) involved in regulating the cellular transcription has not been investigated. NS5A is localized to the endocytoplasmic reticulum (ER), whereas transcriptional trans-activation traditionally requires the protein to be in the nucleus. The NS5A protein must participate in signal transduction pathways that are initiated in the cytoplasm where it resides[6,7]. However, some studies show NS5A protein possesses a nuclear localization-like signal sequence and is present in the nuclear periplasmic membrane fraction related to transcription or translation[8]. The present study shows NS5A protein has transactivating effect on SV40 early promoter.
MATERIALS AND METHODS
Plasmid construction
The HCV-NS5A sequences were generated by PCR amplification of HCV plasmid (HCV strain 1b). The plasmid contains coding sequences for all of the nonstructural proteins. The PCR conditions were as follows: 94 °C for 40 s. 10 ng of PCR product was cloned with pEGM-T vector (Promega). The primary structure of insert was confirmed by direct sequencing. To create pcDNA3.1 (-)-NS5A, the fragments of encoding NS5A were released from the pEGM-T-NS5A by digestion with EcoR I and Kpn I, and ligated to pcDNA3.1 (-)[9].
Cell culture and transfection
The hepatoblastoma cell line HepG2 was propagated in DMEM supplemented with 10% FBS, 200 μmol/L L-glutamine, penicillin, and streptomycin. The HepG2 cells were plated at a density of 1×106 /well in 35-mm dishes. About 60%-70% confluent HepG2 cells were cotransfected with plasmids pcDNA3. 1(-)-NS5A and pCAT3-promoter, transfected with pcDNA3.1 (-)-NS5A, pcDNA3.1 (-) with FuGENE 6(Roche).
Confirmation of protein expression of HCV-NS5A
Expression plasmid pcDNA3.1 (-)-NS5A was transfected using FuGENE 6 into HepG2 cells. The proteins expressed in these cells were analyzed on an immunoblot using the NS5A-specific antibody. The proteins were resolved by electrophoresis on a sodium dodecyl sulfate 125 g/L polyacrylamide gel. The lysate of cells transfected with expression vector pcDNA3. 1(-) served as negative control[10].
CAT assay
Cells were then harvested after 48 h for CAT assay. Lysates of transfected cells were analyzed for CAT density using a commercial enzyme-linked immunosorbent assay (Roche Molecular Biochemicals). The absorbance of the samples was measured at 405 nm[11].
RNA extraction and SSH
mRNAs from HepG2 cells transfected with plasmids pcDNA3.1 (-)-NS5A and pcDNA3.1(-) were extracted by using QuickPrep micro mRNA Purification Kit (Amersham Pharmacia). The amount of mRNA from two samples was 3-4 μg.
SSH was performed with the cDNA Substraction Kit (Clontech) according to the manufacturer’s protocol. cDNA was synthesized from 2 μg of poly A+RNA from two samples being compared. The cDNA from pcDNA3.1 (-)-NS5A acted as the tester, the cDNA from pcDNA3.1 (-) as the driver. The tester and driver cDNAs were digested with Rsa I, which yielded blunt ends. Two different PCR adaptors that could join only 5’ ends DNA were ligated to different aliquots of tester DNA. These ligated DNAs were denatured, mixed with an excess of driver DNA (that had no adaptors), and allowed to anneal. The two DNA pools were then mixed together, and more denatured driver DNAs were added to further bind tester that was also present in the driver. Remaining complementary single strands of tester DNA were allowed to anneal, and the adaptor sequences were copied into their 3’ ends. PCR was then performed to obtain exponential amplification of tester DNAs with different adaptors at each end. PCR amplifications products were directly purified by using Wizard PCR Preps DNA Purification System (Promega), and subcloned into pEGM-T easy vectors (Promega) to set up the subtractive library[12,13].
New gene cloned
On the basis of subtractive cDNA library of genes transactivated by NS5A protein of hepatitis C virus, the coding sequence of a new gene, named NS5ATP2, was obtained by bioinformatics methods. The standard PCR cloning technique was used to amplify NS5ATP2 gene. Cytoplasmic RNA was isolated from HepG2 cells. RNA was used for RT-PCR as described previously, primers were: Sense 5’-GGA TTC ATG GCT TCG GTC TCC TCT GC-3’, antisense 5’-GGT ACC TCA GGA GTG TGG CTC ACT GG -3’ (HepG2 cDNA). The PCR condition was as follows: At 94 °C for 60 s, at 60 °C for 60 s, at 72 °C for 60 s, for 30 cycles. The PCR product was cloned with pGEM-T vector (Promega). The primary structure of insert was confirmed by direct sequencing.
RESULTS
NS5A protein expressed in HepG2 cells
NS5A protein expressed in cells was analyzed by Western blot. The lysates of cells transfected with plasmid pcDNA3.1 (-)-NS5A were specifically detected by NS5A specific antibody (Figure 1).
Figure 1.
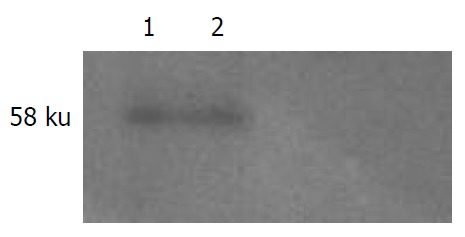
Western blotting of NS5A protein expression in HepG2 cells. Lane 1: Expression plasmid pcDNA3.1 (-)-NS5A; Lane 2: Plasmid pcDNA3.1 (-)-vector.
Transactivating effect of NS5A on SV40 early promoter
To determine whether NS5A protein has transactivating effect, we constructed plasmid pcDNA3.1 (-)-NS5A, and HCV NS5A protein expressed in Hep G2 cells was detected by reverse transcription PCR (RT-PCR) and Western blotting. HepG2 cells were transiently cotransfected with pcDNA3.1 (-)-NS5A/pCAT3-promoter, pcDNA3.1(-)/pCAT3-promoter. Chloramphenicol acetyltransferase (CAT) activity in cells that were cotransfected with pcDNA3.1 (-)-NS5A/pCAT3-promoter is shown in Figure 2.
Figure 2.
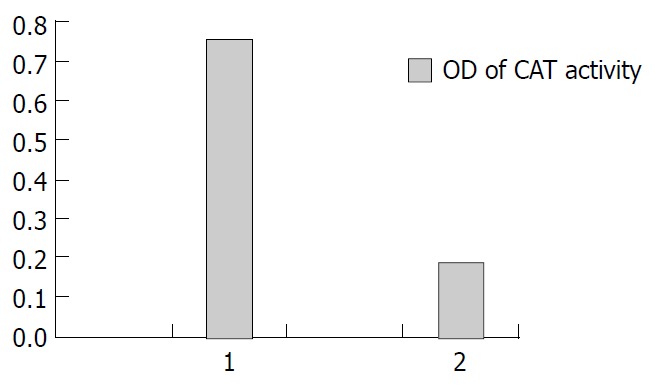
Transactivation on SV40 promoter by NS5A. 1: Plasmid pcDNA3.1 (-)-NS5A was cotransfected with pCAT3-promoter in HepG2 cells. 2: Plasmid pcDNA3.1 (-) was cotransfected with pCAT3-promoter in HepG2 cells.
Construction of subtractive cDNA library
Our studies showed NS5A protein had transactivation effect on SV40 promoter. In order to investigate influence of NS5A protein on cells gene expression, Suppression subtraction hybridization (SSH) was introduced to establish substractive cDNA library of HepG2 transfected with plasmid pcDNA3.1 (-)-NS5A. We performed the PCR experiment to analyse the ligation efficiency. The result showed that at least 25% of the cDNA had adaptors at both ends. The efficiency of subtraction was estimated by PCR experiment. The test was done by comparison of the abundance of G3PDH before and after subtraction. G3PDH primers were provided by the kit (Figure 3).
Figure 3.
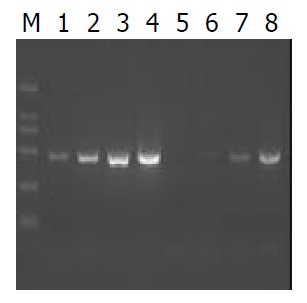
Reduction of G3PDH amount by PCR-selective subtraction. PCR was performed on unsubtracted (Lanes 1-4) and subtracted (Lanes 5-8) secondary PCR products with the G3PDH 5’ and 3’ primers. Lanes 1, 5: 18 cycles; Lanes 2,6: 23 cycles; Lanes 3,7: 28 cycles.
After tester cDNA was hybridized with driver cDNA twice and underwent nested PCR twice, they were then subcloned into pGEM-T easy vectors to set up the subtractive library. Amplification of the library was carried out with E. coli strain JM109. The amplified library contained 121 positive clones.
Colony PCR showed that 115 clones contained 200-1 000 bp inserts (Figure 4). The nucleotide sequences of 90 clones from this cDNA library was analyzed, the full length sequences were obtained with Vector NTI 6 and BLAST database homology search (http://www. ncbi. nlm. nih. gov/). Altogether 44 kinds of coding sequences were obtained, consisting of 29 known and 15 unknown ones. Some genes code for proteins involved in cell cycle regulation, cell apoptosis, signal transduction pathway and tumour (Table 1).
Figure 4.
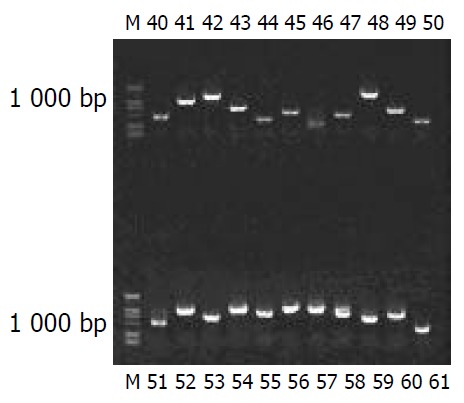
Map of colony PCR on 9 g/L agarose/EtBr gel.
Table 1.
Sequence analysis of 46 clones isolated from subtrac-tive cDNA library
| Known genes | Number of clones | Homology (%) |
| Ribosomal protein | 15 | 99 |
| Eukaryotic translation initiation factor | 4 | 99 |
| HCV NS5A protein | 4 | 98 |
| Sentrin | 4 | 99 |
| Pro-oncosis receptor inducing | 3 | 100 |
| membrane injury (Porimin) | ||
| Importin | 3 | 98 |
| Serine/threonine kinase | 3 | 100 |
| Cadherin-associated protein | 2 | 100 |
| Mitogen-activated protein | 2 | 99 |
| kinase phosphatase | ||
| Adenylyl cyclase-associated protein | 2 | 100 |
| Serum response element | 2 | 100 |
| Rho GTPase activating protein | 2 | 100 |
| Fibronectin | 3 | 99 |
| Laminin | 3 | 99 |
| Lysophospholipase A2 | 2 | 100 |
| Lysophospholipase B | 2 | 100 |
| Dual specificity phosphatase 6 | 1 | 99 |
| Putative homeodomain | 2 | 92 |
| transcription factor | ||
| Transcription factor B2 | 2 | 100 |
| NF-E2-like basic leucine zipper | ||
| Transcriptional activator | 2 | 98 |
| Transcriptional elongation factor (TFIIS) | 2 | 100 |
| MHC-I binding protein | 1 | 100 |
| C response protein binding | 1 | 99 |
| protein (CRPBP) | ||
| Integrin | 2 | 99 |
| Iron-regulated transporter (IREG) | 1 | 99 |
| Tumor associated protein L6 | 2 | 100 |
| WW domain-containing | 1 | 100 |
| protein 1 (WWP1) | ||
| Nascent polypeptide-associate | 1 | 99 |
| complex α (NACA) | ||
| Thioredoxin reductase | 1 | 99 |
Confirmation of new gene expression by RT-PCR
We found the spliced variant of NS5A-TP2 (Figure 5,Figure 6). After EST database homology search (http://www. ncbi. nlm. nih. gov/), the locations of NS5A-TP2 and its spliced variant were detected on chromosome 6q22. 1-23. 3. The exons and introns of two new genes were compared (Figure 7). The direct sequencing showed we acquired the ORF of NS5A-TP2 (Figure 8).
Figure 5.
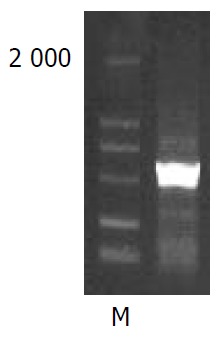
NS5A-TP2 fragment amplified by RT-PCR. M: Marker.
Figure 6.
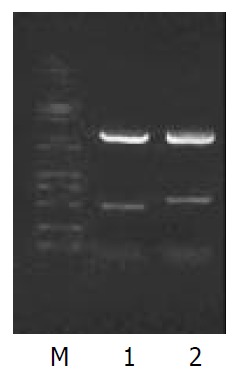
pEGM-T-NS5A-TP2 cut by EcoR I/Kpn I. M: Marker; Lane 1: A 512-bp fragment; Lane 2: A 615-bp fragment.
Figure 7.
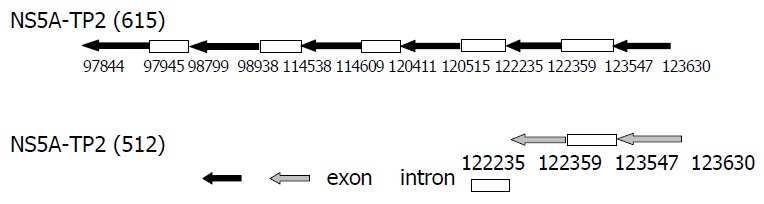
Comparison of exons and introns of NS5A-TP2 (615) and (512) gene.
Figure 8.

ORF comparison of NS5A-TP2 (615) and (512).
DISCUSSION
Hepatitis C virus often causes persistent infection with a significant risk of end-stage cirrhosis and hepatocellular carcinoma. HCV may benefit by regulation of celluar genes leading to the disruption of normal cell growth. Viral genes can override cellular control mechanisms, which in untransformed cells regulate cell cycle progression in response to various antiproliferative signals. In HCV persistently infected cells, the continued presence of viral gene products is likely to be detrimental for host cells. Many studies demonstrated NS5A protein of HCV transcriptionally modulates cellular genes and promotes murine fibroblast cell growth into a tumorigenic phenotype. It may be possible that the NS5A protein plays a role in hepatocarcinogenesis, since many other viral proteins that play a role in carcinogenesis often function as transcriptional activators[14-17]. However, the precise mechanism is still unknown.
In the present study, we investigated the possible mechanism by which NS5A protein transactivated gene expression and its role in hepatocarcinogenesis. NS5A protein in Hep G2 cells was detected by RT-PCR and Western blotting. HepG2 cells were transiently cotransfected with pcDNA3.1 (-)-NS5A/ pCAT3-promoter. CAT activity was evidently higher in the cotransfected cells than in control. It is suggested that NS5A protein has transactivating effect on SV40 early promoter. We predicted that NS5A protein transcriptionally regulated gene expression through regulating promoter activity, either directly or through signal transduction pathways.
On the basis of this study, we constructed subtractive cDNA library by SSH. After sequencing analysis, we obtained coding sequences of 46 genes, which consisted of 26 kinds of known and 15 kinds of unknown ones. Some genes code for proteins involved in cell cycle regulation, cell apoptosis, and tumor angiogenesis. Sentrin is a 101-amino acid ubiquitin-like protein that interacts with the death domains of Fas and TNFR1, with PML, a tumor suppressor implicated in the pathogenesis of promyelocytic leukemia, with Rad51 and Rad52, proteins that are involved in repairing double-stranded DNA breaks, and with RanGAP1, a GTPase-activating protein that is critically involved in nuclear protein transport[18-20]. Overexpression of sentrin in mammalian cells protects them against anti-Fas or tumor necrosis factor-induced cell death[21]. Porimin is a highly glycosylated protein that can be classified as a member of the cell membrane-associated mucin family[22]. Porimin is a membrane mucin that mediates cell death. Although mucins mainly affect cell adhesion and ligand binding, several membrane mucins have also been documented to trigger cell death or inhibit cell proliferation, such as CD43 (leukosialin, sialophorin), CD162 (PSGL-1), and CD164 (MGC-24v)[23]. Likewise, serine/threonine kinase, cadherin-associated protein, adenylyl cyclase-associated protein, mitogen-activated protein kinase phosphatase involving in cell cycle regulation, and cell growth may be correlated with hepatocarcinogenesis of NS5A protein[24-28].
Alternative pre-mRNA splicing is a fundamental mechanism for differential gene expression that has been reported to regulate the tissue distribution, the intracellular localization, and the activity of different protein kinases. In the process of our study on new genes, we accidentally acquired the spliced variant of NS5A-TP2 and confirmed the ORF of NS5A-TP2 (516) and its location on chromosome. Both of NS5A-TP2 (615) and its spliced variant- NS5A-TP2 (516) locate on 6q22.1-23.3, but they have different exons and introns[29-31].
The result of this study shows that the NS5A protein is a potent transcriptional activator and transactivates some genes involved in cell cycle regulation, cell apoptosis, and tumor angiogenesis. The study on new genes NS5A-TP2 (516), and NS5A-TP2 (615) brings some new clues to the biological functions of novel genes and pathogenesis of the viral proteins.
Footnotes
Supported by the National Natural Science Foundation of China, No. C03011402, No. C30070690 and the 9th Five-year Plan Period Research and Development Foundation of PLA, No. 98D063 and the Start-up for Students Studying Overseas of PLA, No. 98H038 and the 10th Five-year period Youth Research and Technology Foundation of PLA, No. 01Q138 and the 10th Five-year period Research and Technology Foundation of PLA, No. 01MB135
Edited by Zhu LH and Chen WW Proofread by Xu FM
References
- 1.Pawlotsky JM. Hepatitis C virus (HCV) NS5A protein: role in HCV replication and resistance to interferon-alpha. J Viral Hepat. 1999;6(Suppl 1):47–48. doi: 10.1046/j.1365-2893.1999.00004.x. [DOI] [PubMed] [Google Scholar]
- 2.Kumar U, Tuthill T, Thomas HC, Monjardino J. Sequence, expression and reconstitution of an HCV genome from a British isolate derived from a single blood donation. J Viral Hepat. 2000;7:459–465. doi: 10.1046/j.1365-2893.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 3.Sandres K, Dubois M, Pasquier C, Payen JL, Alric L, Duffaut M, Vinel JP, Pascal JP, Puel J, Izopet J. Genetic heterogeneity of hypervariable region 1 of the hepatitis C virus (HCV) genome and sensitivity of HCV to alpha interferon therapy. J Virol. 2000;74:661–668. doi: 10.1128/jvi.74.2.661-668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neddermann P, Clementi A, De Francesco R. Hyperphosphorylation of the hepatitis C virus NS5A protein requires an active NS3 protease, NS4A, NS4B, and NS5A encoded on the same polyprotein. J Virol. 1999;73:9984–9991. doi: 10.1128/jvi.73.12.9984-9991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed KE, Rice CM. Identification of the major phosphorylation site of the hepatitis C virus H strain NS5A protein as serine 2321. J Biol Chem. 1999;274:28011–28018. doi: 10.1074/jbc.274.39.28011. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh AK, Steele R, Meyer K, Ray R, Ray RB. Hepatitis C virus NS5A protein modulates cell cycle regulatory genes and promotes cell growth. J Gen Virol. 1999;80(Pt 5):1179–1183. doi: 10.1099/0022-1317-80-5-1179. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh AK, Majumder M, Steele R, Yaciuk P, Chrivia J, Ray R, Ray RB. Hepatitis C virus NS5A protein modulates transcription through a novel cellular transcription factor SRCAP. J Biol Chem. 2000;275:7184–7188. doi: 10.1074/jbc.275.10.7184. [DOI] [PubMed] [Google Scholar]
- 8.Song J, Nagano-Fujii M, Wang F, Florese R, Fujita T, Ishido S, Hotta H. Nuclear localization and intramolecular cleavage of N-terminally deleted NS5A protein of hepatitis C virus. Virus Res. 2000;69:109–117. doi: 10.1016/s0168-1702(00)00206-9. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Cheng J, Lu YY, Wang G, Mou JS, Li L, Zhang LX, Chen JM. [Cloning of genes transactivated by hepatitis B virus X protein] Zhonghua Gan Zang Bing Za Zhi. 2003;11:5–7. [PubMed] [Google Scholar]
- 10.Wang L, Li K, Cheng J, Chen TY, Hong Y, Liu Y, Wang G, Zhong YW. Screening of gene encoding of hepatic protein interacting with Hcbp6 via yeast two hybridization. Shijie Huanren Xiaohua Zazhi. 2003;11:385–388. [Google Scholar]
- 11.Liu Y, Dong J, Cheng J, Lu YY. The study of transactivating ef-fect of HBV X protein on SV40 early promoter. Jiefangjun Yixue Zazhi. 2001;26:404–406. [Google Scholar]
- 12.Shridhar V, Sen A, Chien J, Staub J, Avula R, Kovats S, Lee J, Lillie J, Smith DI. Identification of underexpressed genes in early- and late-stage primary ovarian tumors by suppression subtraction hybridization. Cancer Res. 2002;62:262–270. [PubMed] [Google Scholar]
- 13.Diatchenko L, Lau YF, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED, et al. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majumder M, Ghosh AK, Steele R, Ray R, Ray RB. Hepatitis C virus NS5A physically associates with p53 and regulates p21/waf1 gene expression in a p53-dependent manner. J Virol. 2001;75:1401–1407. doi: 10.1128/JVI.75.3.1401-1407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Mitri MS, Morsica G, Cassini R, Bagaglio S, Zoli M, Alberti A, Bernardi M. Prevalence of wild-type in NS5A-PKR protein kinase binding domain in HCV-related hepatocellular carcinoma. J Hepatol. 2002;36:116–122. doi: 10.1016/s0168-8278(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 16.Park KJ, Choi SH, Choi DH, Park JM, Yie SW, Lee SY, Hwang SB. 1Hepatitis C virus NS5A protein modulates c-Jun N-terminal kinase through interaction with tumor necrosis factor receptor-associated factor 2. J Biol Chem. 2003;278:30711–30718. doi: 10.1074/jbc.M209623200. [DOI] [PubMed] [Google Scholar]
- 17.Reyes GR. The nonstructural NS5A protein of hepatitis C virus: an expanding, multifunctional role in enhancing hepatitis C virus pathogenesis. J Biomed Sci. 2002;9:187–197. doi: 10.1007/BF02256065. [DOI] [PubMed] [Google Scholar]
- 18.Ryu SW, Chae SK, Kim E. Interaction of Daxx, a Fas binding protein, with sentrin and Ubc9. Biochem Biophys Res Commun. 2000;279:6–10. doi: 10.1006/bbrc.2000.3882. [DOI] [PubMed] [Google Scholar]
- 19.Okura T, Gong L, Kamitani T, Wada T, Okura I, Wei CF, Chang HM, Yeh ET. Protection against Fas/APO-1- and tumor necrosis factor-mediated cell death by a novel protein, sentrin. J Immunol. 1996;157:4277–42781. [PubMed] [Google Scholar]
- 20.Kretz-Remy C, Tanguay RM. SUMO/sentrin: protein modifiers regulating important cellular functions. Biochem Cell Biol. 1999;77:299–309. [PubMed] [Google Scholar]
- 21.Kamitani T, Kito K, Nguyen HP, Fukuda-Kamitani T, Yeh ET. Characterization of a second member of the sentrin family of ubiquitin-like proteins. J Biol Chem. 1998;273:11349–11353. doi: 10.1074/jbc.273.18.11349. [DOI] [PubMed] [Google Scholar]
- 22.Ma F, Zhang C, Prasad KV, Freeman GJ, Schlossman SF. Molecular cloning of Porimin, a novel cell surface receptor mediating oncotic cell death. Proc Natl Acad Sci USA. 2001;98:9778–9783. doi: 10.1073/pnas.171322898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Xu Y, Gu J, Schlossman SF. A cell surface receptor defined by a mAb mediates a unique type of cell death similar to oncosis. Proc Natl Acad Sci USA. 1998;95:6290–6295. doi: 10.1073/pnas.95.11.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamari M, Daigo Y, Nakamura Y. Isolation and characterization of a novel serine threonine kinase gene on chromosome 3p22-21.3. J Hum Genet. 1999;44:116–120. doi: 10.1007/s100380050121. [DOI] [PubMed] [Google Scholar]
- 25.Ohteki T, Parsons M, Zakarian A, Jones RG, Nguyen LT, Woodgett JR, Ohashi PS. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. J Exp Med. 2000;192:99–104. doi: 10.1084/jem.192.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratcliffe MJ, Rubin LL, Staddon JM. Dephosphorylation of the cadherin-associated p100/p120 proteins in response to activation of protein kinase C in epithelial cells. J Biol Chem. 1997;272:31894–31901. doi: 10.1074/jbc.272.50.31894. [DOI] [PubMed] [Google Scholar]
- 27.Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- 28.Zelicof A, Gatica J, Gerst JE. Molecular cloning and characterization of a rat homolog of CAP, the adenylyl cyclase-associated protein from Saccharomyces cerevisiae. J Biol Chem. 1993;268:13448–13453. [PubMed] [Google Scholar]
- 29.Shima F, Yamawaki-Kataoka Y, Yanagihara C, Tamada M, Okada T, Kariya K, Kataoka T. Effect of association with adenylyl cyclase-associated protein on the interaction of yeast adenylyl cyclase with Ras protein. Mol Cell Biol. 1997;17:1057–1064. doi: 10.1128/mcb.17.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 31.Cheng J, Li K, Lu YY, Wang L, Liu Y. Bioinformatics analysis of human hepatitis C virus core protein-binding protein 6 gene and protein. Shijie Huanren Xiaohua Zazhi. 2003;11:378–384. [Google Scholar]


