Abstract
AIM: To investigate the pathway (s) mediating rat antral circular smooth muscle contractile responses to the cholinomimetic agent, bethanechol and the subtypes of muscarinic receptors mediating the cholinergic contraction.
METHODS: Circular smooth muscle strips from the antrum of Sprague-Dawley rats were mounted in muscle baths in Krebs buffer. Isometric tension was recorded. Cumulative concentration-response curves were obtained for (+)-cis-dioxolane (cD), a nonspecific muscarinic agonist, at 10-8-10-4 mol/L, in the presence of tetrodotoxin (TTX, 10-7 mol/L). Results were normalized to cross sectional area. A repeat concentration-response curve was obtained after incubation of the muscle for 90 min with antagonists for M1 (pirenzepine), M2 (methoctramine) and M3 (darifenacin) muscarinic receptor subtypes. The sensitivity to PTX was tested by the ip injection of 100 mg/kg of PTX 5 d before the experiment. The antral circular smooth muscles were removed from PTX-treated and non-treated rats as strips and dispersed smooth muscle cells to identify whether PTX-linked pathway mediated the contractility to bethanechol.
RESULTS: A dose-dependent contractile response observed with bethanechol, was not affected by TTX. The pretreatment of rats with pertussis toxin decreased the contraction induced by bethanechol. Lack of calcium as well as the presence of the L-type calcium channel blocker, nifedipine, also inhibited the cholinergic contraction, with a reduction in response from 2.5 ± 0.4 g/mm2 to 1.2 ± 0.4 g/mm2 (P < 0.05). The dose-response curves were shifted to the right by muscarinic antagonists in the following order of affinity: darifenacin (M3) > methocramine (M2) > pirenzepine (M1).
CONCLUSION: The muscarinic receptors-dependent contraction of rat antral circular smooth muscles was linked to the signal transduction pathway(s) involving pertussis-toxin sensitive GTP-binding proteins and to extracellular calcium via L-type voltage gated calcium channels. The presence of the residual contractile response after the treatment with nifedipine, suggests that an additional pathway could mediate the cholinergic contraction. The involvement of more than one muscarinic receptor (functionally predominant type 3 over type 2) also suggests more than one pathway mediating the cholinergic contraction in rat antrum.
INTRODUCTION
The mechanisms involved in the regulation of cholinergic contraction of intestinal smooth muscle are complex and not fully understood, despite the important role of the cholinergic system in the physiology of gastric emptying, and pathophysiology of several motility disorders. Cholinergic agonists activate muscarinic receptors which transduce cholinergic signals by activating G proteins[1,2]. Different signal transduction pathways in different species, as well as different pathways for the circular and longitudinal layers of intestinal smooth muscle have been reported[3-15].
Specific muscarinic receptors are abundantly present in the smooth muscles of gastrointestinal tract[16-22]. Muscarinic receptor subtypes have shown a G-protein coupling specificity, however the published data are inconsistent. In some studies M1, M3, M5 receptor subtypes were preferentially coupled to the Gq/11 protein class, the M2 and M4 receptors were linked to the PTX-sensitive Gi/Go proteins[23-25], Whereas in other studies muscarinic M2 receptors were insensitive to pertussis toxin[26].
Relatively few references are published characterizing rat stomach muscarinic receptor subtypes, again with conflicting results. Prevalence of M3[13,22,27], or of M1[28], or M2 receptors[29-33] has been reported. Which muscarinic receptor is more functionally important in the cholinergic contraction of antral circular muscle has not been determined.
The aim of this study was to examine the signal transduction pathway (s) mediating rat antral smooth muscle cholinergic contraction to fill the existing knowledge gaps: (1) what type of calcium channel was involved and was there a dependence on extracellular Ca2+ influx; (2) whether PTX-sensitive- or PTX-insensitive-G proteins coupled to muscarinic receptors were involved; and (3) what subtypes of specific muscarinic receptors were functionally involved. Determining the physiology of the pathway (s) mediating cholinergic contraction of the antrum should be helpful in understanding the functional motility changes described in models of disease conditions, such as diabetes[34-36]. Preliminary accounts of some of these observations have been published in abstract form[37,38].
MATERIALS AND METHODS
Animals
Young adult male Sprague-Dawley rats (Charles River Breeding Laboratories), weighing 200-450 g were used. Animals were anesthetized by ip injection of sodium pentobarbital (30-65 mg/kg). Anesthesia was given immediately before the tissue removal to avoid the effect of anesthesia on the contractile properties of the tissue. The abdomen was explored through midline incision and the stomach was removed. After the tissue was removed, animals were euthanized by injection of an overdose of pentobarbital. All our studies were approved by the Institutional Animal Care and Use Committee of the PSU College of Medicine.
Smooth muscle strip bath preparation[39]
The antrum tissue was pinned in a dissecting dish in oxygenated Krebs solution, mucosa was gently removed by scraping, and strips were cut in the circular muscle orientation. The muscle was oxygenated in Krebs physiological buffer containing (in mmol/L) 130 Na, 4.7 K, 2.5 Ca, 1.0 Mg2, 140.7 Cl, 19 HCO3, 1.0 PO4 and 10 glucose at 37 °C. The strips were sutured at one end to a glass rod and at the other end to an inelastic wire. The tissue, the rod and a wire were placed in a 10-mL double walled glass chamber with a constant temperature 37 °C. Glass surfaces were silicone coated with Sigmacote to prevent binding of the peptides. The chambers were filled with 5 mL of Krebs buffer and gassed with 95:5 mixture of O2/CO2. The free end of wire was attached to an isometric force transducer (Grass Instrument Co) and recordings were made on a multichannel rectilinear dynograph recorder (Beckman Instruments). The strips were allowed to equilibrate for 1 h. Tissues were stretched to an initial length (Li) from which any additional stretch resulted in an increase in tension. The isometric tension response to bethanechol (10-4 mol/L) was noted. The strips were rinsed and the length increased by 1 mm increment until the maximum response to bethanechol (10-4 mol/L) was recorded. This length was labeled Lo. All subsequent studies were conducted at this length. Drugs were added to the tissue bath and the peak response within 8 min was compared with the maximum tension recorded during 5 min before addition of drugs. Antagonists were added 1 min before the addition of agonist. The peptidase inhibitors bestatin and phosphoramidon (both at 10-6 mol/L) were added at least 5 min before the agonist addition. The response was calculated as the change in the maximal force of contraction in g of tension normalized to the cross sectional area which was calculated as: cross section (mm2) = weight (g)/specific density × length (mm), where the specific density of muscle tissue = 1.056 (g/mm3).
Dispersed single muscle cell preparation[40,41]
Smooth muscle cells were isolated from the circular muscle layer of the antrum. The mucosa was removed by dissection and the longitudinal muscle layer (with enteric plexus ganglia) was removed in strips using a Stadie-Riggs tissue slicer (Thomas TM). The circular muscle layer was minced and incubated for 45 min twice in Hepes buffer containing collagenase (CLS type II, Worthington) and 0.1 g/L soybean trypsin inhibitor at 31 °C. Partially digested strips were washed with enzyme-free Hanks' medium. Muscle cells were allowed to disperse spontaneously under the gentle force of bubbling 950 mL/L O2 + 50 mL/L CO2 for 30 min (no agitation), and then filtered through 500 μmol/L Nitinol mesh, to the culture media. Viability was checked by trypan blue exclusion test. Aliquots of cells were added to solutions containing the agonists at room temperature. The reaction was stopped after 30 min, by addition of acrolein to the final concentration of 1%. Aliquots were sealed under coverslips. Computerized image analysis (NIH Image 1.62) was used for quantification. A scale slide was used for reference. The length of single muscle cells was measured (at least 50 cells per concentration).
Contractile response of antrum smooth muscle strips to cholinergic agonist
To determine the effect of bethanechol on antrum circular smooth muscle contraction, muscle strips were exposed to the increasing doses of muscarinic agonist, bethanechol, at the concentrations of 10-4 to 10-7 mol/L in an organ bath.
Characteristics of effect of extracellular Ca2+ and type of calcium channel involved
To determine whether cholinergic contraction depended on the influx of extracellular Ca2+, the response to bethanechol chloride in the physiological Krebs buffer was tested, then the buffer was changed into Ca-free buffer (in mmol/L: NaCl 132.5; KCl 4.7; MgCl2 1.0; NaH2PO4 1.2; NaHCO3 20.0; D-glucose 10; EGTA (50 μmol/L), and contraction was subsequently recorded after 5 and 10 min.
To characterize the type of calcium channel involved in the cholinergic contraction, nifedipine (an L-type calcium channel antagonist) was used. Nifedipine was dissolved in ethanol and added to the physiological Krebs buffer at the concentration of 10-5 mol/L[42] and contraction was recorded. Statistical significance of the difference between contraction in the presence and absence of nifedipine was calculated by paired t-test, the results were considered statistically significant at P ≤ 0.05.
Treatment with pertussis toxin (PTX)
In order to determine whether PTX-sensitive pathway was involved in cholinergic contraction, strips and dispersed muscle cells (myocytes) isolated from the antrum of PTX-pretreated and non-pretreated animals were compared.
Rats were injected with 100 mg/kg of PTX (dissolved in saline) intraperitoneally 5 d before the study[43]. Muscle strips from PTX-treated and control rats in the tissue bath were exposed to cholinergic agonist, bethanechol, at the concentration of 10-4 to 10-6 mol/L. Statistical significance of the difference between the contraction of the muscle from PTX-pretreated and non-treated rats was defined by non-paired t-test, the results were considered statistically significant at P ≤ 0.05.
The changes in the pattern of contraction of muscle cells in dispersed cell suspension were also measured (detailed description in the “dispersed muscle cell preparation” section of Materials & Methods). Two concentrations of bethanechol (10-7 and 10-8 mol/L) were added to the cell suspensions in the tubes in the physiological buffer. Their contractions were measured as the percentage of the control cell diastolic length[44]. The mean lengths of cells from control rats were compared to those of the cells from PTX-treated animals. Results were presented as mean ± SE. Statistical significance of the difference was calculated by the paired t-test, the results were considered statistically significant at P ≤ 0.05.
Characterization of muscarinic receptor subtypes involved
For the characterization of muscarinic receptor subtypes involved in cholinergic contraction we used a non-selective muscarinic agonist, (+)-cis-Dioxolane[45,46] and relatively specific receptor subtype antagonists.
The conditions of organ bath were described above in the “Smooth muscle strip bath preparation” section of Materials and Methods. At the start of the experimental protocol, the viability of each tissue was assessed by determining the contractile response to bethanechol (10-4 mol/L). After washed, tissues were re-equilibrated for 10 min and allowed to regain baseline tension. Cumulative concentration-effect curves of (+)-cis-Dioxolane, (10-8 to 3 × 10-5 mol/L) were constructed for each tissue. Tissues were then equilibrated in either the absence (control) or presence of the antagonist for 90 min. Subsequently, a second concentration-effect curve to (+)-cis-Dioxolane was constructed. Smooth muscle strips were incubated with increasing concentrations of antagonists demonstrating a relative specificity for M1, M2 or M3 muscarinic receptor subtypes (pirenzepine, methoctramine and darifenacin, respectively). Each antral smooth muscle strip was exposed to only one concentration of antagonists and incubated for 90 min at 37 °C, with a fresh antagonist added to the medium every 30 min[47,48].
The EC50 values for muscarinic antagonists were obtained (i.e. antagonist concentration resulting in 50% of inhibition of the contraction induced by cholinergic agonist, (+)-cis-Dioxolane (10-6 mol/L).
Drugs
Tetrodotoxin (TTX), sigmacote, neurokinin A (NKA), nifedipine, papain, peptidase inhibitors bestatin and phosphoramidon, soybean trypsin inhibitor, acrolein and pirenzepine (predominantly M1 muscarinic receptor antagonist), were from Sigma, St. Louis, MO.
(+)-cis-dioxolane (cholinergic agonist) and methocramine (predominantly M2 muscarinic receptor antagonist) were purchased from RBI Inc., Natick, MA. PTX was purchased from List Biological Labs, Inc., Campbell, CA. Bethanechol chloride was purchased from Merck, West Point, PA and collagenase (CLS type II) from Worthington, PA. Darifenacin (predominantly M3 muscarinic receptor antagonist) was a generous gift from Pfizer Ltd, Sandwich, Kent, GB.
RESULTS
Dose-response curve to cholinergic agonist
A contractile dose-response was observed, when the antral circular smooth muscle strips were exposed to the increasing doses of muscarinic agonist, bethanechol, at the concentrations of 10-4 to 10-7 mol/L in an organ bath. A significant increase of the tension over the baseline was observed at the bethanechol concentrations of 10-4 to 10-6 mol/L (Figure 1, n = 4).
Figure 1.
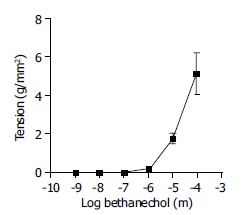
Effect of bethanechol on smooth muscle contraction. Antrum circular smooth muscle strips were incubated with increasing concentrations of bethanechol (10-4 mol/L to 10-7 mol/L). The vertical axis represents the developed tension (in grams per mm2). Bethanechol significantly increased circular muscle tension (P < 0.05; paired t-test). Each data point represents mean ± SE, n = 4.
Effect of tetrodotoxin (TTX)
Antrum circular smooth muscle strips were exposed to bethanechol at the concentration of 10-5 mol/L, in a buffer containing tetrodotoxin (10-5 mol/L), added prior to bethanechol to the organ bath. The cholinergic contraction was not affected by tetrodotoxin, neuronal toxin, with the contraction at 94.9% ± 3.2% of control.
The lack of inhibition of the contraction by TTX confirmed that a cholinergic agonist could induce contraction via muscarinic receptors present at the smooth muscle, without participation of neuronal factor.
Effect of calcium depletion on cholinergic contraction and characterization of a calcium channel subtype involved
Calcium-free buffer Incubation in calcium-free buffer diminished the antral contractile response to bethanechol (10-4 mol/L), with a reduction in response from 2.5 ± 0.4 g/mm2 to 1.2 ± 0.4 g/mm2 (P < 0.05) after 5 min of incubation in calcium-free buffer. After 10 min of incubation in Ca2+ -free buffer contractile activity was almost abolished (Figure 2).
Figure 2.
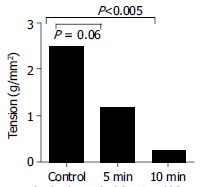
Effect of depletion of Ca2+ from medium on the contraction of antral circular smooth muscle strips to bethanechol (10-4 mol/L). Ca2+ depletion caused a significant decrease in the muscle tension; after 5 min incubation (P < 0.06, paired t-test); and after 10 min incubation (P < 0.005, paired t-test) compared to the base contraction induced by bethanechol before Ca2+ depletion. Each data point represents mean ± SE, n = 3.
Full recovery of the contraction was seen after return of the Ca2+ to the tissue bath medium, indicating that there was no damage to the cell resulting in a decreased contraction. These results showed that cholinergic contraction was dependent on the presence of extracellular calcium, a receptor-operated Ca2+ or voltage-sensitive Ca2+ channels.
Effect of calcium channel blocker The L-type calcium channel blocker, nifedipine at a concentration of 10-5 mol/L inhibited the cholinergic contraction response of antrum to the bethanechol, at the concentration of 10-4 mol/L from 4.02 ± 0.9 to 0.49 ± 0.18 g/mm2 (P < 0.05), indicating that the L-calcium channel could mediate this contraction.
Identification of G-protein-linked signal transduction pathway by PTX
The response to bethanechol of antral circular muscle strips and dispersed smooth muscle cells from PTX-pretreated and control rats was compared (see Methods).
Smooth muscle strips The contractile response to bethanechol of the muscle strips from the PTX-pretreated animals was significantly lower than that of the muscle strip from the non-treated control rats at all concentrations, 10-6 to 10-4 mol/L (Figure 3, P ≤ 0.01).
Figure 3.
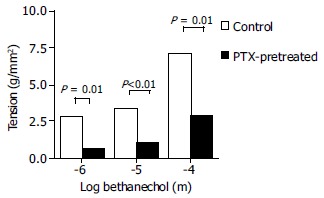
Effect of PTX on antrum circular smooth muscle strips contraction to bethanechol (10-4 mol/L to 10-6 mol/L). PTX significantly inhibited the contractile activity of the smooth muscle (P ≤ 0.01; paired t-test) compared to control rats. Each point represents mean ± SE, n = 7; dotted bars represent control animals; solid bars-PTX-pretreated animals.
The inhibiting effect of PTX on the contraction implied that cholinergic agonist was activating a PTX-sensitive pathway.
A low level of residual contractile activity was observed in the contraction of PTX-treated muscle strips suggesting either that there was a small PTX-insensitive fraction involved, or that the dose of PTX used was insufficient to completely inhibit PTX-sensitive pathways.
Dispersed antral smooth muscle cells When dispersed myocytes from PTX-treated rats and controls were compared, cells from PTX-pretreated rats showed significantly less contraction to bethanechol than the cells isolated from the rats non-treated with PTX, at both bethanechol concentrations, 10-8 and 10-7 mol/L (Figure 4, P ≤ 0.005). The inhibiting effect of PTX on the dispersed myocytes contraction, confirmed the smooth muscle strip data and suggested that the cholinergic agonist involved an occupation of specific muscarinic receptors coupled to PTX-sensitive mediated pathway.
Figure 4.
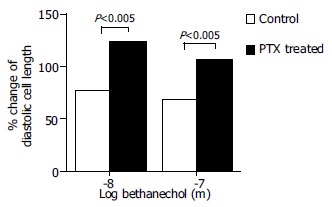
Effect of PTX on dispersed antral circular smooth muscle myocytes from PTX-pretreated rats contraction to bethanechol (10-7 mol/L and 10-8 mol/L). Myocytes from PTX-treated rats contracted less than myocytes from control rats. Each point represents mean ± SE; of the percentage of the control cells diastolic length (no bethanechol). At least 50 myocytes per point were calculated, P < 0.005; n = 7 and 11. Dotted bars represent control animals; solid bars PTX-pretreated animals.
Characterization of the type of muscarinic receptor subtypes involved in contraction to cholinergic agonist To define pharmacologically the muscarinic receptors involved in the cholinergic contraction, specific muscarinic receptor antagonists were used in the presence of tetrodotoxin (10-7 mol/L).
For this functional muscarinic receptor study we chose a non-selective cholinergic agonist, (+)-cis-Dioxolane (see Methods), instead of bethanechol, which has been reported to selectively stimulate M3 receptor[45,46,49].
Pirenzepine, the M1 muscarinic receptor antagonist, was used at the concentrations from 3 × 10-5 to 10-8 mol/L. Pirenzepine caused a significant inhibition of the of the contractile muscle response to the (+)-cis-dioxolane (Figure 5).
Figure 5.
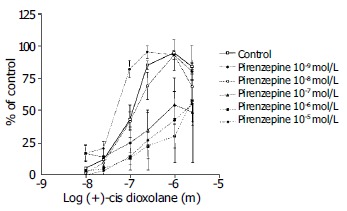
Concentration-response curves for the contractile effect of (+)-cis-Dioxolane alone and in combination with different concentrations of M1 antagonist, pirenzepine (10-8 mol/L to 10-5 mol/L) on the rat antral circular smooth muscle strips. Each point represents the mean ± SE, n = 6, 6, 7 and 8 strips. Cumulative concentration-effect curve was constructed for each strip for (+)-cis-Dioxolane, (10-8 to 3 × 10-5 mol/L). After washing, strips were equilibrated in either the absence (control) or presence of pirenzepine for 90 min. Subsequently, the second concentration-effect curve for (+)-cis-Dioxolane was constructed for each strip. Pirenzepine caused a significant inhibition of the contractile muscle response to (+)-cis-dioxolane.
Methocramine-M2 muscarinic receptor antagonist was used at the concentration range between 3 × 10-5 and 10-8 mol/L. Methocramine caused a significant inhibition of the of the contractile muscle response to the (+)-cis-dioxolane (Figure 6).
Figure 6.
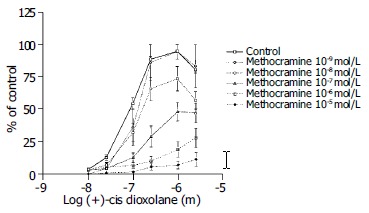
Concentration-response curves for the contractile effect of (+)-cis-Dioxolane alone and in combination with different concentrations of M2 antagonist, methocramine (from 10-8 mol/L to 10-5 mol/L) on the rat antral circular smooth muscle strips. Each point represents the mean ± SE; n = 4, 9, 10 and 11 strips. First, cumulative concentration-effect curve was constructed for each strip for (+)-cis-Dioxolane, (10-8 to 3 × 10-5 mol/L). After washing, tissues were equilibrated in either the absence (control) or presence of methocramine for a 90 min. Subsequently, the second concentration-effect curve for (+)- cis-Dioxolane was constructed for each strip. Methocramine caused a significant inhibition of the contractile muscle response to (+)-cis-Dioxolane.
Darifenacin was used at the concentration range from 3 × 10-5 to 10-9 mol/L (Figure 7). Darifenacin caused a significant inhibition of the contractile muscle response to the (+)-cis-dioxolane.
Figure 7.
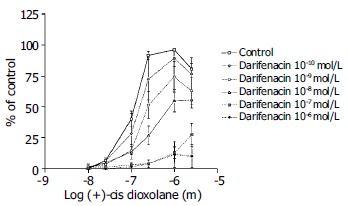
Concentration-response curves for the contractile effect of (+)-cis-Dioxolane alone and in combination with different concentrations of M3 antagonist, darifenacin (from 10-10 mol/L to 10-6 mol/L) on the rat antral circular smooth muscle strips. Each point represents the mean ± SE; n = 4, 7, 11, 10 strips. First, cumulative concentration-effect curve was constructed for each strip for (+)-cis-Dioxolane, (10-8 to 3 × 10-5 mol/L). After wash, tissues were equilibrated in either the absence (control) or presence of darifenacin for a 90 min. Subsequently, the second concentration-effect curve for (+)-cis-Dioxolane was constructed for each strip. Darifenacin caused a significant inhibition of the contractile muscle response to (+)-cis-Dioxolane.
Comparison the muscarinic M1, M2 and M3 receptor antagonists mediating the rat antral circular smooth muscle contraction
All three receptors (M1, M2 and M3) subtype antagonists inhibited the antral circular muscle contraction to cholinergic agonist (+)-cis-dioxolane. Darifenacin (M3) was most potent in inhibiting rat antral smooth muscle cholinergic contraction. The dose-response curves were shifted to the right by muscarinic antagonists in the following order of affinity: darifenacin (M3) > methocramine (M2) > pirenzepine (M1). The EC50 for each antagonist was as follows: darifenacin 7.9, methacramine 7.2 and pirenzepine 6.8 (Figure 8).
Figure 8.
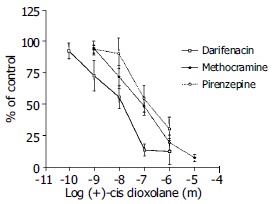
Comparison of the inhibition of the contractile response to cis-Dioxolane by 3 muscarinic receptor antagonists: M3- darifenacin, M2- methocramine, and M1- pirenzepine. The M3 antagonist, darifenacin, was the most potent inhibitor of the contraction induced by (+)-cis Dioxolane (10-6 mol/L). The EC50 values for each were: for darifenacin (M3) -7.9; for methocramine (M2) -7.2; and for pirenzepine (M1) -6.8.
DISCUSSION
It is well established that cholinergic agonists are of major importance for the stimulation of antral smooth muscle contraction and that their effect is mediated by muscarinic receptors. In various smooth muscle cells, the action of cholinergic agonists involved the occupation of specific receptors, activation of receptor-coupled G-proteins and subsequent activation of the second messenger systems, which would lead to the mobilization of Ca2+ from intracellular storage pools[10]. Certain steps of the signaling pathway (s) for the cholinergic contraction of antral circular smooth muscle in rats are still not understood, for example, the second messenger system or the functional characteristics of the muscarinic receptor subtypes involved. In our study the cholinergic rat antral circular muscle contraction in vitro was sensitive to the depletion from the extracellular Ca2+ that significantly diminished the cholinergic contraction. This finding along with the inhibitory effect of nifedipine indicated a dependence on extracellular Ca2+ influx via L-type calcium channels[50].
It is still not clear whether cholinergic contractile pathways of antral circular smooth muscle are mediated by PTX-sensitive GTP-binding protein through an increased inflow of extracellular calcium, or mediated via PTX-insensitive, inositol triphosphate (IP3)-dependent pathway. The circular muscle of cat esophagus and the lower esophageal sphincter (LES) were reported to be linked to different pathways. LES circular muscle cholinergic contraction was shown to be linked to the PTX-insensitive pathway, whereas esophageal circular muscle cholinergic contraction has been reported to be linked to the PTX-sensitive pathway[11-14].
In the present study, pretreatment with PTX significantly inhibited the contractile response to bethanechol of the antral smooth muscle strips in the organ bath and of the dissociated myocytes.
More than one muscarinic receptor may be responsible for cholinergically mediated contractility at a particular area[6,22,51-56]. A major role for M3 and a minor role for M2 receptors in cholinergic-induced smooth muscle contraction have been shown at the tissue of various species and organs[57-59], but it is still not clear how different receptors may interact to mediate a specific function. For example, M2 receptor might serve as an inhibitory presynaptic autoreceptor[60], or M2 receptor could act by gating cation channels that were subsequently modulated by M3 receptors[61].
The functional characteristics of muscarinic receptor subtypes regulating antrum circular muscle cholinergic contraction have not been previously reported. In our functional studies, cholinergic contraction in response to cis-dioxolane in rat antral circular muscles was mediated mostly through M3, less by M2, and the least by M1 muscarinic receptor subtypes.
The involvement of PTX-sensitive GTP-binding proteins in mediating the contractile response to muscarinic agonists has been reported to be linked to muscarinic M2 receptor in many studies, such as in cat esophageal circular muscle[11,12]. In contrast, our experiments in rat antral circular muscles indicated that the M3 muscarinic receptor was functionally prevalent, and yet, PTX-sensitive pathways were shown to be activated in the process of antral cholinergic contraction. A few published reports were in agreement with our results[13,22,26].
Possible explanations of the reported differences include: (1) Different experimental systems were used to compare the homogenous suspension of smooth muscle cells was compared with smooth muscle strips. (2) M3 and M2 receptors were expressed together at the shared site of the signaling pathways[22,54,57,60,62]. (3) The cholinergic agonist (+/- cis-Dioxolane) was used in our studies. Although, it has been reported to be non-selective[46,48,52], +/- cis-Dioxolane might be more selective for M3 muscarinic receptor than it was originally described[63]. (4) It has been suggested that seven transmembrane spanning receptors including muscarinic are promiscuous in that they could form interactions with multiple G-proteins[64] or activate many different transduction pathways at the same time[65]. (5) M2 receptor might be masked by M3 receptor, thus detectable when M3 receptor was inhibited. Separating out these possibilities would require more specific antagonists than the currently available.
In conclusion, the rat antral circular smooth muscle contracts to cholinergic agonists (bethanechol chloride and (+)-cis- Dioxolane) in a dose-dependent fashion through activation of muscarinic receptors at the smooth muscle. The contractile responses to cholinergic agonists are dependent on the increase in intracellular calcium induced by the influx of extracellular calcium via L-type voltage gated calcium channels. Bethanechol activates M3 and M2 muscarinic receptors coupled to pertussis-toxin sensitive GTP-binding protein (s). The presence of a residual contractile cholinergic response after 5 and 10 min in calcium-free buffer, as well as a residual contractile response after the treatment with nifedipine, suggests a role for an additional pathway (s). M3 receptor is predominant out of three functionally tested M1, M2, and M3 muscarinic receptors regulating cholinergic contraction. The involvement of more than one muscarinic receptor indicates more than one pathway (s) regulating the cholinergic contraction of rat antrum circular smooth muscle.
The results of these studies have important clinical implications for possible treatment of gastric dysfunction with muscarinic subtype-selective agents.
ACKNOWLEDGEMENTS
Authors would like to thank John Ellis, Ph.D, for helpful discussions. An excellent technical assistance of Rohini Polavarapu is acknowledged.
Footnotes
Supported by NIH grant RO1-DK-34148
Edited by Chen WW and Wang XL Proofread by Xu FM
References
- 1.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Bygrave FL, Roberts HR. Regulation of cellular calcium through signaling cross-talk involves an intricate interplay between the actions of receptors, G-proteins, and second messengers. FASEB J. 1995;9:1297–1303. doi: 10.1096/fasebj.9.13.7557019. [DOI] [PubMed] [Google Scholar]
- 3.Akbulut H, Gören Z, Iskender E, Eraslan A, Ozdemir O, Oktay S. Subtypes of muscarinic receptors in rat duodenum: a comparison with rabbit vas deferens, rat atria, guinea-pig ileum and gallbladder by using imperialine. Gen Pharmacol. 1999;32:505–511. doi: 10.1016/s0306-3623(98)00231-6. [DOI] [PubMed] [Google Scholar]
- 4.Grider JR, Makhlouf GM. Contraction mediated by Ca++ release in circular and Ca++ influx in longitudinal intestinal muscle cells. J Pharmacol Exp Ther. 1988;244:432–437. [PubMed] [Google Scholar]
- 5.Murthy KS, Grider JR, Makhlouf GM. InsP3-dependent Ca2+ mobilization in circular but not longitudinal muscle cells of intestine. Am J Physiol. 1991;261:G937–G944. doi: 10.1152/ajpgi.1991.261.6.G937. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Ruan Y, Guo ZD, Cong H, Zhang KY, Takemura H. Function and localization of high and low affinity binding sites to muscarinic receptors in longitudinal and circular smooth muscles of human stomach. Res Commun Chem Pathol Pharmacol. 1990;67:31–42. [PubMed] [Google Scholar]
- 7.Kerr PM, Hillier K, Wallis RM, Garland CJ. Characterization of muscarinic receptors mediating contractions of circular and longitudinal muscle of human isolated colon. Br J Pharmacol. 1995;115:1518–1524. doi: 10.1111/j.1476-5381.1995.tb16645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucchesi PA, Romano FD, Scheid CR, Yamaguchi H, Honeyman TW. Interaction of agonists and selective antagonists with gastric smooth muscle muscarinic receptors. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:145–151. doi: 10.1007/BF00165136. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki H, Koyama I, Nakamura H, Taneike T, Ohga A. Regional differences in cholinergic innervation and drug sensitivity in the smooth muscles of pig stomach. J Auton Pharmacol. 1991;11:255–265. doi: 10.1111/j.1474-8673.1991.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 10.Makhlouf GM, Murthy KS. Signal transduction in gastrointestinal smooth muscle. Cell Signal. 1997;9:269–276. doi: 10.1016/s0898-6568(96)00180-5. [DOI] [PubMed] [Google Scholar]
- 11.Sohn UD, Harnett KM, De Petris G, Behar J, Biancani P. Distinct muscarinic receptors, G proteins and phospholipases in esophageal and lower esophageal sphincter circular muscle. J Pharmacol Exp Ther. 1993;267:1205–1214. [PubMed] [Google Scholar]
- 12.Sohn UD, Han B, Tashjian AH, Behar J, Biancani P. Agonist-independent, muscle-type-specific signal transduction pathways in cat esophageal and lower esophageal sphincter circular smooth muscle. J Pharmacol Exp Ther. 1995;273:482–491. [PubMed] [Google Scholar]
- 13.Lin S, Kajimura M, Takeuchi K, Kodaira M, Hanai H, Kaneko E. Expression of muscarinic receptor subtypes in rat gastric smooth muscle: effect of M3 selective antagonist on gastric motility and emptying. Dig Dis Sci. 1997;42:907–914. doi: 10.1023/a:1018808329603. [DOI] [PubMed] [Google Scholar]
- 14.Cao W, Chen Q, Sohn UD, Kim N, Kirber MT, Harnett KM, Behar J, Biancani P. Ca2+-induced contraction of cat esophageal circular smooth muscle cells. Am J Physiol Cell Physiol. 2001;280:C980–C992. doi: 10.1152/ajpcell.2001.280.4.C980. [DOI] [PubMed] [Google Scholar]
- 15.Bitar KN, Saffouri B, Makhlouf GM. Cholinergic and peptidergic receptors on isolated human antral smooth muscle cells. Gastroenterology. 1982;82:832–837. [PubMed] [Google Scholar]
- 16.Konturek SJ, Jaworek J, Tasler J, Cieszkowski M, Yanaihara N. Subtypes of muscarinic receptors in canine pancreatic secretion in vivo and in vitro. Int J Pancreatol. 1987;2:11–22. doi: 10.1007/BF02788345. [DOI] [PubMed] [Google Scholar]
- 17.Liebmann C, Nawrath S, Schnittler M, Schubert H, Jakobs KH. Binding characteristics and functional G protein coupling of muscarinic acetylcholine receptors in rat duodenum smooth muscle membranes. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:7–15. doi: 10.1007/BF00175462. [DOI] [PubMed] [Google Scholar]
- 18.Brann MR, Ellis J, Jørgensen H, Hill-Eubanks D, Jones SV. Muscarinic acetylcholine receptor subtypes: localization and structure/function. Prog Brain Res. 1993;98:121–127. doi: 10.1016/s0079-6123(08)62388-2. [DOI] [PubMed] [Google Scholar]
- 19.Preiksaitis HG, Krysiak PS, Chrones T, Rajgopal V, Laurier LG. Pharmacological and molecular characterization of muscarinic receptor subtypes in human esophageal smooth muscle. J Pharmacol Exp Ther. 2000;295:879–888. [PubMed] [Google Scholar]
- 20.Eglen RM. Muscarinic receptors and gastrointestinal tract smooth muscle function. Life Sci. 2001;68:2573–2578. doi: 10.1016/s0024-3205(01)01054-2. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 22.Stengel PW, Yamada M, Wess J, Cohen ML. M(3)-receptor knockout mice: muscarinic receptor function in atria, stomach fundus, urinary bladder, and trachea. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1443–R1449. doi: 10.1152/ajpregu.00486.2001. [DOI] [PubMed] [Google Scholar]
- 23.Migeon JC, Thomas SL, Nathanson NM. Differential coupling of m2 and m4 muscarinic receptors to inhibition of adenylyl cyclase by Gi alpha and G(o)alpha subunits. J Biol Chem. 1995;270:16070–16074. doi: 10.1074/jbc.270.27.16070. [DOI] [PubMed] [Google Scholar]
- 24.Murthy KS, Makhlouf GM. Differential coupling of muscarinic m2 and m3 receptors to adenylyl cyclases V/VI in smooth muscle. Concurrent M2-mediated inhibition via Galphai3 and m3-mediated stimulation via Gbetagammaq. J Biol Chem. 1997;272:21317–21324. doi: 10.1074/jbc.272.34.21317. [DOI] [PubMed] [Google Scholar]
- 25.Kostenis E, Zeng FY, Wess J. Structure-function analysis of muscarinic receptors and their associated G proteins. Life Sci. 1999;64:355–362. doi: 10.1016/s0024-3205(98)00574-8. [DOI] [PubMed] [Google Scholar]
- 26.Yokotani K, Osumi Y. Cholinergic M2 muscarinic receptor-mediated inhibition of endogenous noradrenaline release from the isolated vascularly perfused rat stomach. J Pharmacol Exp Ther. 1993;264:54–60. [PubMed] [Google Scholar]
- 27.Milovanović DR, Janković SM. Pharmacologic characterization of muscarine receptor subtypes in rat gastric fundus mediating contractile responses. Indian J Med Res. 1997;105:239–245. [PubMed] [Google Scholar]
- 28.Nelson DK, Pieramico O, Dahmen G, Dominguez-Muñoz JE, Malfertheiner P, Alder G. M1-muscarinic mechanisms regulate interdigestive cycling of motor and secretory activity in human upper gut. Dig Dis Sci. 1996;41:2006–2015. doi: 10.1007/BF02093604. [DOI] [PubMed] [Google Scholar]
- 29.Hammer R. Muscarinic receptors in the stomach. Scand J Gastroenterol Suppl. 1980;66:5–11. [PubMed] [Google Scholar]
- 30.Herawi M, Lambrecht G, Mutschler E, Moser U, Pfeiffer A. Different binding properties of muscarinic M2-receptor subtypes for agonists and antagonists in porcine gastric smooth muscle and mucosa. Gastroenterology. 1988;94:630–637. doi: 10.1016/0016-5085(88)90233-8. [DOI] [PubMed] [Google Scholar]
- 31.Moummi C, Magous R, Strosberg D, Bali JP. Muscarinic receptors in isolated smooth muscle cells from gastric antrum. Biochem Pharmacol. 1988;37:1363–1369. doi: 10.1016/0006-2952(88)90795-2. [DOI] [PubMed] [Google Scholar]
- 32.Hasler WL, Heldsinger A, Owyang C. Cisapride acts on muscarinic (glandular M2) receptors to induce contraction of isolated gastric myocytes: mediation via a calcium-phosphoinositide pathway. J Pharmacol Exp Ther. 1991;259:1294–1300. [PubMed] [Google Scholar]
- 33.Rhee JC, Rhee PL, Park MK, So I, Uhm DY, Kim KW, Kang TM. Muscarinic receptors controlling the carbachol-activated nonselective cationic current in guinea pig gastric smooth muscle cells. Jpn J Pharmacol. 2000;82:331–337. doi: 10.1254/jjp.82.331. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi T, Kojima Y, Tsunoda Y, Beyer LA, Kamijo M, Sima AA, Owyang C. Impaired intracellular signal transduction in gastric smooth muscle of diabetic BB/W rats. Am J Physiol. 1996;270:G411–G417. doi: 10.1152/ajpgi.1996.270.3.G411. [DOI] [PubMed] [Google Scholar]
- 35.Xue L, Suzuki H. Electrical responses of gastric smooth muscles in streptozotocin-induced diabetic rats. Am J Physiol. 1997;272:G77–G83. doi: 10.1152/ajpgi.1997.272.1.G77. [DOI] [PubMed] [Google Scholar]
- 36.Maruyama Y, Sakai Y, Nobe K, Momose K. Subcellular distribution of protein kinase C isoforms in gastric antrum smooth muscle of STZ-induced diabetic rats. Life Sci. 1999;64:1933–1940. doi: 10.1016/s0024-3205(99)00138-1. [DOI] [PubMed] [Google Scholar]
- 37.Wrzos HF, Polavarapu R, Ouyang A. The signal transduction pathway involved in rat antral and colonic circular muscle response to muscarinic and neurokinin receptor agonists. Abstract. Gastroenterology. 1997;112:A853. [Google Scholar]
- 38.Wrzos HF, Tandon T, Ouyang A. Functional significance of muscarinic receptor subtypes on rat antral circular muscle. Abstract. Neurogastroenterol Motil 1998 [Google Scholar]
- 39.Bertiger G, Reynolds JC, Ouyang A, Cohen S. Properties of the feline pyloric sphincter in vitro. Gastroenterology. 1987;92:1965–1972. doi: 10.1016/0016-5085(87)90631-7. [DOI] [PubMed] [Google Scholar]
- 40.Bitar KN, Makhlouf GM. Receptors on smooth muscle cells: characterization by contraction and specific antagonists. Am J Physiol. 1982;242:G400–G407. doi: 10.1152/ajpgi.1982.242.4.G400. [DOI] [PubMed] [Google Scholar]
- 41.Biancani P, Hillemeier C, Bitar KN, Makhlouf GM. Contraction mediated by Ca2+ influx in esophageal muscle and by Ca2+ release in the LES. Am J Physiol. 1987;253:G760–G766. doi: 10.1152/ajpgi.1987.253.6.G760. [DOI] [PubMed] [Google Scholar]
- 42.Maggi CA, Zagorodnyuk V, Giuliani S. Specialization of tachykinin NK1 and NK2 receptors in producing fast and slow atropine-resistant neurotransmission to the circular muscle of the guinea-pig colon. Neuroscience. 1994;63:1137–1152. doi: 10.1016/0306-4522(94)90579-7. [DOI] [PubMed] [Google Scholar]
- 43.Thomas EA, Ehlert FJ. Pertussis toxin blocks M2 muscarinic receptor-mediated effects on contraction and cyclic AMP in the guinea pig ileum, but not M3-mediated contractions and phosphoinositide hydrolysis. J Pharmacol Exp Ther. 1994;271:1042–1050. [PubMed] [Google Scholar]
- 44.von Schrenck T, Mackensen B, Mende U, Schmitz W, Sievers J, Mirau S, Raedler A, Greten H. Signal transduction pathway of the muscarinic receptors mediating gallbladder contraction. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:346–354. doi: 10.1007/BF00170879. [DOI] [PubMed] [Google Scholar]
- 45.Choppin A, Eglen RM. Pharmacological characterization of muscarinic receptors in dog isolated ciliary and urinary bladder smooth muscle. Br J Pharmacol. 2001;132:835–842. doi: 10.1038/sj.bjp.0703901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith CM, Wallis RM. Characterisation of [3H]-darifenacin as a novel radioligand for the study of muscarinic M3 receptors. J Recept Signal Transduct Res. 1997;17:177–184. doi: 10.3109/10799899709036602. [DOI] [PubMed] [Google Scholar]
- 48.Choppin A, Eglen RM, Hegde SS. Pharmacological characterization of muscarinic receptors in rabbit isolated iris sphincter muscle and urinary bladder smooth muscle. Br J Pharmacol. 1998;124:883–888. doi: 10.1038/sj.bjp.0701920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barocelli E, Morini G, Ballabeni V, Lavezzo A, Impicciatore M. Effects of two new pirenzepine analogs on the contractile response of the guinea-pig oesophageal muscularis mucosae to acetylcholine, bethanechol, histamine and high potassium. Eur J Pharmacol. 1990;179:89–96. doi: 10.1016/0014-2999(90)90405-u. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto H, Inoue K, Kamisaki T, Takahashi K, Sato M. Regional differences in calcium sensitivity in the guinea-pig intestine. J Pharm Pharmacol. 1997;49:981–984. doi: 10.1111/j.2042-7158.1997.tb06027.x. [DOI] [PubMed] [Google Scholar]
- 51.Dietrich C, Kilbinger H. Prejunctional M1 and postjunctional M3 muscarinic receptors in the circular muscle of the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:237–243. doi: 10.1007/BF00233242. [DOI] [PubMed] [Google Scholar]
- 52.Eglen RM, Harris GC. Selective inactivation of muscarinic M2 and M3 receptors in guinea-pig ileum and atria in vitro. Br J Pharmacol. 1993;109:946–952. doi: 10.1111/j.1476-5381.1993.tb13712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehlert FJ, Sawyer GW, Esqueda EE. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal smooth muscle. Life Sci. 1999;64:387–394. doi: 10.1016/s0024-3205(98)00584-0. [DOI] [PubMed] [Google Scholar]
- 54.Sawyer GW, Ehlert FJ. Muscarinic M3 receptor inactivation reveals a pertussis toxin-sensitive contractile response in the guinea pig colon: evidence for M2/M3 receptor interactions. J Pharmacol Exp Ther. 1999;289:464–476. [PubMed] [Google Scholar]
- 55.Wang J, Krysiak PS, Laurier LG, Sims SM, Preiksaitis HG. Human esophageal smooth muscle cells express muscarinic receptor subtypes M(1) through M(5) Am J Physiol Gastrointest Liver Physiol. 2000;279:G1059–G1069. doi: 10.1152/ajpgi.2000.279.5.G1059. [DOI] [PubMed] [Google Scholar]
- 56.Ren J, Harty RF. Presynaptic muscarinic receptors modulate acetylcholine release from rat antral mucosal/submucosal nerves. Dig Dis Sci. 1994;39:1099–1106. doi: 10.1007/BF02087564. [DOI] [PubMed] [Google Scholar]
- 57.Chen Q, Yu P, de Petris G, Biancani P, Behar J. Distinct muscarinic receptors and signal transduction pathways in gallbladder muscle. J Pharmacol Exp Ther. 1995;273:650–655. [PubMed] [Google Scholar]
- 58.Bellido I, Fernández JL, Gómez A, Sánchez de la Cuesta F. Otenzepad shows two populations of binding sites in human gastric smooth muscle. Can J Physiol Pharmacol. 1995;73:124–129. doi: 10.1139/y95-017. [DOI] [PubMed] [Google Scholar]
- 59.Hanack C, Pfeiffer A. Upper gastrointestinal porcine smooth muscle expresses M2- and M3-receptors. Digestion. 1990;45:196–201. doi: 10.1159/000200246. [DOI] [PubMed] [Google Scholar]
- 60.Parkman HP, Pagano AP, Ryan JP. Subtypes of muscarinic receptors regulating gallbladder cholinergic contractions. Am J Physiol. 1999;276:G1243–G1250. doi: 10.1152/ajpgi.1999.276.5.G1243. [DOI] [PubMed] [Google Scholar]
- 61.Bolton TB, Zholos AV. Activation of M2 muscarinic receptors in guinea-pig ileum opens cationic channels modulated by M3 muscarinic receptors. Life Sci. 1997;60:1121–1128. doi: 10.1016/s0024-3205(97)00056-8. [DOI] [PubMed] [Google Scholar]
- 62.Braverman AS, Tallarida RJ, Ruggieri MR. Interaction between muscarinic receptor subtype signal transduction pathways mediating bladder contraction. Am J Physiol Regul Integr Comp Physiol. 2002;283:R663–R668. doi: 10.1152/ajpregu.00116.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lanzafame A, Christopoulos A, Mitchelson F. Interactions of agonists with an allosteric antagonist at muscarinic acetylcholine M2 receptors. Eur J Pharmacol. 1996;316:27–32. doi: 10.1016/s0014-2999(96)00639-5. [DOI] [PubMed] [Google Scholar]
- 64.Nahorski SR, Tobin AB, Willars GB. Muscarinic M3 receptor coupling and regulation. Life Sci. 1997;60:1039–1045. doi: 10.1016/s0024-3205(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 65.Parekh AB, Brading AF. The M3 muscarinic receptor links to three different transduction mechanisms with different efficacies in circular muscle of guinea-pig stomach. Br J Pharmacol. 1992;106:639–643. doi: 10.1111/j.1476-5381.1992.tb14388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


