Abstract
AIM: To more clearly define the clinical and pathological characteristics and appropriate diagnosis and treatment of nonfunctioning (NFICTs) islet cell tumors, and to review our institutional experience over the last 30 years.
METHODS: The records of 43 patients confirmed to have nonfunctioning islet cell tumors of pancreas were retrospectively reviewed. Survival was estimated by the Kaplan-Meier methods and potential risk factors for survival were compared with the log-rank tests.
RESULTS: The mean age was 31.63 years (range, 8 to 67 years). There were 7 men and 36 women. Twenty-eight patients had a confirmed diagnosis of nonfunctioning islet cell carcinoma (NFICC) and benign islet cell tumors were found in 15 patients. The most common symptoms in patients with NFICTs were abdominal pain (55.8%), nausea and/or vomiting (32.6%), fatigue (25.6%) and abdominal mass (23.3%). Preoperative ultrasonic and computed tomography localized the tumors in all patients. Forty-three NFICTs were distributed throughout the pancreas, with 21 located to the right of the superior mesenteric vessels, 10 in the body of the pancreas, 6 in the tail of the pancreas, and multiple tumors were found in one patient. Thirty-nine of 43 patients (91%) underwent surgical resection. Surgical treatment was curative in 30 patients (70%) and palliative in 9 (21%). The resectability and curative resection rate in patients with NFICC of pancreas were 89% and 61%, respectively. The overall cumulative 5-year and 10-year survival rates for patients with NFICC were 58.05% and 29.03%, respectively. Radical operation and diameter of cancer small than 10 cm were positive prognostic factors in females younger than 30 years old. Multivariate Cox regression analysis indicated that radical operation was the only independent prognostic factor, P = 0.007.
CONCLUSION: Nonfunctioning islet cell tumors of pancreas are found mainly in young women. The long-term results for patients undergone surgery, especially curative resection are good.
INTRODUCTION
Tumors arising from the pancreatic islet cell are rare and represent a heterogeneous group of benign or malignant lesions. Most tumors present with well characterized syndromes, whereas others appear to be nonfunctioning. Functioning tumors, such as gastrinomas, insulinomas, and vasoactive intestinal peptide-secreting tumour (VIPomas), are characterized by extensive production of single polypeptide hormones which lead to specific clinical syndromes. Nonfunctioning tumors, which account for 15% and 52% of tumors, are not associated with clinical syndromes, as they do not produce an excess of any active polypeptide hormones[1,2]. These Lesions tend to occur later and have a different clinical course from their functioning counterparts.
The purpose of this study was to analyze the clinical presentations, diagnosis, management, and prognosis of a large group of patients with nonfunctioning islet cell tumors (NFICTs) of pancreas managed at a single cancer center to determine the natural history and optimum management of such lesions.
MATERIALS AND METHODS
Patients
A total of 43 patients with NFICTs were treated between 1972-2002. The diagnosis of islet cell tumors of pancreas was established by histopathologic examination. Tumors were considered nonfunctional if no clinical symptoms of excess hormone were present.
The disease was considered regional if no evidence of metastases in the liver or elsewhere was found either during surgery or by imaging techniques. Metastases in the liver or elsewhere were considered distant.
Methods
Clinical records were reviewed with regard to patient demographics, clinical features, diagnostic procedures, pathologic findings, operative details, medical treatment, and long-term survival. All patients were followed up by return visits, letters from the follow-up department, correspondence with primary surgeons, or telephone contact within the last months from the conclusion of this study. Survival rate was estimated by the Kaplan-Meier methods, and potential risk factors for survival were compared with the log-rank tests and Cox methods. Ninety-five percent confidence intervals were calculated for specific estimates of survival.
RESULTS
Clinical characteristics
There were 36 females and 7 males with a mean age of 31.63 years and a range of 8 to 67 years and the details are shown in Figure 1. At the time of diagnosis, the median age of male and female patients was 33.57 and 31.25 years, respectively (P > 0.05). The clinical presentations are illustrated in Table 1. The predominant presentations were abdominal pain, nausea and/or vomiting, fatigue and abdominal pain. None of the patients displayed the clinical features of an endocrine disorder. The duration from the onset of symptoms to diagnosis for all patients ranged from 3 d to 10 years (median 6 mo). Only three patients were asymptomatic, with the tumor found incidentally.
Figure 1.
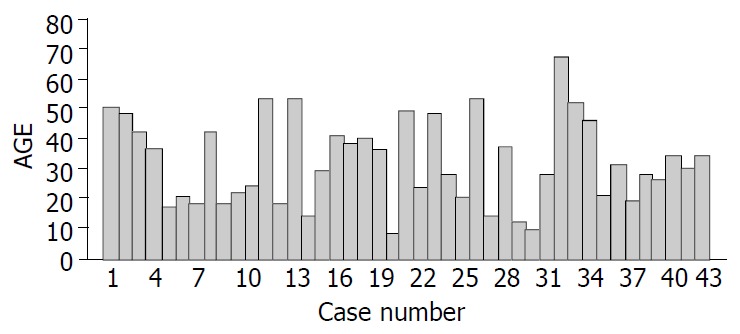
Age distribution in 43 NFICTs patients.
Table 1.
Clinical presentations
| Symptoms | Patients(n=43) | (%) |
| Abdominal pain | 24 | 55.8 |
| Nausea and/or vomiting | 14 | 32.6 |
| Fatigue | 11 | 25.6 |
| Abdominal mass | 10 | 23.3 |
| Back pain | 9 | 21.0 |
| Jaundice | 8 | 18.6 |
| Weight loss | 5 | 11.6 |
| No symptoms | 3 | 7.0 |
| Acut abdomen | 1 | 2.3 |
Abdominal ultrasonography (US) and spiral CT were the most commonly employed to detect the primary tumor in all but the patient with emergency operation. Correct prediction of an islet cell tumors based on CT and US results was achieved only in 6 (20%) patients. The common diagnosis of CT was carcinoma of pancreas (in 20 patients). Endoscopic retrograde cholangiopancreaticography(ERCP) was performed in 6 patients to determine the cause of obstructive jaundice. Magnetic resonance imaging (MRI) and endoscopic ultrasonography (EUS) localized the tumor and the local extension correctly in 5 and 4 patients, respectively.
Pathological findings
Confirmation of malignancy was mainly based on the demonstration of local invasion, microscopic vascular or perineural invasion, lymph node metastases, hepatic metastasis or distant metastases. Twenty-eight patients were histopathologically diagnosed as nonfunctioning islet cell carcinoma (NFICC) of pancreas.
The size of islet malignant and benign tumors ranged from 2.5 cm to 15 cm (mean 10 cm) and from 4 cm to 30 cm (mean 9.9 cm), respectively (P > 0.05). Metastases were presented in 6 patients at the time of operation. There were 21 tumors located in the head of the pancreas, 10 in tail, 6 in the body, 5 in body and tail (Table 2). Multiple tumors were presented only in one patient.
Table 2.
Location of the tumors
| Location | Malignant (n = 28) | Benign (n = 15) |
| Head | 13 | 8 |
| Tail | 8 | 2 |
| Body | 2 | 4 |
| Body+Tail | 4 | 1 |
| Multiple | 1 | 0 |
Operative detail and outcome
We performed 30 (70%) potentially curative resections, 9 (21%) palliative resections, and 4 (9.3%) bypass procedures, resulting in a resection rate of 91%. Of the 28 patients with islet cell carcinoma of pancreas, potentially curative resection, palliative resection, palliative bypass and biopsy were performed in 17 (61%), 8 (28.6%), 3 (10.7%) and 1 (3.6%), respectively (Table 3). The potentially curative resection was performed in 87% of patients with benign islet cell tumors compared to only 61% of those with NICC (P<0.05). Table 4 shows the type of operative procedures for patients with benign and malignant islet cell tumors. There was no 30-day mortality.
Table 3.
Nature operative procedures
| Nature | Malignant (n = 28) | (%) | Benign (n = 15) | (%) |
| Curative resection | 17 | 61.0 | 13 | 87.0 |
| Palliative resection | 8 | 28.6 | 1 | 6.7 |
| Palliative bypass | 2 | 5.1 | 1 | 6.7 |
| Biopsy only | 1 | 3.6 | 0 | 0 |
Table 4.
Primary operative procedures
| Procedure | Malignant (n = 28) | (%) | Benign (n = 15) | (%) |
| Pancreatoduodenectomy | 8 | 28.5 | 2 | 13.3 |
| Pylorus-preserving pancreatoduodenectomy | 0 | 0 | 1 | 6.7 |
| Distal pancreatectomy + splenectomy | 7 | 25.0 | 5 | 33.3 |
| Subtotal pancreatectomy + splenectomy | 4 | 14.3 | 2 | 13.3 |
| Distal pancreatectomy | 2 | 7.1 | 0 | 0 |
| Enucleation | 1 | 3.6 | 4 | 26.7 |
| Double bypass | 1 | 3.6 | 0 | 0 |
| Biliary bypass | 3 | 10.7 | 1 | 6.7 |
| Gastric bypass | 1 | 3.6 | 0 | 0 |
| Biopsy only | 1 | 3.6 | 0 | 0 |
Other treatment modalities
A total of 13 cases of NFICC received adjuvant therapy after curative operation, of them 9 patients with NFICC underwent chemotherapy (5 of them with the regimen of FAM and 4 others with 5-FU only) after a curative operation. One of them developed liver metastasis one year later, the another patient died 23 mo postoperatively. The remaining 7 patients were alive with no evidence of disease for a median 45.5 mo (range 15-96 mo). Another 4 patients with malignant tumors underwent radiation plus chemotherapy (three with FAM, and one with 5-FU). All these 4 patients were alive with no evidence of disease for 55.6 mo in average (range 14-124 mo).
Among the patients with NFICC who underwent palliative resection, 4 (50%) were given chemotherapy with 5-FU and CF, one developed multiple liver metastasis 13 mo later.
Survival
The cumulative 5-year and 10-year survival rates for all patients with NFICC were 58.05% and 29.03%, respectively (Figure 2). Table 5 demonstrates the survival in detail. The median survival of female patients was 120 mo, which was better than the survival for male patients (45 mo, P = 0.312, Figure 3). Patients who were younger than 30 years had a better long-term survival than those elder than 30 years, but the difference was not statistical significant (Figure 4, P = 0.758). Figure 5 compares survival curves for the curative operations and noncurative groups. Survivals at 5-years and 10-years were significant better for patients who underwent PD and STRP than those who underwent palliative operation, P = 0.007. As demonstrated in Figure 6, survivals were better for patients with smaller tumors than those with larger ones, but the difference was not statistically significant, P = 0.1169.(Table 6).
Figure 2.
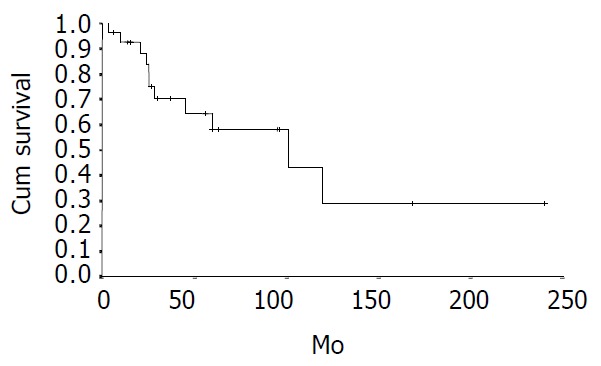
Actual survival for all patients with NFICC (n = 28).
Table 5.
Survival of patients with NFICC
| Patients | n | 5-yr | 10-yr | Median |
| survival (%) | survival (%) | survival (mo) | ||
| ALL cases | 28 | 58.05 | 29.03 | 60 ± 4.35 |
| Male | 5 | 40.00 | 20.00 | 45 ± 21.92 |
| Female | 23 | 63.49 | 31.75 | 120 ± 44.05 |
| ≤ 30 yr | 14 | 54.17 | 36.11 | 120 ± 64.02 |
| > 30 yr | 14 | 57.69 | 0 | 101 |
| D1 ≤ 10 cm | 18 | 67.57 | 49.05 | 101 |
| D1 > 10 cm | 10 | 36.00 | 0 | 60 ± 21.75 |
| PD2 | 7 | 71.43 | 35.71 | 101 ± 56.73 |
| STRP3 | 1 4 | 72.00 | 36.00 | 120 ± 44.94 |
| PO4 | 7 | 0 | 0 | 24 ± 3.19 |
Diameter of the cancer,
Pancreatoduodenectomy,
Subtotal resection of pancreas,
palliative operation.
Figure 3.
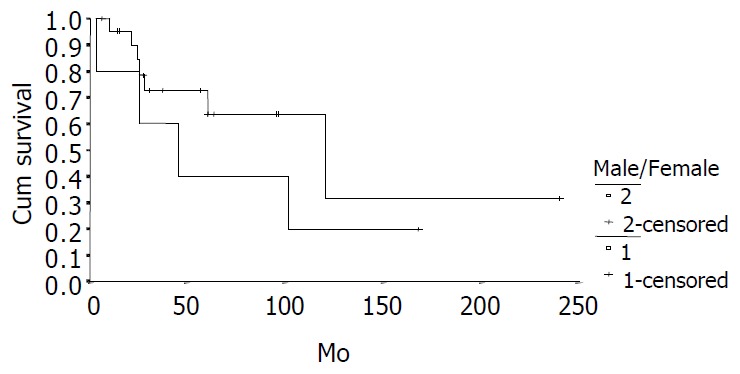
Survival comparison between male (n = 5) and female (n = 23) patients with NFICC (P = 0.312).
Figure 4.
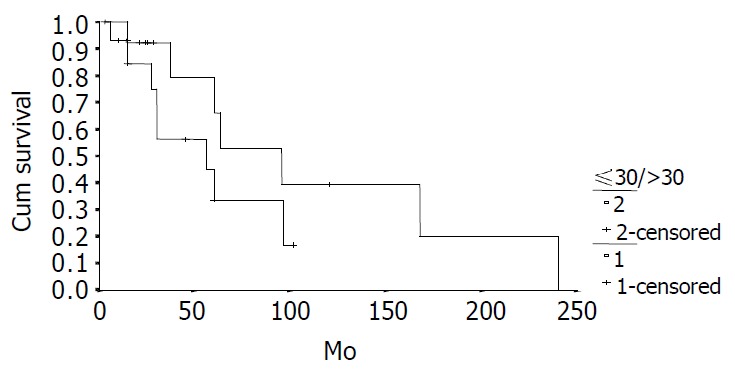
Survival comparison between younger (30 years, n = 14) and elder ( > 30 years, n = 14) patients with NFICC P = 0.7582).
Figure 5.
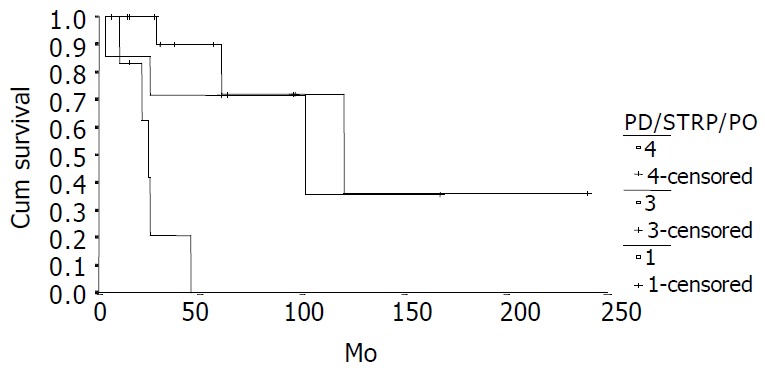
Survival comparison between patients who under-went different operations (PD, n = 7; Subtotal resection of pancreas, n = 14, palliative operation, n = 7, P = 0.0005).
Figure 6.
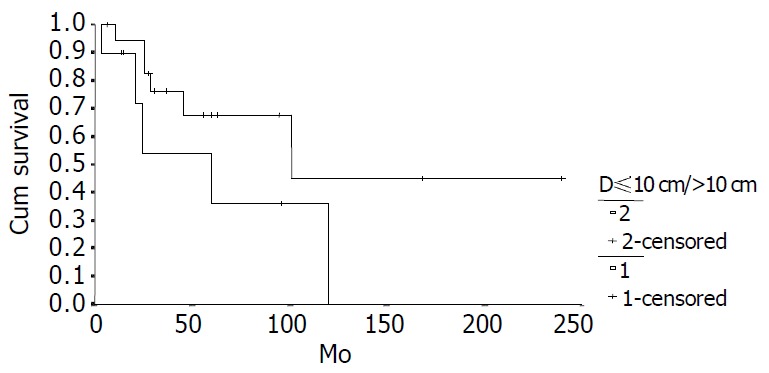
Survival comparison between patients with cancer of different diameters (D10 cm, n = 18; D > 10 cm, n = 10, P = 0.1169).
Table 6.
Multivariate Cox regression analysis
| Factors | Standard Error | Degree of freedom | P value |
| Sex | 2.190 | 1 | 0.679 |
| Age | 1.292 | 1 | 0.821 |
| Location | 35.889 | 1 | 0.917 |
| Operation | 1.528 | 1 | 0.007 |
| Tumor Size | 117.237 | 1 | 0.903 |
| Radial/palliative | 117.261 | 1 | 0.884 |
DISCUSSION
NFICTs are developmentally and histologically identical to functioning tumors but differ in their clinical course and outcome. In this group, the mean age at diagnosis (31.63 years) was 20 years younger than those reported by other researchers[3-5], but it was similar to some domestic reports[6-8]. The mean age of the females was 31.25 years, a little bit younger than that of males (33.57 years). It was considered that more male than female patients suffered from islet cell tumors. The ratio of males: females was 1.29:1[9-12]. In contrast to other reports, we found there were more females than males with this disease (F:M = 36:7). It was similar to domestic reports [6-8]. The reason is unknown. The difference races may be responsible for this phenomenon.
The clinical presentations of NFICTs were mainly due to mass effects. When tumors were smaller than 5 cm in diameter, most patients were asymptomatic[16]. The symptoms or signs at the time of diagnosis were abdominal pain, weight loss, nausea/vomiting, jaundice and abdominal mass[5-7,10].
Abdominal US and contrast-enhanced spiral CT were used. In this series, abdominal US and CT were used to detect the primary tumor in all but the patients with emergency operation. Correct prediction of a NFICT based on CT and US results was achieved only in 6 (20%) patients. It is believed that the following features appear to be characteristic for NFICTs: a well defined pancreatic mass with a unusually large size, moderate to strong mass hyperdense in the arterial contrastographic phase of either the primary or the hepatic metastases, and no infiltration of vessels of the celiac axis or the proximal superior mesenteric artery[4,13]. A recent report showed that in patients with NFICT, a hypoechoic mass with an irregular central echogenic area on EUS or complete obstruction of the main pancreatic duct on ERCP suggested a malignancy[14].
EUS and laparoscopy could provide accurate preoperative and intraoperative locatization to enhance laparoscopic or open operation[15]. Magnetic resonance imaging, angiography, transabdominal sonography and portal venous sampling could yield an additional accuracy for the diagnosis.
NFICTs of pancreas were believed to be malignant in the presence of distant metastases, or histologic evidence of vascular, lymphatic, or perineural invasion[3,10]. However, because all NFICTs had a similar histologic appearance, the absence of lymphovascular or perineural invasion could not exclude the potential for malignant behavior. Patients who had locally advanced nonmetastatic diseases at diagnosis subsequently died of complications of the primary tumor[11]. It was believed that neither vascular or perineural invasion nor electron microscopy snd any other histologic criteria could accurately predict whether tumor behavior was benign or malignant[5]. Most previous reports have suggested that NFICTs are biologically very aggressive, with a malignancy rate ranging from 82% to 100%[1,11]. However, our findings do not support these previous observations, as we found that the evidence of malignancy based upon evidence of local invasion or presence of metastases could accurately predict the clinical outcome. We believe that the diagnosis of malignancy in NFICTs should be mainly based upon gross findings of local tumor invasion or the presence of metastases. Some reports showed that patients with benign islet tumors were alive at the follow-up time point[16]. The 5 year survival rate of patients with benign tumors in our group was 100%.
Despite of advances in the multidisciplinary management of NFICC of pancreas in recent decades, surgery plays still an important role in providing potentially curative resections, relieving symptoms, and improving long-term survival. The resectable rate of this disease was 24% to 100%[3,4,17] and curative resection was performed only in 61% patients[5]. In current series, the resectable rate was 91%, the curative resection rate for the patients was 61%, which was comparable to that in literature reports. Radical operation could yield a best survival for patients with NFICC, palliative debulking could reduce the mass of tumor, enhance other treatment modalities, it might also prevent other complications such as recurrent pancreatitis, intestinal obstruction, and gastrointestinal bleeding due to direct tumor invasion or varices secondary to splenic vein obstruction[7]. For this reason, noncurative debulking of tumors might improve survival. Repeated resections for respectable recurrences or metastases might be indicated to improve survival also[8].
Tumor size is not included in the diagnostic criteria, but when NFICC is definitely diagnosed, it is helpful to predict the survival. When the diameter was larger than 10 cm, the long-term survival would be poor. On the other hand, female and younger (≤ 30 years) patients would have a better survival.
One third of our patients received adjuvant chemotherapy after radical resection of the primary tumors. Obviously, this was too small a number to make any meaningful observations regarding the efficacy of any specific chemotherapy regimen. Moertel and colleagues[18] reported a large prospective, randomized multi-institutional trial of two regimens using 162 patients with advanced islet cell carcinomas. They concluded that the combination of streptozotocin and 5-fluorouracil could produce a response rate of 63%, which was significantly higher than 34% seen with streptozotocin alone.
Footnotes
Edited by Wang XL and Xu JY Proofread by Xu FM
References
- 1.Kent RB, van Heerden JA, Weiland LH. Nonfunctioning islet cell tumors. Ann Surg. 1981;193:185–190. doi: 10.1097/00000658-198102000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broughan TA, Leslie JD, Soto JM, Hermann RE. Pancreatic islet cell tumors. Surgery. 1986;99:671–678. [PubMed] [Google Scholar]
- 3.Eriksson B, Skogseid B, Lundqvist G, Wide L, Wilander E, Oberg K. Medical treatment and long-term survival in a prospective study of 84 patients with endocrine pancreatic tumors. Cancer. 1990;65:1883–1890. doi: 10.1002/1097-0142(19900501)65:9<1883::aid-cncr2820650902>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Bartsch DK, Schilling T, Ramaswamy A, Gerdes B, Celik I, Wagner HJ, Simon B, Rothmund M. Management of nonfunctioning islet cell carcinomas. World J Surg. 2000;24:1418–1424. doi: 10.1007/s002680010234. [DOI] [PubMed] [Google Scholar]
- 5.White TJ, Edney JA, Thompson JS, Karrer FW, Moor BJ. Is there a prognostic difference between functional and nonfunctional islet cell tumors. Am J Surg. 1994;168:627–629; discussion 627-629;. doi: 10.1016/s0002-9610(05)80134-5. [DOI] [PubMed] [Google Scholar]
- 6.Zheng X, Guo K, Tian Y, Li J, Guo R, Zhan Y, Song M, Shen K. Cellular composition and anatomic distribution in nonfunctioning pancreatic endocrine tumors: immunohistochemical study of 30 cases. Chin Med J (Engl) 1998;111:373–376. [PubMed] [Google Scholar]
- 7.Lo CY, van Heerden JA, Thompson GB, Grant CS, Söreide JA, Harmsen WS. Islet cell carcinoma of the pancreas. World J Surg. 1996;20:878–883; discussion 884. doi: 10.1007/s002689900134. [DOI] [PubMed] [Google Scholar]
- 8.Jiang XS, Shou NH, LI ZY. Diagnosis and treatment of non-functional islet cell tumors. Zhongguo Putong Waike Zazhi. 1998;7:87–89. [Google Scholar]
- 9.Cheslyn-Curtis S, Sitaram V, Williamson RC. Management of non-functioning neuroendocrine tumours of the pancreas. Br J Surg. 1993;80:625–627. doi: 10.1002/bjs.1800800528. [DOI] [PubMed] [Google Scholar]
- 10.Legaspi A, Brennan MF. Management of islet cell carcinoma. Surgery. 1988;104:1018–1023. [PubMed] [Google Scholar]
- 11.Evans DB, Skibber JM, Lee JE, Cleary KR, Ajani JA, Gagel RF, Sellin RV, Fenoglio CJ, Merrell RC, Hickey RC. Nonfunctioning islet cell carcinoma of the pancreas. Surgery. 1993;114:1175–1181; discussion 1175-1181;. [PubMed] [Google Scholar]
- 12.Furukawa H, Mukai K, Kosuge T, Kanai Y, Shimada K, Yamamoto J, Mizuguchi Y, Ushio K. Nonfunctioning islet cell tumors of the pancreas: clinical, imaging and pathological aspects in 16 patients. Jpn J Clin Oncol. 1998;28:255–261. doi: 10.1093/jjco/28.4.255. [DOI] [PubMed] [Google Scholar]
- 13.Procacci C, Carbognin G, Accordini S, Biasiutti C, Bicego E, Romano L, Guarise A, Minniti S, Pagnotta N, Falconi M. Nonfunctioning endocrine tumors of the pancreas: possibilities of spiral CT characterization. Eur Radiol. 2001;11:1175–1183. doi: 10.1007/s003300000714. [DOI] [PubMed] [Google Scholar]
- 14.Sugiyama M, Abe N, Izumisato Y, Yamaguchi Y, Yamato T, Tokuhara M, Masaki T, Mori T, Atomi Y. Differential diagnosis of benign versus malignant nonfunctioning islet cell tumors of the pancreas: the roles of EUS and ERCP. Gastrointest Endosc. 2002;55:115–119. doi: 10.1067/mge.2002.119604. [DOI] [PubMed] [Google Scholar]
- 15.Spitz JD, Lilly MC, Tetik C, Arregui ME. Ultrasound-guided laparoscopic resection of pancreatic islet cell tumors. Surg Laparosc Endosc Percutan Tech. 2000;10:168–173. doi: 10.1097/00019509-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Yeo CJ, Wang BH, Anthone GJ, Cameron JL. Surgical experience with pancreatic islet-cell tumors. Arch Surg. 1993;128:1143–1148. doi: 10.1001/archsurg.1993.01420220063008. [DOI] [PubMed] [Google Scholar]
- 17.Thompson GB, van Heerden JA, Grant CS, Carney JA, Ilstrup DM. Islet cell carcinomas of the pancreas: a twenty-year experience. Surgery. 1988;104:1011–1017. [PubMed] [Google Scholar]
- 18.Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1980;303:1189–1194. doi: 10.1056/NEJM198011203032101. [DOI] [PubMed] [Google Scholar]


