Abstract
AIM: Activation of transcription factor nuclear factor-κB (NF-κB) has been shown to play a role in cell proliferation, apoptosis, cytokine production, and oncogenesis. The purpose of this study was to determine whether NF-κB was constitutively activated in human colorectal tumor tissues and, if so, to determine the role of NF-κB in colorectal tumorigenesis, and furthermore, to determine the association of RelA expression with tumor cell apoptosis and the expression of Bcl-2 and Bcl-xL.
METHODS: Paraffin sections of normal epithelial, adenomatous and adenocarcinoma tissues were analysed immunohisto- chemically for expression of RelA, Bcl-2 and Bcl-xL proteins. Electrophoretic mobility shift assay (EMSA) was used to confirm the increased nuclear translocation of RelA in colorectal tumor tissues. The mRNA expressions of Bcl-2 and Bcl-xL were determined by reverse transcription polymerase chain reaction (RT-PCR) analysis. Apoptotic cells were detected by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate fluorescence nick end labeling (TUNEL) method.
RESULTS: The activity of NF-κB was significantly higher in adenocarcinoma tissue in comparison with that in adenomatous and normal epithelial tissues. The apoptotic index (AI) significantly decreased in the transition from adenoma to adenocarcinoma. Meanwhile, the expressions of Bcl-2 and Bcl-xL protein and their mRNAs were significantly higher in adenocarcinoma tissues than that in adenomatous and normal epithelial tissues.
CONCLUSION: NF-κB may inhibit apoptosis via enhancing the expression of the apoptosis genes Bcl-2 and Bcl-xL. And the increased expression of RelA/nuclear factor-κB plays an important role in the pathogenesis of colorectal carcinoma.
INTRODUCTION
Rel/NF-κB is a family of dimeric transcription factors that control the expression of numerous genes involved in cell growth, differentiation, regulation of apoptosis, cytokine production, and neoplastic transformation[1,2]. The Rel/NF-κB family comprises NF-κB1 (p50), NF-κB2 (p52), and the Rel proteins, RelA (p65), RelB, and c-Rel, which have a high level of sequence homology within their NH2-terminal 300 amino acids, the Rel homology domain[3]. The p50 and p52 can interact with the RelA proteins to form all possible homo- and heterodimer combinations. The most common dimer is the RelA (p65)/NF-κB1 (p50) heterodimer, i.e., NF-κB. In most unstimulated cells, Rel/NF-κB proteins are sequestered in the cytoplasm and are complexed with specific inhibitor proteins called IκB that render the Rel/NF-κB proteins inactive[3]. Stimulation of cells leads to phosphorylation and degradation of IκB and allows translocation of Rel/NF-κB to the nucleus, resulting in expression of target genes[4]. A surprising variety of inducers have been found to activate Rel/NF-κB[3]. These pathways are involved in innate immune responses that involve cytokines such as tumor necrosis factor (TNF) -α and IL-1, responses to physical stresses such as UV light and ionizing radiation (χ and γ), and responses to oxidative stresses such as hydrogen peroxide and butyl peroxide[5].
Several investigators have reported constitutive activation of NF-κB in various types of human tumor cell lines, including those of lymphoid origin such as Hodgkin/Reed Sternberg cells[6], T-cell lymphoma Hut 78 cells[7], and multiple myeloma cells[8]. In addition, nonlymphoid cell lines including ovarian cancer cells[9], lung carcinoma cells[10], breast cancer cells[11], thyroid carcinoma cells[12], melanomas[13], and bladder cancer cells[14] exhibited enhanced NF-κB activity.
Recent studies also indicated that NF-κB was constitutively activated in tumors such as pancreatic cancer and breast cancer[15,16]. However, little information is available concerning NF-κB activation in colorectal carcinoma, which is one of the most aggressive forms of cancer. The major objective of this study was to determine whether NF-κB was constitutively activated in colorectal carcinoma tissues, to examine whether the expression of Bcl-2 and Bcl-xL was regulated by NF-κB activation, and to evaluate the correlation between NF-κB activity and apoptosis in colorectal carcinoma.
MATERIALS AND METHODS
Materials
Ten normal colorectal mucosa, thirty colorectal adenoma (average age of the patients: 56.8 years), and thirty colorectal carcinoma (average age of the patients: 58 years) patients who gave informed consent before surgical treatment were entered into the present study. Specimens were obtained from the Department of Pathology, Renmin Hospital, Wuhan University (Wuhan, China). The patients had received neither chemotherapy nor radiation therapy before tumor resection. To justify comparisons, we excluded lesions from patients with familial colon carcinoma syndrome and suspected de novo cancers. Tissues were fixed with 40 g/L formaldehyde and embedded in paraffin for H & E staining and immunohistochemistry. Tissue specimens were snap-frozen immediately in liquid N2 and stored at -80 °C for EMSA assays and RT-PCR analysis. The study was approved by the Institutional Board of the Ethics Committee of Wuhan University under full consideration of the declaration on human rights of Helsinki.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue blocks were cut into 5 μm thick and mounted onto glass slides. After that, they were kept in an oven at 4 °C overnight. Immunostaining was performed as previously described with a slight modification[17]. Sections were deparaffinized in xylene and rehydrated. Endogenous peroxidase activity was blocked with 1% hydrogen peroxide for 20 min. To improve the quality of staining, microwave oven-based antigen retrieval was performed. Slides were probed with either anti-RelA (1:50, mouse monoclonal, Santa Cruz Biotechnology), anti-Bcl-2 (1:100, mouse monoclonal, Santa Cruz Biotechnology) or anti-Bcl-xL (1:100, mouse monoclonal, Santa Cruz Biotechnology). Sections were washed three times with PBS for 10 min each and incubated with biotin-labeled anti-mouse IgG for 1 h at room temperature. After three washes with PBS for 10 min each, sections were stained with a streptavidin-peroxidase detection system. Incubation with PBS instead of the primary antibody served as a negative control. In specimens containing positive cells, the positive cells were counted in ten randomly selected fields under high power microscope (200-fold or 400-fold magnification) for each sample, and the average was expressed as the density of positive cells.
Determination of apoptosis
The TUNEL assay, originally described by Gavrieli et al[18], was used with minor modifications. Briefly, tissue sections of 5 μm were mounted onto glass slides, deparaffinized, hydrated, and treated for 15-30 min at 37 °C with proteinase-K (Roche Co.; 20 μg/mL in 10 mmol/L Tris-HCl buffer, pH7.4). Slides were rinsed twice with PBS. Then, 50 μL of TUNEL reaction mixture (450 μL nucleotide mixture containing fluoresceinated dUTP in reaction buffer plus 50 μL enzyme TdT from calf thymus, Roche Co.) were added to the samples. To ensure homogeneous distribution of the TUNEL reaction mixture on tissue sections and to avoid evaporative loss, slides were covered with coverslips during incubation. Slides were incubated in a humidified chamber for 60 min at 37 °C. After rinsed, slides were incubated with anti-fluorescein antibody, with Fab fragment from sheep, conjugated with horse-radish peroxidase for 30 min at 37 °C. Slides were rinsed twice with PBS. Then, 50-100 μL of DAB substrate was added and incubated for 10 min at room temperature. Samples can be counterstained prior to analysis by light microscope. Positive signals were defined as presence of a distinct brown color nuclear staining of the neoplastic cells or morphologically defined apoptotic bodies. The apoptotic index (AI) was determined by counting a total of at least 1000 neoplastic nuclei in 10 randomly chosen fields at 400-fold magnification. Apoptotic cells were identified using a TUNEL assay in conjunction with characteristic morphological changes such as cell shrinkage, membrane blebbing, and chromatin condensation, to distinguish apoptotic cells and apoptotic bodies from necrotic cells.
EMSA
Nuclear extracts were harvested according to protocols described previously.[19] In brief, fresh samples were minced and homogenized in 400 μL of hypotonic lysis buffer A (10 mmol/L HEPES pH7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA,1 mmol/L DTT, and 1 mmol/L PMSF). Homogenized tissues were incubated on ice for 5 min, NP-40 was added to a final concentration of 5 g/L, and samples were vigorously mixed and centrifuged. The cytoplasmic proteins were removed and the pellet nuclei were resuspended in 50 μL buffer C (20 mmol/L HEPES pH7.9, 0.4 mol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, and 1 mmol/L PMSF). After 30 min agitation at 4 °C, the samples were centrifuged and supernatants, containing nuclear proteins, were transferred to a fresh vial. The protein concentrations of nuclear extracts were determined by Bio-Rad protein assay. The nuclear extracts were stored at -80 °C until use. Nuclear protein extracts of carcinomas, adenomas, and normal tissues were analyzed by EMSA for NF-κB nuclear translocation as previously described[20-22]. EMSA binding reaction mixture contained 8 μg protein of nuclear extracts, 2 μg of poly (deoxyinosinic- deoxycytidylic acid) (Sigma Co.), and [32P]-labeled double-stranded oligonucleotide containing the binding motif of NF-κB probe (4000 cpm) in binding buffer (10 mmol/L HEPES pH7.9, 50 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L DTT, 100 mL/L glycerol, and 0.2 g/L albumin). The sequence of the double-stranded oligomer used for EMSA was 5’-AGTTGAGGGGACTTTCCCAGGC-3’. The reaction was incubated for 30 min at room temperature before separation on a 50 g/L acrylamide gel, followed by autoradiography. For supershift experiments, 2 μg of mouse monoclonal antibodies against the p65 subunit (Santa Cruz Biotechnology) of NF-κB was incubated with the nuclear extracts 10 min before the addition of the [32P]-labeled probe and then analyzed as described.
RT-PCR
The mRNA expressions of Bcl-2 and Bcl-xL were assessed using RT-PCR standardized by coamplifying housekeeping gene β-actin, which served as an internal control. Total RNA was isolated from the normal epithelial, adenomatous and adenocarcinoma tissues by the single-step method[23]. Total RNA was reversely transcribed into cDNA and used for PCR with human specific primers for Bcl-2, Bcl-xL and β-actin. Sequences of Bcl-2 primers were 5’-CAGCTGCACCTGACGCCCTT –3’ (forward primer) and 5’-GCCTCCGTTATCCTGGATCC-3’ (reverse primer), generating a 199 bp PCR product; for Bcl-xL , the forward primer was 5’-AAGGATACAGCTGGAGTCAG-3’ and the reverse primer was 5’-ATCAATGGCAACCCATCCTG-3’, generating a 316 bp PCR product; for β-actin, the forward primer was 5’-AGCGGGAAATCGTGCGTGAC-3’ and the reverse primer was 5’-ACTCCTGCTTGCTGATCCACATC-3’, producing a 471 bp PCR product[24,25]. Briefly, the PCR was amplified by 32 repeat denaturation cycles at 95 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. During the first cycle, the denaturation was extended to 2 min, and in the final cycle the extension step was extended to 5 min. PCR products were separated on 15 g/L agarose gels containing 0.5 g/L of ethidium bromide and visualized by UV transillumination.
Statistical analysis
All statistical analyses were performed with SPSS10.0 statistical package for Microsoft Windows. Student’s t test and one-way analysis of variance (ANOVA) were used to compare continuous variables among groups. Correlation coefficients between continuous variables were calculated by the method of Pearson’s correlation coefficient. The χ2 test was used to compare binomial proportions. A P value of < 0.05 was considered significant.
RESULTS
RelA/NF-κB expression in colorectal carcinoma tissues
To investigate whether RelA/NF-κB-DNA binding activities were altered in human colorectal carcinoma tissues, we first carried out immunohistochemical analyses. The monoclonal antibodies used in this study detected only activated RelA proteins[17]. RelA staining was shown as brown color and detected in normal colorectal mucosa, colorectal adenoma and colorectal adenocarcinoma specimens. In colorectal adenoma and adenocarcinoma, positive staining of RelA was mainly observed in the cytoplasm, and nuclear staining for RelA was also detected (Figure 1). Tissues of colorectal adenocarcinoma showed more cells with nuclear staining for RelA than those in colorectal adenoma tissues. No nuclear staining for RelA was found in normal colorectal mucosa. As shown in Table 1, the density of RelA-positive cells was significantly increased (P < 0.01) in the transition from normal mucosa to adenoma and adenocarcinoma.
Figure 1.
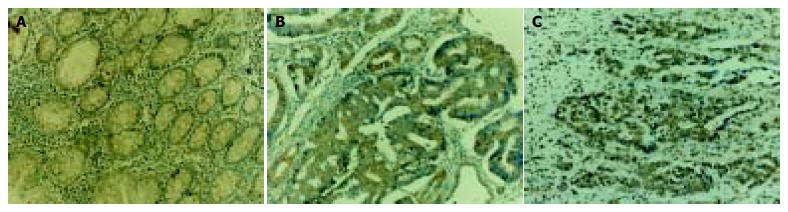
Immunohistochemical staining of RelA in tissue sections of colorectal adenoma (A) and adenocarcinoma (B, C). RelA protein is mainly expressed in the cytoplasm of tumor cells and nuclear accumulation of RelA is also detected. × 200.
Table 1.
Changes in expression of RelA, Bcl-2, Bcl-xL and AI in transition from normal mucosa to tumor tissues
| Group | n | RelA | Bcl-2 | Bcl-xL | AI |
| Normal | 10 | 9.31 ± 0.56 | 15.62 ± 0.75 | 11.35 ± 0.71 | 13.09 ± 0.78 |
| Adenoma | 30 | 54.01 ± 4.53 | 55.64 ± 6.51 | 56.43 ± 6.14 | 105.91 ± 6.11 |
| Adenocarcinoma | 30 | 70.92 ± 7.23 | 78.23 ± 8.33 | 77.32 ± 6.51 | 31.53 ± 3.71 |
EMSA
To confirm the finding that RelA/NF-κB-DNA binding activities were activated in human colorectal carcinoma tissues, we carried out EMSA analyses. Figure 2 shows increased NF-κB DNA binding activity in adenocarcinoma tissues compared with that in adenoma and normal tissues. HPIAS-1000 SOFTWARE ANALYSIS took the image of electrophoresis. The absorbance of EMSA brands showed that the RelA/NF-κB complexes were not presented in normal colorectal epithelium, 0.6587 ± 0.0021 in adenocarcinoma, and 0.2153 ± 0.0013 in adenoma. The RelA expressions were significantly increased (P < 0.05) in the transition from normal colorectal epithelium to colon tumor tissues. To confirm the specificity of NF-κB DNA binding, we performed supershift analysis with antibodies specific for RelA (p65) and a competitive study with a 50-fold excess of unlabeled oligonucleotide. An antibody specific for RelA which recognizes RelA/NF-κB heterodimer, unlabeled oligonucleotide diminished the intensity of RelA/NF-κB complexes, indicating that complex was the NF-κB binding-specific band. Our results showed that RelA was frequently activated in human colorectal tumor tissues but not in normal colon tissue.
Figure 2.
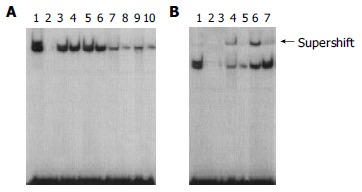
Electrophoretic mobility shift assay demonstrating increased nuclear translocation and DNA binding of NF-kB. A: lane 1, positive control (using Hela nuclear extract); lane 2, normal; lanes 3-6, adenocarcinoma; lanes 7-10, adenoma. B: lane 1, positive control (using Hela nuclear extract); lanes 2-3, specific competitor (using excess of unlabeled oligonucleotide); lanes 4-5, adenoma; lanes 6-7, adenocarcinoma; lane 4 and 6, supershift (addition of p65 antibodies to the nuclear extracts).
Bcl-2 and Bcl-xL protein expression in colorectal carcinoma tissues
In the present study, the expressions of Bcl-2 and Bcl-xL were also investigated using immunohistochemistry. Immunostaining specific for Bcl-2 and Bcl-xL was cytoplasmic and shown as brown color (Figures 3, Figures 4). The expressions of Bcl-2 and Bcl-xL were significantly increased (P < 0.01) from normal mucosa to tumor tissue (Table 1). Expressions of Bcl-2 and Bcl-xL were both significantly associated (r = 0.95,0.88; P < 0.05) with RelA expression in adenoma and adenocarcinoma.
Figure 3.
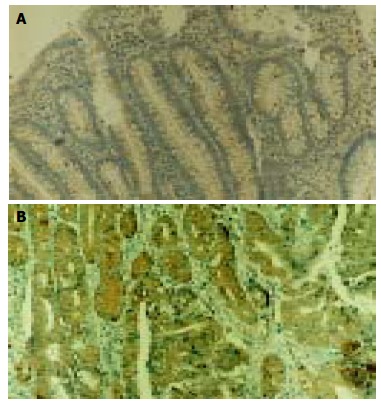
Immunohistochemical staining of Bcl-2 in tissue sections of colorectal adenoma (A) and adenocarcinoma (B). Bcl-2 expression is restricted to the cytoplasm of cancer cells. × 200.
Figure 4.
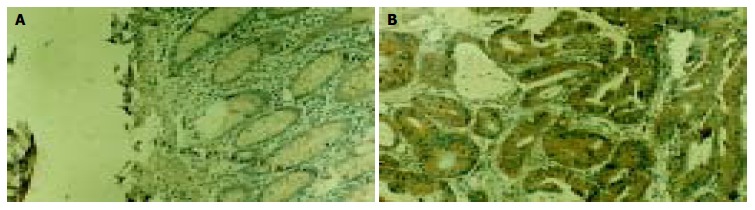
Immunohistochemical staining of Bcl-xL in tissue sections of colorectal adenoma (A) and adenocarcinoma (B). Bcl-xL expression is restricted to the cytoplasm of cancer cells. × 200.
Bcl-2 and Bcl-xL mRNA expression in colorectal carcinoma tissues
RT-PCR analysis of mRNA expressions of Bcl-2 and Bcl-xL was standardized by co-amplifying these genes with the housekeeping gene β-actin. HPIAS-1000 SOFTWARE ANALYSIS took the image of electrophoresis with β-action as internal standard. The relative absorbance of mRNA expression for Bcl-2: 2.43% ± 0.27% in normal tissues, 17.96% ± 1.51% in adenoma, and 36.71% ± 2.17% in adenocarcinoma; for Bcl-xL: 3.54% ± 0.33% in normal tissues, 23.02% ± 2.11% in adenoma, and 39.71% ± 2.49% in adenocarcinoma (Figure 5). Our results showed that colon tumor tissue constitutively expressed Bcl-2 and Bcl-xL. The mRNA expressions of Bcl-2 and Bcl-xL were significantly increased (P < 0.05) in the transition from normal colorectal epithelium to colon tumor tissue.
Figure 5.
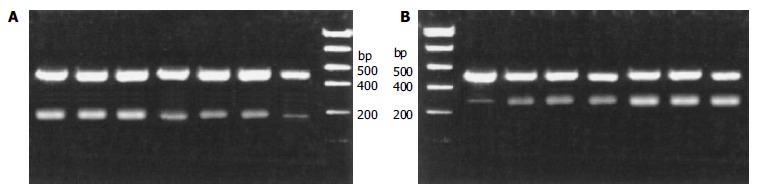
The mRNA expressions of Bcl-2 and Bcl-xL were assessed using RT-PCR standardized by coamplifying the housekeeping gene b-actin. A: the mRNA expression of Bcl-2. lanes 1-3, adenocarcinoma; lanes 4-6, adenoma; lane 7, normal; lane 8, marker. B: the mRNA expression of Bcl-2. lane 1, marker; lane 2, normal; lanes 3-5, adenoma; lanes 6-8, adenocarcinoma.
Cell apoptosis
In this study, TUNEL staining was restricted to the nucleus of apoptotic cells. TUNEL-positive staining cells were detected in normal colorectal mucosa, adenoma, and adenocarcinoma. The AI was significantly increased (P < 0.01) in the transition from normal colorectal mucosa to adenoma, but decreased from adenoma to adenocarcinoma (Figure 6). There was no association between the AI and the histological classification of adenoma and adenocarcinoma. The density of RelA-positive cells inversely correlated with the AI in the transition from adenoma to adenocarcinoma (r = -0.89; P < 0.001).
Figure 6.
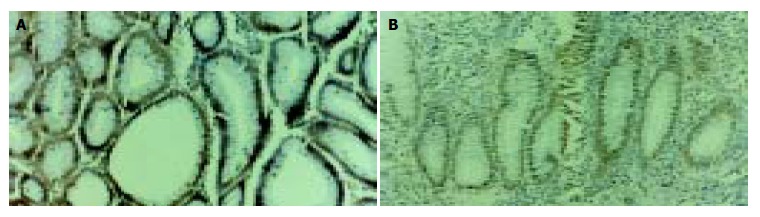
TUNEL staining in tissue sections of colorectal adenoma (A) and adenocarcinoma (B). TUNEL staining is restricted to the nucleus of apoptotic cells. × 200.
DISCUSSION
We have demonstrated that RelA-DNA binding activity was constitutively activated in the majority of human colorectal carcinomas. Whereas the role for RelA/NF-κB in tumorigenesis has not firmly established, recent work has suggested that it may play a role in this process. RelA/NF-κB activation has been shown to be necessary for tumor formation in Hodgkin lymphoma cells[26,27]. More recently, the inhibition of RelA/NF-κB activity through the use of specific NF-κB inhibitors (gliotoxin and MG132) resulted in spontaneous caspase-independent apoptosis in Hodgkin and Reed-Sternberg cells[28]. Also, an increase in RelA/NF-κB levels was identified in breast cancer cell lines, primary human breast cancer, hepatocellular carcinoma, pancreatic adenocarcinoma, and gastric carcinoma when compared with nontransformed controls or normal tissues[29-32]. In addition, NF-κB transcriptional activity was required for oncogenic Ras-induced cellular transformation[33], which likely occurred through the inhibition of transformation-associated apoptosis[34]. However, the role of NF-κB in colorectal tumorigenesis is unknown and currently under investigation.
We primarily used immunohistochemistry to detect NF-κB activation in human colorectal carcinoma tissues. Its expression was significantly increased in the transition from normal colorectal mucosa to adenoma and adenocarcinoma. In our immunohistochemical analyses, we used monoclonal antibodies to detect RelA/NF-κB-DNA binding activities, and their sensitivity and specificity have been characterized previously. They were useful in differentiating between activated and inactivated forms of RelA and facilitated the detection of the activated RelA proteins. In the current investigation, only 10%-20% of RelA/NF-κB protein was detectable in the nucleus, which was consistent with previous reports[32]. And 80%-90% of RelA still remained in the cytoplasm when RelA proteins were activated. It is unclear why the majority of RelA/ NF-κB proteins that were freed from IκB remained in the cytoplasm. Possible explanations for this[32] are: (1) IκB was mutated and therefore could not bind to RelA and masked the nuclear translocation signal in RelA; (2) mutations in RelA prohibited IκB binding to RelA, and (3) the RelA upstream signal transduction cascades were constitutively activated.
The Bcl-2 proto-oncogene is an apoptosis inhibitor originally described in association with the t (14; 18) (q32; q21) translocation in follicular B cell lymphoma, which places the Bcl-2 gene under the stimulatory control of the IgH promoter- enhancer at 14q32, resulting in increased Bcl-2 mRNA and protein[35] and inhibition of apoptosis. The Bcl-xL proto- oncogene, a member of Bcl-2 family, is a homologue of Bcl-2 and is an apoptosis inhibitor. The Bcl-2 and Bcl-xL oncoproteins have been described in normal colonic mucosa[35], where these were restricted to the epithelial regenerative compartment and the intestinal crypt bases. In our study, the expression of Bcl-2 and Bcl-xL was increased in the transition from normal mucosa to adenoma and adenocarcinoma. These results also show that the increased RelA/NF-κB expression occurred concomitantly with an increased expression of Bcl-2 and Bcl-xL. To date, a number of gene products that inhibit apoptosis have been identified. These include Bcl-2 and Bcl-xL genes. Indeed, Bcl-2 and Bcl-xL have been identified as NF-κB target gene[36], but the exact role NF-κB plays in its regulation remains controversial.
In recent years, increasing evidence indicates that activation of NF-κB plays an important role in coordinating the control of apoptotic cell death. NF-κB has been shown to prevent Fas-induced death in B cells through the upregulation of Bcl-2 and Bcl-xL expression[36], but has also been demonstrated to promote apoptosis in thymocytes by downregulating Bcl-2 and Bcl-xL gene expression[37]. However, the exact mechanism of NF-κB in the regulation of apoptosis is not entirely clear. There are at least two distinct mechanisms by which NF-κB blocks apoptosis[38]: (1) induction of antiapoptosis factors including IEX-1L, TRAF1, TRAF2, c-IAP-1, c-IAP-2 etc; (2) interference of apoptotic pathway by protein-protein interaction. However, these two distinct mechanisms are not mutually exclusive since either mechanism alone cannot fully explain the antiapoptotic action of NF-κB. In our study, apoptosis was significantly decreased in the transition from adenoma to adenocarcinoma, which was in contrast to the expressions of RelA, Bcl-2, and Bcl-xL. We also observed an inverse relation between AI and the expression of RelA in the transition from adenoma to adenocarcinoma, implying that increased RelA protein expression to a certain level might be anti-apoptotic and thus promote tumorigenic cell behavior. The anti-apoptotic role of NF-κB has been well characterized and various down-stream targets of NF-κB, including Bcl-2 and Bcl-xL have been identified. In the present study, the statistical correlation between the increased expression of RelA, elevated Bcl-2 and Bcl-xL expression implies that in colorectal tissue, activation of RelA might exhibit anti-apoptotic effects at least in part through upregulation of Bcl-2 and Bcl-xL expression. This has been shown to decrease mitochondrial permeability changes and cytochrome C release and thus to block apoptosis[39].
To date, NF-κB has been believed to play an important role in coordinating the control of apoptotic cell death. However, the mechanism by which NF-κB blocks apoptosis is still controversial. Some laboratories have reported that activation of NF-κB is able to either promote or prevent apoptosis, depending on different stimuli and different cell types[40-42]. For example, Grimm et al[43] reported that serum starvation activated NF-κB and induced human embryonic kidney cells into apoptosis. Qin et al[44] found that NF-κB activation contributed to the excitotoxin-induced death of striatal neurons. However, somewhat inconsistent results have also been presented by Beg and Baltimore[45] that NF-κB activation generally inhibited apoptosis in embryonic fibroblasts. A question arises: who is right on earth We think the answer is expected by further studies.
In conclusion, our results demonstrate that the RelA/NF-κB pathway is activated constitutively in colorectal carcinoma tissues, suggesting that activation of RelA/NF-κB might play an important role in colorectal tumorigenesis. Further studies are required to elucidate the mechanisms of NF-κB activation and to determine whether NF-κB might serve as a therapeutic target in the anti-neoplastic treatment of colorectal cancer.
Footnotes
Supported by Grants Grom National Natural Science Foundation of China No.39470330, and Natural Science Foundation of Hubei Province, China (SJ-97J083)
Edited by Zhang JZ and Zhu LH Proofread by Xu FM
References
- 1.Zhu JW, Yu BM, Ji YB, Zheng MH, Li DH. Upregulation of vascular endothelial growth factor by hydrogen peroxide in human colon cancer. World J Gastroenterol. 2002;8:153–157. doi: 10.3748/wjg.v8.i1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loncar MB, Al-azzeh ED, Sommer PS, Marinovic M, Schmehl K, Kruschewski M, Blin N, Stohwasser R, Gött P, Kayademir T. Tumour necrosis factor alpha and nuclear factor kappaB inhibit transcription of human TFF3 encoding a gastrointestinal healing peptide. Gut. 2003;52:1297–1303. doi: 10.1136/gut.52.9.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 4.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 5.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 6.Bargou RC, Leng C, Krappmann D, Emmerich F, Mapara MY, Bommert K, Royer HD, Scheidereit C, Dörken B. High-level nuclear NF-kappa B and Oct-2 is a common feature of cultured Hodgkin/Reed-Sternberg cells. Blood. 1996;87:4340–4347. [PubMed] [Google Scholar]
- 7.Giri DK, Aggarwal BB. Constitutive activation of NF-kappaB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells. Autocrine role of tumor necrosis factor and reactive oxygen intermediates. J Biol Chem. 1998;273:14008–14014. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 8.Feinman R, Koury J, Thames M, Barlogie B, Epstein J, Siegel DS. Role of NF-kappaB in the rescue of multiple myeloma cells from glucocorticoid-induced apoptosis by bcl-2. Blood. 1999;93:3044–3052. [PubMed] [Google Scholar]
- 9.Xiao CW, Yan X, Li Y, Reddy SA, Tsang BK. Resistance of human ovarian cancer cells to tumor necrosis factor alpha is a consequence of nuclear factor kappaB-mediated induction of Fas-associated death domain-like interleukin-1beta-converting enzyme-like inhibitory protein. Endocrinology. 2003;144:623–630. doi: 10.1210/en.2001-211024. [DOI] [PubMed] [Google Scholar]
- 10.Mukhopadhyay T, Roth JA, Maxwell SA. Altered expression of the p50 subunit of the NF-kappa B transcription factor complex in non-small cell lung carcinoma. Oncogene. 1995;11:999–1003. [PubMed] [Google Scholar]
- 11.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Sledge GW. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visconti R, Cerutti J, Battista S, Fedele M, Trapasso F, Zeki K, Miano MP, de Nigris F, Casalino L, Curcio F, et al. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFkappaB p65 protein expression. Oncogene. 1997;15:1987–1994. doi: 10.1038/sj.onc.1201373. [DOI] [PubMed] [Google Scholar]
- 13.Ivanov VN, Bhoumik A, Ronai Z. Death receptors and melanoma resistance to apoptosis. Oncogene. 2003;22:3152–3161. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- 14.Sumitomo M, Tachibana M, Ozu C, Asakura H, Murai M, Hayakawa M, Nakamura H, Takayanagi A, Shimizu N. Induction of apoptosis of cytokine-producing bladder cancer cells by adenovirus-mediated IkappaBalpha overexpression. Hum Gene Ther. 1999;10:37–47. doi: 10.1089/10430349950019174. [DOI] [PubMed] [Google Scholar]
- 15.Zelvyte I, Ohlsson B, Axelson J, Janciauskiene S. Diverse responses between human pancreatic cancer cell lines to native alpha 1-antitrypsin and its C-terminal fragment. Anticancer Res. 2003;23:2267–2273. [PubMed] [Google Scholar]
- 16.Sovak MA, Arsura M, Zanieski G, Kavanagh KT, Sonenshein GE. The inhibitory effects of transforming growth factor beta1 on breast cancer cell proliferation are mediated through regulation of aberrant nuclear factor-kappaB/Rel expression. Cell Growth Differ. 1999;10:537–544. [PubMed] [Google Scholar]
- 17.Zabel U, Henkel T, Silva MS, Baeuerle PA. Nuclear uptake control of NF-kappa B by MAD-3, an I kappa B protein present in the nucleus. EMBO J. 1993;12:201–211. doi: 10.1002/j.1460-2075.1993.tb05646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Johnson KR, Norris JS, Fan W. Nuclear factor-kappaB/IkappaB signaling pathway may contribute to the mediation of paclitaxel-induced apoptosis in solid tumor cells. Cancer Res. 2000;60:4426–4432. [PubMed] [Google Scholar]
- 20.Wong BC, Jiang Xh, Fan XM, Lin MC, Jiang SH, Lam SK, Kung HF. Suppression of RelA/p65 nuclear translocation independent of IkappaB-alpha degradation by cyclooxygenase-2 inhibitor in gastric cancer. Oncogene. 2003;22:1189–1197. doi: 10.1038/sj.onc.1206234. [DOI] [PubMed] [Google Scholar]
- 21.Li-Weber M, Laur O, Dern K, Krammer PH. T cell activation-induced and HIV tat-enhanced CD95(APO-1/Fas) ligand transcription involves NF-kappaB. Eur J Immunol. 2000;30:661–670. doi: 10.1002/1521-4141(200002)30:2<661::AID-IMMU661>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Šošić D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–180. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura S, Kondo S, Shinomura Y, Kanayama S, Miyazaki Y, Kiyohara T, Hiraoka S, Matsuzawa Y. Met/HGF receptor modulates bcl-w expression and inhibits apoptosis in human colorectal cancers. Br J Cancer. 2000;83:668–673. doi: 10.1054/bjoc.2000.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima-Iijima S, Hamada H, Reddy P, Kakunaga T. Molecular structure of the human cytoplasmic beta-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci USA. 1985;82:6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W, Royer HD, Grinstein E, Greiner A, Scheidereit C, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenkein D. Proteasome inhibitors in the treatment of B-cell malignancies. Clin Lymphoma. 2002;3:49–55. doi: 10.3816/clm.2002.n.011. [DOI] [PubMed] [Google Scholar]
- 28.Izban KF, Ergin M, Huang Q, Qin JZ, Martinez RL, Schnitzer B, Ni H, Nickoloff BJ, Alkan S. Characterization of NF-kappaB expression in Hodgkin's disease: inhibition of constitutively expressed NF-kappaB results in spontaneous caspase-independent apoptosis in Hodgkin and Reed-Sternberg cells. Mod Pathol. 2001;14:297–310. doi: 10.1038/modpathol.3880306. [DOI] [PubMed] [Google Scholar]
- 29.Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonenshein GE. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tai DI, Tsai SL, Chang YH, Huang SN, Chen TC, Chang KS, Liaw YF. Constitutive activation of nuclear factor kappaB in hepatocellular carcinoma. Cancer. 2000;89:2274–2281. [PubMed] [Google Scholar]
- 31.Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka M, et al. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7:4136–4142. [PubMed] [Google Scholar]
- 32.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 33.Finco TS, Westwick JK, Norris JL, Beg AA, Der CJ, Baldwin AS. Oncogenic Ha-Ras-induced signaling activates NF-kappaB transcriptional activity, which is required for cellular transformation. J Biol Chem. 1997;272:24113–24116. doi: 10.1074/jbc.272.39.24113. [DOI] [PubMed] [Google Scholar]
- 34.Mayo MW, Wang CY, Cogswell PC, Rogers-Graham KS, Lowe SW, Der CJ, Baldwin AS. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 35.Popescu RA, Lohri A, de Kant E, Thiede C, Reuter J, Herrmann R, Rochlitz CF. bcl-2 expression is reciprocal to p53 and c-myc expression in metastatic human colorectal cancer. Eur J Cancer. 1998;34:1268–1273. doi: 10.1016/s0959-8049(98)00057-4. [DOI] [PubMed] [Google Scholar]
- 36.Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:9136–9141. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hettmann T, DiDonato J, Karin M, Leiden JM. An essential role for nuclear factor kappaB in promoting double positive thymocyte apoptosis. J Exp Med. 1999;189:145–158. doi: 10.1084/jem.189.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kajino S, Suganuma M, Teranishi F, Takahashi N, Tetsuka T, Ohara H, Itoh M, Okamoto T. Evidence that de novo protein synthesis is dispensable for anti-apoptotic effects of NF-kappaB. Oncogene. 2000;19:2233–2239. doi: 10.1038/sj.onc.1203560. [DOI] [PubMed] [Google Scholar]
- 39.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 40.Glasgow JN, Wood T, Perez-Polo JR. Identification and characterization of nuclear factor kappaB binding sites in the murine bcl-x promoter. J Neurochem. 2000;75:1377–1389. doi: 10.1046/j.1471-4159.2000.0751377.x. [DOI] [PubMed] [Google Scholar]
- 41.O'Connor L, Huang DC, O'Reilly LA, Strasser A. Apoptosis and cell division. Curr Opin Cell Biol. 2000;12:257–263. doi: 10.1016/s0955-0674(99)00084-8. [DOI] [PubMed] [Google Scholar]
- 42.Yoneda T, Imaizumi K, Maeda M, Yui D, Manabe T, Katayama T, Sato N, Gomi F, Morihara T, Mori Y, et al. Regulatory mechanisms of TRAF2-mediated signal transduction by Bcl10, a MALT lymphoma-associated protein. J Biol Chem. 2000;275:11114–11120. doi: 10.1074/jbc.275.15.11114. [DOI] [PubMed] [Google Scholar]
- 43.Grimm S, Bauer MK, Baeuerle PA, Schulze-Osthoff K. Bcl-2 down-regulates the activity of transcription factor NF-kappaB induced upon apoptosis. J Cell Biol. 1996;134:13–23. doi: 10.1083/jcb.134.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin ZH, Wang Y, Nakai M, Chase TN. Nuclear factor-kappa B contributes to excitotoxin-induced apoptosis in rat striatum. Mol Pharmacol. 1998;53:33–42. doi: 10.1124/mol.53.1.33. [DOI] [PubMed] [Google Scholar]
- 45.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]


